Introduction
Postoperative pancreatic fistula (POPF) is generally
recognized as one of the most common complications following
pancreaticoduodenectomy (PD) (1–3).
Although perioperative management and operative techniques used
during pancreatic surgery have improved, it occurs in approximately
5 to 30% of all PD procedures (2,4–6). Clinically-relevant POPF (CR-POPF) can
trigger subsequent postoperative complications such as
intraabdominal abscess and pseudoaneurysm associated with
postpancreatectomy hemorrhage, resulting in longer hospital stays,
increased treatment costs, and even postoperative mortality.
Therefore, identifying patients at high risk for developing CR-POPF
is considered important for improving the clinical outcome in
patients undergoing PD. In this regard, many studies have
identified predictive factors for developing CR-POPF such as body
mass index, diameter of the main pancreatic duct (MPD), and
pancreatic consistency (7–11). In addition to such predictors of
CR-POPF development, treatment of POPF after it develops is also
important for improving clinical outcomes. When considering the
treatment for CR-POPF, while the therapeutic options themselves are
actually important, healing time is the most important issue
regardless of the choice of the therapeutic options. However, few
studies have focused on the time needed for CR-POPF healing, though
many investigators have studied risk factors for developing this
complication. Therefore, in the present study, our aim was to
assess the time needed for CR-POPF healing after PD and,
furthermore, to investigate factors affecting the healing time
based on our experiences.
Patients and methods
A total of consecutive 158 cases underwent PD
between 2009 and 2017 for periampullary diseases at the Department
of Surgery, Toyonaka Municipal Hospital. This study excluded cases
where curative resection was not achieved and those which required
resection of other organs in addition to PD. Among the 158 cases,
CR-POPF developed after PD in 38 cases (24.1%), and these patients
were enrolled in the present study. The clinical and surgical
characteristics of these patients are summarized in Table I. The time needed for CR-POPF healing
was assessed, and factors affecting the healing time were
investigated.
 | Table I.Comparison of the perioperative
factors of patients with and without CR-POPF. |
Table I.
Comparison of the perioperative
factors of patients with and without CR-POPF.
| Factor | All cases
(n=158) | CR-POPF (−)
(n=120) | CR-POPF (+)
(n=38) | P-value |
|---|
| Preoperative
factors |
| Age
(years) | 70±9 | 70±10 | 70±7 | 0.8397 |
| Sex
(male/female) | 93/65 | 65/55 | 28/10 | 0.0331 |
| Height
(cm) | 159.6±9.6 | 159.2±9.7 | 160.6±9.5 | 0.4277 |
| Weight
(kg) | 55.0±9.8 | 54.0±9.6 | 58.1±9.9 | 0.0223 |
| Body mass
index (kg/m2) | 21.5±2.8 | 21.2±2.8 | 22.4±2.6 | 0.0205 |
|
Hemoglobin (g/dl) | 12.2±1.5 | 12.1±1.4 | 12.4±1.5 | 0.2708 |
|
Lymphocyte (/µl) | 1535±736 | 1507±764 | 1623±642 | 0.3958 |
| PT
(%) | 93±15 | 93±14 | 94±16 | 0.7402 |
| Total
protein (g/dl) | 6.8±0.7 | 6.8±0.7 | 6.8±0.5 | 0.3081 |
| Albumin
(g/dl) | 3.5±0.5 | 3.6±0.5 | 3.6±0.4 | 0.4271 |
| Total
cholesterol (mg/dl) | 190±45 | 188±46 | 196±44 | 0.4019 |
|
Cholinesterase (U/l) | 257±84 | 251±87 | 273±69 | 0.1669 |
| Amylase
(U/l) | 107±81 | 102±89 | 98±39 | 0.5639 |
|
Prognostic nutritional
index | 43.0±6.6 | 42.8±6.7 | 44.0±5.7 | 0.2360 |
| Disease |
|
|
| 0.0009 |
|
Pancreatic cancer | 55 | 52 | 3 |
|
| Bile duct
cancer | 43 | 30 | 13 |
|
| Ampullary
cancer | 17 | 11 | 6 |
|
|
Others | 43 | 27 | 16 |
|
| Diameter of MPD
(mm) | 4.1±1.9 | 4.5±2.0 | 2.8±1.1 | <0.0001 |
| Biliary drainage |
|
|
| 0.3186 |
| None | 77 | 60 | 17 |
|
|
External | 16 | 14 | 2 |
|
|
Internal | 65 | 46 | 19 |
|
| Intraoperative
factors |
|
Pancreatic consistency
(soft/hard) | 89/69 | 52/68 | 37/1 | <0.0001 |
|
Pancreaticojejunostomy
procedure (Kakita/Blumgart) | 85/73 | 60/60 | 25/13 | 0.0889 |
| Combined
resection of portal vein (−/+) | 118/40 | 86/34 | 32/6 | >0.9999 |
| Operation
time (min) | 428±99 | 425±100 | 439±98 | 0.4618 |
|
Intraoperative blood loss
(mL) | 844±566 | 814±538 | 936±644 | 0.2489 |
|
Homologous blood transfusion
(−/+) | 132/26 | 102/18 | 30/8 | 0.3805 |
| Postoperative
factors |
|
Postoperative complication
other than CR-POPF (−/+) |
| 101/19 | 28/10 | 0.1457 |
Subtotal stomach-preserving PD was basically adopted
as the PD procedure in the cases. With regard to reconstruction
after the resection, a modified Child method, with
pancreaticojejunostomy (PJ), choledochoduodenostomy, and
gastrojejunostomy with Braun's anastomosis, were performed. The
Kakita method or a modified Blumgart anastomosis method was applied
as techniques for pancreaticojejunostomy based on the surgeon's
preference (12,13). Briefly, in patients with the Kakita
method, in addition to duct-to-mucosa PJ anastomosis, the
pancreatic parenchyma of the stump was approximated to jejunal
seromuscular layer with nonabsorbable interrupted penetrating
sutures. In patients with the modified Blumgart anastomosis group,
a double-armed nonabsorbable suture was used to place a U-suture
with both arms through the pancreatic stump and a longitudinal
suture through the jejunal seromuscular layer. After the
duct-to-mucosa PJ anastomosis, the outer anterior horizontal
mattress sutures on the jejunum with the U-sutures were completed
and tied on the anterior surface of the pancreas to cover the
duct-to-mucosa anastomosis by jejunal serosa. Before completing the
surgery, we placed 2 and 1 closed intraabdominal drainage tubes at
the vicinity of pancreaticojejunostomy and hepaticojejunostomy,
respectively. POPF was diagnosed and stratified by severity into
biochemical leakage (BL), grade B, or grade C according to the
International Study Group of Pancreatic Fistula definition
(14). CR-POPF was defined as grade
B or grade C POPF. Our treatment for CR-POPF was performed
uniformly in all the patients. Briefly, for the treatment, the
abdominal drainage was continued until disappearance of the
abdominal cavity was confirmed at imaging modalities. Cure was
judged to be when the intraabdominal drainage tubes used for the
CR-POPF treatment were removed. The drainage tubes were changed
every 1–2 weeks until their removal. During the treatment, oral
intake was continued. No patients received somatostatin analogs. In
this study, CR-POPF healing time was defined as the length of time
(in days) from the day of the surgery to the day when CR-POPF was
cured. The diameter of the MPD was measured at the resection line
of the pancreas on enhanced CT images. Pancreatic consistency was
judged as soft or hard based on intraoperative findings of the
pancreas.
On the basis of extensive dialogue with the Human
Ethics Review Committee of Toyonaka Municipal Hospital, approval
for an opt-out consent method was given. The study received ethical
approval for the use of an opt-out methodology, and the
participation of the patients was obtained through the opt-out
methodology (certificate no. 2018-05-07).
Data were expressed as mean ± standard deviation.
Differences between groups were assessed by Chi-square test,
Fisher's exact test, or Mann-Whitney U test. Analysis of the
healing time was performed based on cumulative healing rate
calculated with the Kaplan-Meier method. Cumulative healing rates
in subgroups were compared with the log-rank test in univariate
analysis. Multivariate analysis was performed using Cox
proportional hazard regression for identifying independent
variables associated with healing time. A median was used for
turning a continuous variable into a categorical one. Statistical
analyses were performed using StatView (version 5.0; SAS Institute
Inc., Cary, NC, USA). A P<0.05 was considered significant.
Results
Among the 158 patients who underwent PD, CR-POPF
developed in 38 cases (24.1%), while CR-POPF was not identified
postoperatively in the remaining 120 cases (75.9%). Among the 38
cases with CR-POPF, there were 35 cases with grade B and 3 cases
with grade C. Outcome of the patients with grade C was death
derived from POPF. The remaining 120 cases without CR-POPF included
35 cases with BL and 85 cases without BL or CR-POPF. The clinical
and surgical characteristics of the cases with and without CR-POPF
are shown in Table I. Cases with and
without CR-POPF were significantly different in age, weight, body
mass index, disease, diameter of MPD, and pancreatic consistency,
which is consistent with previous reports. CR-POPF was treated
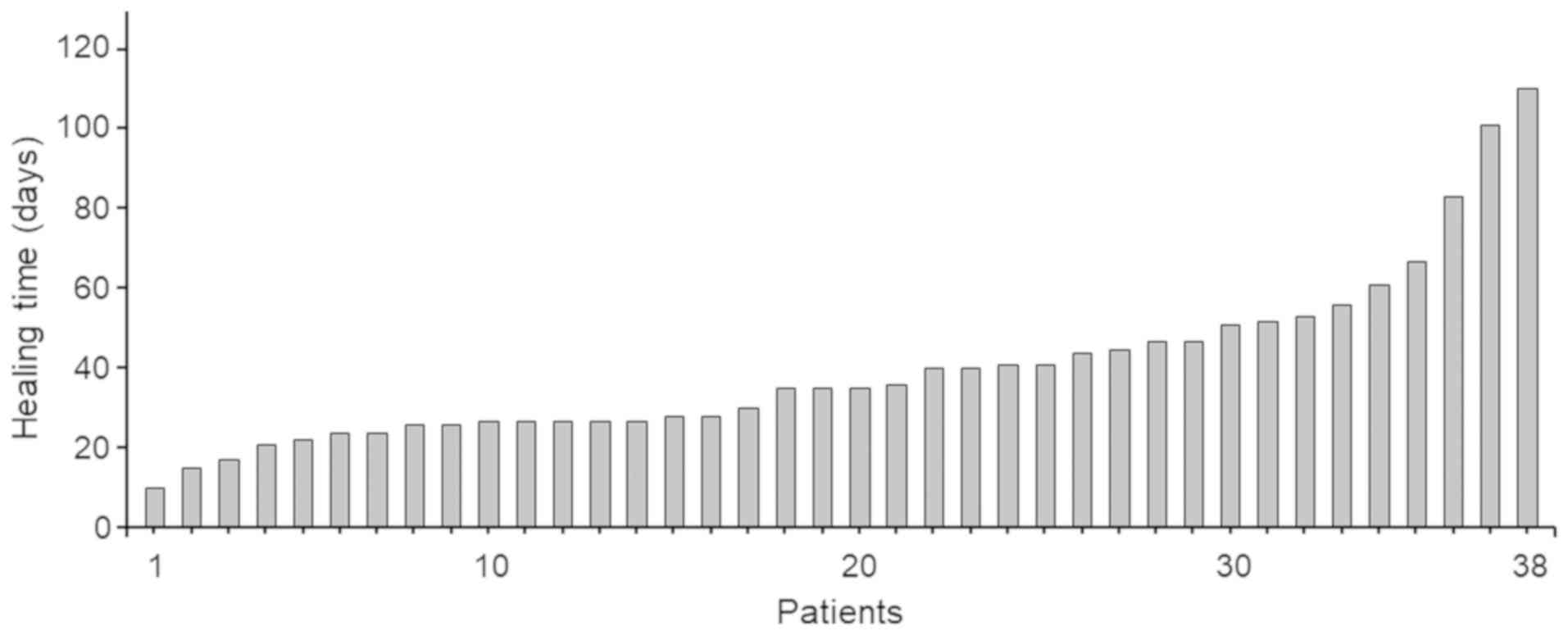
during hospitalization in all patients, with a mean duration of
40.2±21.7 days (median, 35 days; range 10–110 days). The time
needed for healing of CR-POPF in individual cases is shown in
Fig. 1. Univariate analysis using
various factors associated with CR-POPF healing time is summarized
in Table II. The univariate
analysis showed a significant relationship between sex (male vs.
female), procedure for pancreaticojejunostomy (Kakita vs. modified
Blumgart anastomosis), and intraoperative blood loss (<696 vs.
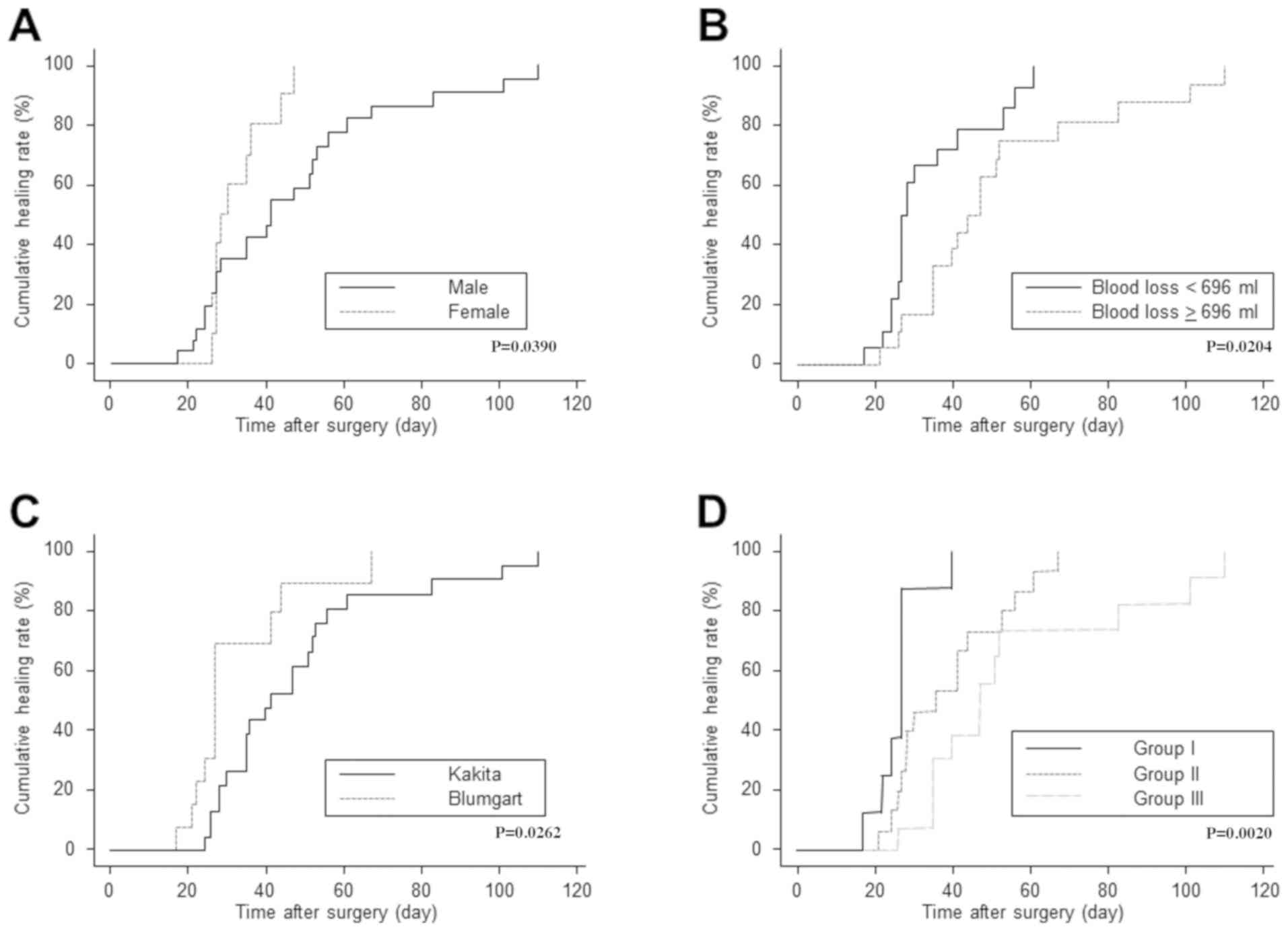
≥696 ml) (P=0.0390, P=0.0262 and P=0.0204, respectively).
Cumulative healing rates after the surgery calculated with the
Kaplan-Meier method and stratified by these significant factors are
shown in Fig. 2A-C. The remaining
factors including age, height, weight, body mass index,
preoperative laboratory parameters such as hemoglobin, lymphocytes,
prothrombin time, total protein, albumin, total cholesterol, and
cholinesterase level, prognostic nutritional index defined by
Onodera et al (15), disease,
diameter of MPD, presence of preoperative biliary drainage,
pancreatic consistency, pancreaticojejunostomy procedure, presence
of combined resection of the portal vein, operation time, presence
of homologous blood transfusion, and presence of postoperative
complication other than CR-POPF were not significantly associated
with CR-POPF healing time.
 | Table II.Determinants of CR-POPF healing time
in patients with CR-POPF. |
Table II.
Determinants of CR-POPF healing time
in patients with CR-POPF.
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Preoperative
factors | No. of patients | P-value | OR | 95% CI | P-value |
|---|
| Age
(year) (<65 vs. ≥65) | 19/19 | 0.9280 |
|
|
|
| Sex (male
vs. female) | 28/10 | 0.0390 | 1.873 | 0.799–4.388 | 0.1487 |
| Height
(cm) (<162 vs. ≥162) | 19/19 | 0.8332 |
|
|
|
| Weight
(kg) (<57 vs. ≥57) | 19/19 | 0.5485 |
|
|
|
| Body
mass index (kg/m2) (<22 vs. ≥22) | 19/19 | 0.9604 |
|
|
|
|
Hemoglobin (g/dl) (<12.9
vs. ≥12.9) | 19/19 | 0.3433 |
|
|
|
|
Lymphocyte (/µl) (<1525 vs.
≥1525) | 19/19 | 0.8480 |
|
|
|
| PT (%)
(<93 vs. ≥93) | 19/19 | 0.2522 |
|
|
|
| Total
protein (g/dl) (<6.9 vs. ≥6.9) | 19/19 | 0.3166 |
|
|
|
| Albumin
(g/dl) | 19/19 | 0.3185 |
|
|
|
| Total
cholesterol (mg/dl) (<190 vs. ≥190) | 19/19 | 0.4927 |
|
|
|
|
Cholinesterase (U/l) (<273
vs. ≥273) | 19/19 | 0.3956 |
|
|
|
| Amylase
(U/l) (<85 vs. ≥85) | 19/19 | 0.2151 |
|
|
|
|
Prognostic nutritional index
(<46 vs. ≥46) | 19/19 | 0.7374 |
|
|
|
| Disease
(pancreatic cancer vs. others) | 3/35 | 0.5887 |
|
|
|
|
Diameter of MPD (mm) (<2.8
vs. ≥2.8) | 19/19 | 0.5152 |
|
|
|
| Biliary
drainage (− vs. +) | 17/21 | 0.9065 |
|
|
|
| Intraoperative
factors |
|
Pancreatic consistency
(Soft/Hard) | 37/1 | 0.7975 |
|
|
|
|
Pancreaticojejunostomy
procedure (Kakita/Blumgart) | 25/13 | 0.0262 | 2.225 | 1.026–4.825 | 0.0429 |
|
Combined resection of portal
vein (− vs. +) | 32/6 | 0.2095 |
|
|
|
|
Operation time (min) (<430
vs. ≥430) | 19/19 | 0.4896 |
|
|
|
|
Intraoperative blood loss (ml)
(<696 vs. ≥696) | 19/19 | 0.0204 | 0.429 | 0.200–0.923 | 0.0305 |
|
Homologous blood transfusion
(− vs. +) | 30/8 | 0.9297 |
|
|
|
| Postoperative
factors |
|
Postoperative complication
other than CR-POPF (−/+) | 28/10 | 0.5091 |
|
|
|
Next, to identify significant independent factors
for the time needed for CR-POPF healing, multivariate Cox
regression analysis was performed using factors identified as
significant variables in the univariate analysis (Table II). The multivariate analysis
demonstrated that intraoperative blood loss (<696 vs. ≥696ml)
and procedure for pancreaticojejunostomy (Kakita vs. modified
Blumgart anastomosis) were significant independent factors for
CR-POPF healing time (P=0.0305 and P=0.0429, respectively)
(Table II). Using these independent
factors, we proposed a practical stratification of the CR-POPF
patients for the prediction of the healing time of CR-POPF. The
stratification was as follows; Group I included patients with
smaller intraoperative blood loss (<696 ml) and adoption of
modified Blumgart anastomosis for the reconstruction, Group II
included those with larger intraoperative blood loss (/≥696 ml) and
adoption of modified Blumgart anastomosis and those with smaller
intraoperative blood loss (<696 ml) and adoption of Kakita
method, and Group III included those with larger intraoperative
blood loss (/≥696 ml) and adoption of Kakita method. When
stratified by the groups, cumulative healing rates after the
surgery were significantly different among the groups (P=0.0020)
(Fig. 2D). Median healing time of
CR-POPF was 24 days in Group I, 36 days in Group II, and 45 days in
Group III.
Discussion
The present study focused on the time needed for
CR-POPF healing in patients who had developed this complication
after PD. We clarified the actual healing time and successfully
identified two statistically significant independent factors
associated with the time needed for healing of CR-POPF. Based on
the fact that CR-POPF potentially causes subsequent critical
postoperative complications such as intraabdominal abscess and
pseudoaneurysm associated with postpancreatectomy hemorrhage, both
predicting and treating CR-POPF are clinically significant issues.
With regard to its prediction, owing to previous studies some
factors have been identified as being predictive for CR-POPF
development such as body mass index, indication disease for the
surgical treatment, diameter of the main pancreatic duct, texture
of the pancreatic parenchyma, and amount of intraoperative bleeding
(7–11,16,17). On
the other hand, few studies have focused on treatment of CR-POPF.
Especially, to the best of our knowledge, the time needed for
CR-POPF healing has not yet been discussed. Based on the
above-mentioned nature of CR-POPF, and considering that CR-POPF
develops in some patients regardless of the predictive factors,
investigation into the time needed for healing should be discussed
more frequently. Earlier recovery after CR-POPF develops could
potentially lead to a lower incidence of the subsequent critical
problems related to CR-POPF as mentioned above. This background led
us to feel that the present study was worth reporting.
The two factors identified as significant
independent factors associated with CR-POPF healing time in the
present study were intraoperative blood loss and the procedure used
for pancreaticojejunostomy. While these factors were identified as
being associated with healing time for the first time, some studies
have reported a significant relationship between these factors and
CR-POPF development. For example, with regard to intraoperative
blood loss, Kawai et al reported that intraoperative
bleeding >1,000 ml is a significant predictive factor for POPF
based on a study by the Japanese Society of
Hepato-Biliary-Pancreatic Surgery (16). Yang et al also reported a
similar study (17). As for the
procedure used for pancreaticojejunostomy, Fujii et al
reported that the rate of CR-POPF formation was significantly lower
in patients where the modified Blumgart anastomosis was used than
in patients where the Kakita method was used (12). Thus, it can be considered that these
two factors are significantly associated not only with POPF
development, but also with CR-POPF healing time.
As for the clinical application of the results of
this study, it is important to consider the merits of a shorter
healing time for CR-POPF. Shortening the time needed for CR-POPF
healing has some potential clinical advantages of which one is
lowering the incidence of subsequent critical postoperative
complications due to CR-POPF. The second is decreasing the cost of
hospitalization due to the shortened hospitalized period. Finally,
shorter healing allows for smoother administration of adjuvant
therapy when the patients have cancers for which adjuvant therapy
is proven to be oncologically effective (18,19). In
this regard, keeping the effect of intraoperative blood loss and
the procedure used for pancreaticojejunostomy and the effect of
these factors on CR-POPF healing time in mind could potentially
ensure these advantages.
The present study has several limitations. The most
obvious one is the study design, which was retrospective and it was
not a prospective, randomized, controlled study. Therefore, there
could be certain biases in several points. For example, a bias
exists in the selection of the procedure for
pancreaticojejunostomy. As mentioned above, the Kakita method or
modified Blumgart anastomosis methods were applied based on the
surgeon's preference, but the latter is more recently developed
than the former, which suggest that patients' backgrounds in the
cases where each method was used are potentially different even
though there were no statistically significant differences in other
background factors between the two groups. The second limitation is
that the data for the present study were based on the experience of
a single institution. This may produce some bias in the
preoperative management of the patients such as surgical
procedures, method for measuring intraoperative blood loss, or
management of the drainage tubes. Taken together, the study has
several potential limitations and, furthermore, the results of the
study are not validated. In future, these results should be
validated in a multi-institutional, prospective, randomized,
controlled trial, using certain criteria as mentioned above.
In summary, we examined the time needed for CR-POPF
healing after PD and identified two statistically significant
independent factors associated with the healing time;
intraoperative blood loss and the type of procedure used for
pancreaticojejunostomy. The findings could provide information that
leads to earlier recovery from CR-POPF.
Acknowledgements
Not applicable.
Funding
No funding was relieved.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YT was responsible for the concept and design of the
study and for data interpretation. KN assisted with data
interpretation and production of the article. SN, HI and TI
assisted in data interpretation and article preparation. KD
supervised the project.
Ethics approval and consent to
participate
Ethical approval for this study was obtained from
the Human Ethics Review Committee of Toyonaka Municipal Hospital
(Certificate no. 2018-05-07). Written informed consent was obtained
from the patients.
Patient consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no competing
interests.
References
|
1
|
de Castro SM, Busch OR, van Gulik TM,
Obertop H and Gouma DJ: Incidence and management of pancreatic
leakage after pancreatoduodenectomy. Br J Surg. 92:1117–1123. 2005.
View Article : Google Scholar
|
|
2
|
Kirihara Y, Takahashi N, Hashimoto Y,
Sclabas GM, Khan S, Moriya T, Sakagami J, Huebner M, Sarr MG and
Farnell MB: Prediction of pancreatic anastomotic failure after
pancreatoduodenectomy: The use of preoperative, quantitative
computed tomography to measure remnant pancreatic volume and body
composition. Ann Surg. 257:512–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu SJ, Shen SL, Li SQ, Hu WJ, Hua YP,
Kuang M, Liang LJ and Peng BG: Risk factors and outcomes of
postoperative pancreatic fistula after pancreatico-duodenectomy: An
audit of 532 consecutive cases. BMC Surg. 15:342015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hashimoto Y and Traverso LW: Pancreatic
anastomotic failure rate after pancreaticoduodenectomy decreases
with microsurgery. J Am Coll Surg. 211:510–521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pecorelli N, Balzano G, Capretti G, Zerbi
A, Di Carlo V and Braga M: Effect of surgeon volume on outcome
following pancreaticoduodenectomy in a high-volume hospital. J
Gastrointest Surg. 16:518–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poon RT, Fan ST, Lo CM, Ng KK, Yuen WK,
Yeung C and Wong J: External drainage of pancreatic duct with a
stent to reduce leakage rate of pancreaticojejunostomy after
pancreaticoduodenectomy: A prospective randomized trial. Ann Surg.
246:425–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aoki S, Miyata H, Konno H, Gotoh M, Motoi
F, Kumamaru H, Wakabayashi G, Kakeji Y, Mori M, Seto Y, et al: Risk
factors of serious postoperative complications after
pancreaticoduodenectomy and risk calculators for predicting
postoperative complications: A nationwide study of 17,564 patients
in Japan. J Hepatobiliary Pancreat Sci. 24:243–251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gaujoux S, Cortes A, Couvelard A, Noullet
S, Clavel L, Rebours V, Lévy P, Sauvanet A, Ruszniewski P and
Belghiti J: Fatty pancreas and increased body mass index are risk
factors of pancreatic fistula after pancreaticoduodenectomy.
Surgery. 148:15–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu BY, Wan T, Zhang WZ and Dong JH: Risk
factors for postoperative pancreatic fistula: Analysis of 539
successive cases of pancreaticoduodenectomy. World J Gastroenterol.
22:7797–7805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shubert CR, Wagie AE, Farnell MB, Nagorney
DM, Que FG, Reid Lombardo KM, Truty MJ, Smoot RL and Kendrick ML:
Clinical Risk Score to Predict Pancreatic Fistula after
Pancreatoduodenectomy: Independent External Validation for Open and
Laparoscopic Approaches. J Am Coll Surg. 221:689–698. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tranchart H, Gaujoux S, Rebours V,
Vullierme MP, Dokmak S, Levy P, Couvelard A, Belghiti J and
Sauvanet A: Preoperative CT scan helps to predict the occurrence of
severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg.
256:139–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujii T, Sugimoto H, Yamada S, Kanda M,
Suenaga M, Takami H, Hattori M, Inokawa Y, Nomoto S, Fujiwara M, et
al: Modified Blumgart anastomosis for pancreaticojejunostomy:
Technical improvement in matched historical control study. J
Gastrointest Surg. 18:1108–1115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kakita A, Yoshida M and Takahashi T:
History of pancreaticojejunostomy in pancreaticoduodenectomy:
Development of a more reliable anastomosis technique. J
Hepatobiliary Pancreat Surg. 8:230–237. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bassi C, Marchegiani G, Dervenis C, Sarr
M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink
MG, et al International Study Group on Pancreatic Surgery (ISGPS),
: The 2016 update of the International Study Group (ISGPS)
definition and grading of postoperative pancreatic fistula: 11
Years After. Surgery. 161:584–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
16
|
Kawai M, Kondo S, Yamaue H, Wada K, Sano
K, Motoi F, Unno M, Satoi S, Kwon AH, Hatori T, et al: Predictive
risk factors for clinically relevant pancreatic fistula analyzed in
1,239 patients with pancreaticoduodenectomy: Multicenter data
collection as a project study of pancreatic surgery by the Japanese
Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary
Pancreat Sci. 18:601–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang H, Lu XF, Xu YF, Liu HD, Guo S, Liu Y
and Chen YX: Application of air insufflation to prevent clinical
pancreatic fistula after pancreaticoduodenectomy. World J
Gastroenterol. 21:1872–1879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al JASPAC 01 Study Group, : Adjuvant chemotherapy of S-1
versus gemcitabine for resected pancreatic cancer: A phase 3,
open-label, randomised, non-inferiority trial (JASPAC 01). Lancet.
388:248–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neoptolemos JP, Palmer DH, Ghaneh P,
Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA,
Cunningham D, Wadsley J, et al European Study Group for Pancreatic
Cancer, : Comparison of adjuvant gemcitabine and capecitabine with
gemcitabine monotherapy in patients with resected pancreatic cancer
(ESPAC-4): A multicentre, open-label, randomised, phase 3 trial.
Lancet. 389:1011–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|