Introduction
Small-cell lung cancer (SCLC) is a high-grade
neuroendocrine lung cancer and comprises about 15% of all lung
cancers (1). SCLC is characterized
by rapid growth and early metastasis to distant organs, and most
patients are diagnosed with extensive disease. Systemic
chemotherapy is the standard treatment, and the response rate to
first-line platinum-based chemotherapy is high. However, most
patients experience disease progression while on or after
first-line chemotherapy (2). The
response of second-line chemotherapy depends on the interval time
from the last day of first-line chemotherapy to the day of
confirmed relapse: Sensitive disease (the interval >90 days) has
an overall response rate (ORR) of approximately 25% and refractory
disease (the interval <90 days) has an ORR of approximately 10%
(3,4). Topotecan is safe and effective for the
sensitive disease (5,6). Subgroup analysis of a phase III trial
suggested the effectiveness of amrubicin for patients with the
refractory disease (7). Furthermore,
a recent study demonstrated the antitumor activity with durable
response of immune-checkpoint inhibitors, nivolumab monotherapy and
nivolumab plus ipilimumab in patients with both sensitive and
refractory disease (8). However,
patients with severe complications including idiopathic
interstitial pneumonias (IIPs) were excluded from these clinical
trials, and therefore the survival benefit of chemotherapy for SCLC
patients with IIPs is unclear.
IIPs are a common comorbidity of lung cancer and
affect 5.8% of surgically-resected lung cancer patients (9). Among patients with IIPs, the prevalence
of lung cancer at the diagnosis of IIPs was 6–17% (10). The presence of IIPs is an obstacle to
systemic chemotherapy because of the risk of death and the
deterioration of quality of life attributed to acute exacerbation
(AE) of IIPs (11). A combination of
platinum agents, cisplatin or carboplatin, plus etoposide is the
standard treatment regimen for chemotherapy-naïve SCLC patients
with extensive disease (2,3). The safety and efficacy of these
regimens for SCLC patients with IIPs or interstitial lung disease
were evaluated in two retrospective studies and one prospective
study in Japan. AE related to platinum agents plus etoposide was
observed in 5.9% (1/17) of patients with IIPs and 1.9% (1/52) of
patients with preexisting interstitial lung disease (12,13). The
ORRs, the median progression-free survival (PFS), and the median
survival time (MST) of the combination of platinum agents plus
etoposide for SCLC patients with IIPs were 63.6–88.2%, 4.5–5.5
months, and 7.0–9.4 months, respectively (12–14).
Compared to the MST of SCLC patients who received only supportive
care, which is 2–3 months (15),
these results reveal the benefit of first-line chemotherapy for
SCLC patients with IIPs. In the second-line setting,
topotecan-induced AE occurred in 20.0–23.8% of SCLC patients with
interstitial lung disease (16,17). Two
retrospective studies reported that the incidences of
amrubicin-induced AE were 10% (1/10 patients) and 17.6% (3/17
patients) in lung cancer patients with preexisting interstitial
lung disease (13,18). Thus, the standard chemotherapy
regimens for relapsed SCLC may be ineligible for patients with IIPs
because of the high incidence of AE.
The efficacy of single agent paclitaxel (PTX) for
previously treated SCLC patients was assessed in two phase II
studies, and the ORRs of PTX were 23.8 and 29.2% (19,20). PTX
in combination with carboplatin is a candidate regimen for advanced
non-small cell lung cancer (NSCLC) patients with IIPs in terms of
the low incidence of AE (21).
Furthermore, a retrospective study suggested the safety and
effectiveness of second-line chemotherapy including PTX in relapsed
SCLC patients with IIPs (16). Thus,
these results suggested that PTX may be a treatment option for
relapsed SCLC patients with IIPs. However, no study has examined
the safety and efficacy of PTX in relapsed SCLC patients with IIPs.
Therefore, we conducted a retrospective analyses to evaluate the
safety and efficacy of PTX in relapsed SCLC patients with IIPs.
Materials and and methods
Study population and design
Between January 2010 and August 2017, we enrolled 32
patients (all Japanese) who were diagnosed with both SCLC and IIPs
and received first-line chemotherapy at Tokushima University
hospital. Of the 32 patients, three died during first-line
chemotherapy: Two died from tumor-progression and another died from
respiratory infection following febrile neutropenia. They were
excluded from the analysis. We retrospectively analyzed clinical
features including age, sex, smoking history, performance status
(PS), and clinical stage at the end of first-line chemotherapy.
Radiologic features of IIPs on high-resolution computed tomography
(HRCT) and respiratory function test at the diagnosis of SCLC were
also analyzed. The clinical stage was determined on the basis of
the international TNM criteria for cancer staging. PS was assessed
according to the Eastern Cooperative Oncology Group (ECOG)
classification. The cumulative cigarette exposure (pack-years) was
calculated by multiplying the average number of packs of cigarettes
smoked per day by the number of years for smoking. The diagnosis of
IIPs was made according to the reported criteria (22). IIPs were classified into two groups:
Idiopathic pulmonary fibrosis (IPF), which consists of usual
interstitial pneumonia (UIP) or a possible UIP pattern by HRCT, and
non-IPF, which consists of an inconsistent UIP pattern by HRCT
according to the official ATS/ERS/JRS/ALAT statement (23). AE of IIPs was diagnosed using the
international working group report (24).
Statistical analysis
All comparisons between populations were performed
by the Fisher's exact test or Student's t-test, as
appropriate. ORR was defined using Response Evaluation Criteria in
Solid Tumors (25). Disease control
rate (DCR) was defined as the proportion of patients with complete
response, partial response, and stable disease. Overall survival
(OS) was defined as the time from the date of initiation of
PTX-containing regimens until the date of death from any cause. PFS
was defined as the time from the date of the initiation of
PTX-containing regimens until the date of death or evidence of
tumor progression. Patients who were alive or no evidence of tumor
progression at the time of analysis were censored at the last known
date of follow-up. OS and PFS were estimated using the Kaplan-Meier
method, and the log-rank test was used to assess differences
between groups. Results are reported as the mean ± standard error
of mean (SEM). Adverse events were graded using National Cancer
Institute Common Terminology Criteria for Adverse Events, version
4.0. P-values less than 0.05 were considered significant.
Statistical analyses were performed using GraphPad PRISM (5.01;
GraphPad Software, Inc., La Jolla, CA, USA).
Results
Characteristics of SCLC patients with
IIPs
We identified 29 SCLC patients with IIPs who
received first-line chemotherapy of a platinum-based drug plus
etoposide. Of the 29 patients, 17 received PTX-containing regimens
(PTX group) and 12 received best supportive care (BSC: BSC group)
after disease progression. AE of IIPs related to first-line
chemotherapy occurred in one patient in the BSC group. Patients in
the BSC group had poor PS compared to those in the PTX group, and
the ORR to first-line chemotherapy was similar between the groups
(Table I). Of the 17 patients that
received PTX, all but one were refractory-relapsed cases; 12
received PTX monotherapy, four received nanoparticle albumin-bound
PTX monotherapy, and one received combination therapy with
carboplatin plus PTX (Tables I and
II).
 | Table I.Comparison of the characteristics of
SCLC patients with IIPs. |
Table I.
Comparison of the characteristics of
SCLC patients with IIPs.
| Variables | PTX n=17 | BSC n=12 | P-value |
|---|
| Age (years) |
|
| 0.365a |
| Mean (SEM) | 72.0±2.3 | 74.8±1.3 |
|
| Sex |
|
| 1.000b |
| Male | 16 (94%) | 11 (92%) |
|
|
Female | 1 (6%) | 1 (8%) |
|
| ECOG PS |
|
| 0.119b |
| 0, 1 | 13 (76%) | 5 (42%) |
|
| ≥2 | 4 (24%) | 7 (58%) |
|
| Clinical stage |
|
| 0.422b |
| LD | 4 (24%) | 5 (42%) |
|
| ED | 13 (76%) | 7 (58%) |
|
| Cigarette exposure
(pack-years) |
|
| 0.477c |
| Mean
(SEM) | 68.8±10.2 | 80.3±12.2 |
|
| Type of IIPs |
|
| 1.000b |
| IPF | 6 (35%) | 5 (42%) |
|
|
Non-IPF | 11 (65%) | 7 (58%) |
|
| Response to
first-line therapy |
|
| 1.000b |
| CR,
PR | 12 (71%) | 9 (75%) |
|
| SD,
PD | 5 (29%) | 3 (25%) |
|
| Type of
relapse |
|
| 0.279b |
|
Sensitive | 1 (6%) | 3 (25%) |
|
|
Refractory | 16 (94%) | 9 (75%) |
|
 | Table II.Response of second-line chemotherapy
for SCLC patients with IIPs. |
Table II.
Response of second-line chemotherapy
for SCLC patients with IIPs.
| Regimen | n | ORR n
(%) | DCR n
(%) |
|---|
| PTX | 12 | 3 (25.0) | 6 (50.0) |
| nab-PTX | 4 | 2 (50.0) | 2 (50.0) |
| CBDCA+PTX | 1 | 0 (0) | 0 (0) |
| All | 17 | 5 (29.4) | 8 (47.1) |
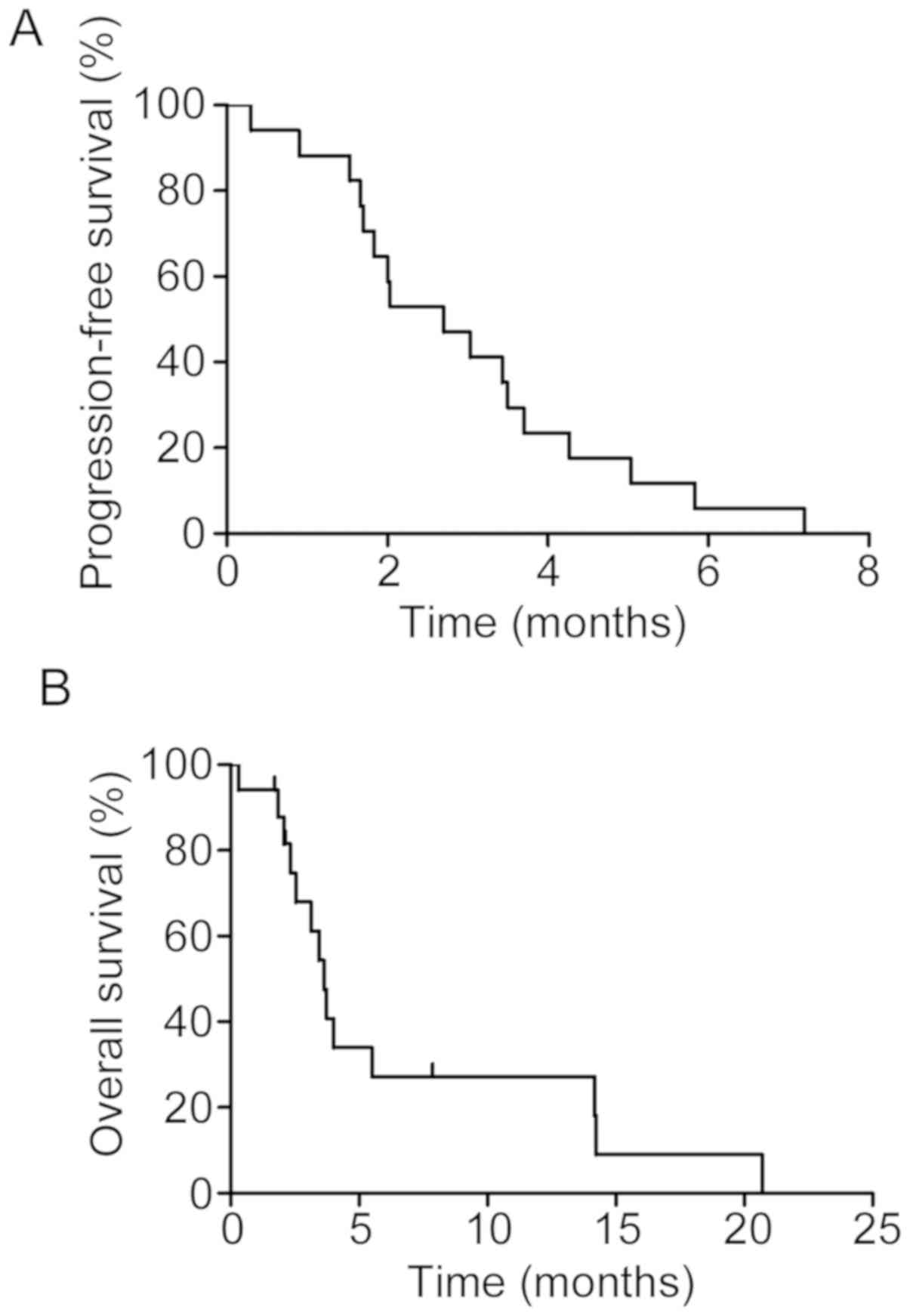
Efficacy of PTX in SCLC patients with
IIPs
During the observation period, 14 patients (82.4%)
in the PTX group and 10 (83.3%) patients in the BSC group died. The
ORR and the DCR of PTX-containing regimens were 29.4 and 47.1%,
respectively (Table II). The median
PFS and the MST of PTX-containing regimens were 2.7 months [95%
confidence interval (CI), 1.6–3.6 months] and 3.6 months (95% CI,
2.3–14.0 months), respectively (Fig.
1).
Toxicity of PTX in SCLC patients with
IIPs
The median number of PTX treatment cycles was two
(range 1–7). The most common grade 3–4 adverse events were
hematological toxicities in 12 (70.6%) and seven (47.1%) patients
with neutropenia and leukopenia, respectively (Table III). Two patients experienced
febrile neutropenia (11.8%) and one of them died from respiratory
infection. For non-hematologic toxicity, sensory neuropathy of
grade 2 or lower and AE of IIPs were observed in four (23.5%) and
five patients (29.4%), respectively. The characteristics of five
patients that experienced AE of IIPs are summarized in Table IV. All received broad-spectrum
antibiotics and high-dose intravenous methylprednisolone pulse
therapy followed by oral prednisolone. Four recovered from AE of
IIPs, but one died from respiratory failure. No significant factor
associated with PTX-induced AE of IIPs was observed (Table V). During the observation period, AE
of IIPs was not observed in the BSC group.
 | Table III.PTX-related adverse events in SCLC
patients with IIPs. |
Table III.
PTX-related adverse events in SCLC
patients with IIPs.
| Adverse events | n (%) |
|---|
| Grade ≥3 |
|
|
Hematologic |
|
|
Leukopenia | 7 (47.1) |
|
Neutropenia | 12 (70.6) |
|
Anemia | 2 (11.8) |
|
Thrombocytopenia | 2 (11.8) |
|
Febrile
neutropenia | 2 (11.8) |
| Any grade |
|
|
Non-hematologic |
|
|
Neuropathy | 4 (23.5) |
|
Pneumonitis | 5 (29.4) |
 | Table IV.The characteristics of patients who
developed PTX-induced AE of IIPs. |
Table IV.
The characteristics of patients who
developed PTX-induced AE of IIPs.
|
| Case |
|---|
|
|
|
|---|
| Variables | 1 | 2 | 3 | 4 | 5 |
|---|
| Age (years) | 67 | 68 | 69 | 69 | 84 |
| Sex | Male | Male | Male | Male | Female |
| ECOG PS | 2 | 0 | 1 | 1 | 1 |
| Clinical stage | IVB | IVB | IVB | IVB | IVA |
| Cigarette exposure
(pack-years) | 45 | 64 | 37 | 84 | 20 |
| Type of IIPs | Inconsistent | Inconsistent | UIP | UIP | Inconsistent |
| History of thoracic
radiotherapy | No | No | No | No | No |
| KL-6 (U/ml) | 520 | 2,210 | 387 | 2,107 | 377 |
| LDH (IU/l) | 638 | 228 | 346 | 256 | 221 |
| CRP (mg/dl) | 7.9 | 0.4 | 1.5 | 0.3 | 0.2 |
| %VC (%) | 104.0 | 86.3 | 87.9 | 94.0 | 56.6 |
| CTCAE Grade | 5 | 3 | 3 | 3 | 3 |
| OS (months) | 0.3 | 2.1a | 3.4 | 3.6 | 4.0 |
 | Table V.Comparison of characteristics between
SCLC patients with or without PTX-induced AE of IIPs. |
Table V.
Comparison of characteristics between
SCLC patients with or without PTX-induced AE of IIPs.
| Variables | Without AE of IIPs
n=12 | With AE of IIPs
n=5 | P-value |
|---|
| Age (years) |
|
| 0.779a |
| Mean (SEM) | 72.5±3.1 | 71.0±3.3 |
|
| Sex |
|
| 0.294b |
|
Male | 12 (100%) | 1 (20%) |
|
|
Female | 0 | 4 (80%) |
|
| ECOG PS |
|
| 0.538b |
| 0,
1 | 10 (83%) | 3 (60%) |
|
| ≥2 | 2 (17%) | 2 (40%) |
|
| Type of IIPs |
|
| 1.000b |
|
IPF | 4 (33%) | 3 (60%) |
|
|
Non-IPF | 8 (67%) | 2 (40%) |
|
| KL-6 (U/ml) | 484.7±87.5 | 1120.0±424.9 | 0.217c |
| LDH (IU/l) | 265.3±21.5 | 337.8±78.3 | 0.422c |
| CRP (mg/dl) | 2.7±1.2 | 2.1±1.5 | 0.758a |
| %VC (%) | 80.5±8.8 | 85.8±7.9 | 0.708a |
Discussion
In this study, we evaluated the safety and efficacy
of PTX in relapsed SCLC patients with IIPs and showed that
PTX-containing regimens had promising anti-tumor activity against
refractory-relapsed SCLC patients with IIPs. However, the survival
benefit of PTX in relapsed SCLC patients with IIPs appeared to be
limited.
Two phase II studies evaluated the effectiveness of
PTX for previously treated SCLC patients without IIPs (19,20), and
the ORRs of PTX were 20.0 and 29.2% (19,20).
Furthermore, the ORR of PTX with or without carboplatin for both
sensitive (39%) and refractory (61%) SCLC patients with IIPs in the
second line setting was 27.8% (16).
We found that the ORR of PTX-containing regimens in
refractory-relapsed SCLC patients with IIPs was 29.4%, which was
comparable to previous reports. Because the ORR of second line
chemotherapy for refractory-relapsed SCLC patients was 14.8%, PTX
may be effective for refractory-relapsed SCLC patients with or
without IIPs (4). However, we found
that the MST of second-line PTX in refractory-relapsed SCLC
patients with IIPs was 3.6 months. The MST of PTX in both sensitive
(52%) and refractory (48%) relapsed SCLC patients without IIPs was
5.8 months (20). Furthermore, the
MST of second-line chemotherapy including PTX in both sensitive
(39%) and refractory (61%) SCLC patients with IIPs was 7.1 months
(16). Compared to previous reports,
the survival benefit of PTX in this study was limited, which may be
because all patients except for one were refractory-relapsed
cases.
Severe toxicities related to PTX may also limit the
survival benefit of PTX. Grade 3 or higher pneumonitis related to
PTX-induced AE of IIPs was observed in five patients (29.4%) and
one of them died from respiratory failure in spite of high-dose
corticosteroid therapy. AE of IIPs related to second-line PTX was
observed in 11% (2/18 patients) of SCLC patients with IPF and at
least one of two patients died from pneumonitis (16). In NSCLC patients with IIPs, AE of
IIPs related to PTX administered as first-line chemotherapy was
observed in 9.5% (10/105 patients), and the incidence of AE of IIPs
related to PTX was lower than that related to other agents
(21,26–28).
Therefore, PTX in combination with carboplatin is a good candidate
regimen for NSCLC patients with IIPs. An increased risk of AE of
IIPs was observed in lung cancer patients with IIPs that received
second- or more-line chemotherapy compared to those that received
first-line chemotherapy (13,29).
Therefore, the risk of AE of IIPs related to PTX in relapsed SCLC
patients may be higher compared to that in NSCLC patients that
received PTX as a first-line chemotherapy. Although, we did not
find any factors associated with PTX-induced AE of IIPs, UIP
pattern was reported to be an independent risk factor for
chemotherapy-related AE of interstitial lung disease (ILD)
(30). In addition, the baseline
serum Krebs von den Lungen-6 (KL-6) level with cut-off values of
1300 U/ml and a disease severity based on the partial pressure of
arterial oxygen (PaO2) were associated with the
development of AE of IPF (31,32).
Consistent with a previous study, baseline serum KL-6 levels were
higher in patients with AE of IIPs than those without AE of IIPs,
and all but two patients with baseline serum KL-6 levels above 1300
U/ml developed AE of IIPs. The GAP index helps predicts mortality
in patients with IPF (33). A recent
study demonstrated that modified GAP index score, which was
calculated by four predictors: ILD subtype, sex, age, and forced
vital capacity, predicted the risk of AE of ILD associated with
chemotherapy in NSCLC patients with ILD (34). The 1-year incidence of AE of ILD in
patients with modified GAP index stage I was 14%, and an increased
risk of AE of ILD was observed in patients with modified GAP stage
II and III. The incidence rate in patients with GAP index stage I
was equivalent to that observed in the natural course of IPF
patients, and therefore patients with modified GAP index stage I
may be indicated for chemotherapy against lung cancer (34). In this study, all five patients who
experienced AE of IIPs associated with PTX corresponded to modified
GAP stage II (data not shown). The indication of PTX in relapsed
SCLC patients with IIPs should be carefully considered, especially
in those with UIP pattern, high baseline serum KL-6 level, low
baseline PaO2, and modified GAP stage II or III.
In this study, grade 3 or 4 neutropenia was observed
in 12 (70.6%) of 17 patients. The high incidence of neutropenia was
comparable to past clinical trials of weekly PTX or topotecan for
relapsed SCLC patients (6,20). Although most adverse events
associated with myelosuppression were manageable, one patient died
from respiratory infection caused from febrile neutropenia. A
clinical trial of weekly PTX for relapsed SCLC patients also
reported one case (4.8%) of treatment-related death caused by
neutropenic pneumonia (20). When we
administer PTX-containing regimens in relapsed SCLC patients,
caution is warranted for neutropenia and infection associated with
neutropenia.
There were several limitations in the present study.
First, this study did not have sufficient power to evaluate the
precise clinical benefit of PTX and may have overestimated the
incidence of PTX-related AE of IIPs because of the small number of
patients and the retrospective nature of the study at a single
institution. Second, the diagnoses of IIPs were made clinically
with no histological confirmation in all patients. Because SCLC
patients usually have no indications for surgery at diagnosis and
should receive chemotherapy as soon as possible because of its
rapid growth, it is usually difficult to obtain a specimen from
non-tumor-bearing fibrotic areas.
In conclusion, this was the first retrospective
study to evaluate the safety and efficacy of PTX-containing
regimens in relapsed SCLC patients with IIPs. Although
PTX-containing regimens demonstrated promising anti-tumor activity
against relapsed SCLC with IIPs, the survival benefit was limited
because of the high incidence of PTX-related AE of IIPs and
treatment-related death. The administration of PTX in relapsed SCLC
patients with IIPs should carefully considered and be performed
with particular care because of pulmonary toxicity.
Ackonowledgements
Not applicapable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AS, MH and YN designed the study. AS, HO and KO
contributed to the data acquisition. AS, MH, HG and HN analyzed and
interpreted the patient data. AS, MH and YN drafted the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of Tokushima University Hospital (IRB approval number: 2973).
For this type of study formal consent was not required. Information
about the current study was disclosed to patients instead of
obtaining their written informed consent, and patients who declined
to participate were excluded.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gazdar AF, Bunn PA and Minna JD:
Small-cell lung cancer: What we know, what we need to know and the
path forward. Nat Rev Cancer. 17:725–737. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Früh M, De Ruysscher D, Popat S, Crinò L,
Peters S and Felip E; ESMO Guidelines Working Group, : Small-cell
lung cancer (SCLC): ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 6 (24
Suppl):vi99–vi105. 2013.
|
|
3
|
Kalemkerian GP, Loo BW, Akerley W, Attia
A, Bassetti M, Boumber Y, Decker R, Dobelbower MC, Dowlati A,
Downey RJ, et al: NCCN guidelines insights: Small cell lung cancer,
Version 2.2018. J Natl Compr Cancer Netw. 16:1171–1182. 2018.
View Article : Google Scholar
|
|
4
|
Owonikoko TK, Behera M, Chen Z, Bhimani C,
Curran WJ, Khuri FR and Ramalingam SS: A systematic analysis of
efficacy of second-line chemotherapy in sensitive and refractory
small-cell lung cancer. J Thorac Oncol. 7:866–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk
Y, Čučeviá B, Juhasz G, Thatcher N, Ross GA, Dane GC and Crofts T:
Phase III trial comparing supportive care alone with supportive
care with oral topotecan in patients with relapsed small-cell lung
cancer. J Clin Oncol. 24:5441–5447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eckardt JR, von Pawel J, Pujol JL, Papai
Z, Quoix E, Ardizzoni A, Poulin R, Preston AJ, Dane G and Ross G:
Phase III study of oral compared with intravenous topotecan as
second-line therapy in small-cell lung cancer. J Clin Oncol.
25:2086–2092. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Von Pawel J, Jotte R, Spigel DR, O'Brien
ME, Socinski MA, Mezger J, Steins M, Bosquee L, Bubis J, Nackaerts
K, et al: Randomized phase III trial of Amrubicin versus topotecan
as second-line treatment for patients with small-cell lung cancer.
J Clin Oncol. 32:4012–4019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antonia SJ, López-Martin JA, Bendell J,
Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F,
et al: Nivolumab alone and nivolumab plus ipilimumab in recurrent
small-cell lung cancer (CheckMate 032): A multicentre, open-label,
phase 1/2 trial. Lancet Oncol. 17:883–895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sato T, Teramukai S, Kondo H, Watanabe A,
Ebina M, Kishi K, Fujii Y, Mitsudomi T, Yoshimura M, Maniwa T, et
al: Impact and predictors of acute exacerbation of interstitial
lung diseases after pulmonary resection for lung cancer. J Thorac
Cardiovasc Surg. 147:1604–1611.e3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raghu G, Nyberg F and Morgan G: The
epidemiology of interstitial lung disease and its association with
lung cancer. Br J Cancer. 2 Suppl 91:S3–S10. 2004. View Article : Google Scholar
|
|
11
|
Togashi Y, Masago K, Handa T, Tanizawa K,
Okuda C, Sakamori Y, Nagai H, Kim YH and Mishima M: Prognostic
significance of preexisting interstitial lung disease in Japanese
patients with small-cell lung cancer. Clin Lung Cancer. 13:304–311.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Minegishi Y, Kuribayashi H, Kitamura K,
Mizutani H, Kosaihira S, Okano T, Seike M, Azuma A, Yoshimura A,
Kudoh S and Gemma A: The feasibility study of carboplatin plus
etoposide for advanced small cell lung cancer with idiopathic
interstitial pneumonias. J Thorac Oncol. 6:801–807. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida T, Yoh K, Goto K, Niho S, Umemura
S, Ohmatsu H and Ohe Y: Safety and efficacy of platinum agents plus
etoposide for patients with small cell lung cancer with
interstitial lung disease. Anticancer Res. 33:1175–1180.
2013.PubMed/NCBI
|
|
14
|
Watanabe N, Taniguchi H, Kondoh Y, Kimura
T, Kataoka K, Nishiyama O, Kondo M and Hasegawa Y: Chemotherapy for
extensive-stage small-cell lung cancer with idiopathic pulmonary
fibrosis. Int J Clin Oncol. 19:260–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pelayo Alvarez M, Westeel V, Cortés-Jofré
M and Bonfill Cosp X: Chemotherapy versus best supportive care for
extensive small cell lung cancer. Cochrane Database Syst Rev.
27:CD0019902013.
|
|
16
|
Fujimoto D, Shimizu R, Kato R, Sato Y,
Kogo M, Ito J, Teraoka S, Otoshi T, Nagata K, Nakagawa A, et al:
Second-line chemotherapy for patients with small cell lung cancer
and interstitial lung disease. Anticancer Res. 35:6261–6266.
2015.PubMed/NCBI
|
|
17
|
Enomoto Y, Inui N, Imokawa S, Karayama M,
Hasegawa H, Ozawa Y, Matsui T, Yokomura K and Suda T: Safety of
topotecan monotherapy for relapsed small cell lung cancer patients
with pre-existing interstitial lung disease. Cancer Chemother
Pharmacol. 76:499–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miura Y, Saito Y, Atsumi K, Takeuchi S,
Miyanaga A, Mizutani H, Minegishi Y, Noro R, Seike M, Shinobu K, et
al: Interstitial lung disease associated with amrubicin
chemotherapy in patients with lung cancer: A single institutional
study. Jpn J Clin Oncol. 46:674–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smit EF, Fokkema E, Biesma B, Groen HJ,
Snoek W and Postmus PE: A phase II study of paclitaxel in heavily
pretreated patients with small-cell lung cancer. Br J Cancer.
77:347–351. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamamoto N, Tsurutani J, Yoshimura N, Asai
G, Moriyama A, Nakagawa K, Kudoh S, Takada M, Minato Y and Fukuoka
M: Phase II study of weekly paclitaxel for relapsed and refractory
small cell lung cancer. Anticancer Res. 26:777–781. 2006.PubMed/NCBI
|
|
21
|
Minegishi Y, Sudoh J, Kuribayasi H,
Mizutani H, Seike M, Azuma A, Yoshimura A, Kudoh S and Gemma A: The
safety and efficacy of weekly paclitaxel in combination with
carboplatin for advanced non-small cell lung cancer with idiopathic
interstitial pneumonias. Lung Cancer. 71:70–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Travis WD, Costabel U, Hansell DM, King TE
Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU,
et al: An official American Thoracic Society/European Respiratory
Society statement: Update of the international multidisciplinary
classification of the idiopathic interstitial pneumonias. Am J
Respir Crit Care Med. 188:733–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J Respir Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Collard HR, Ryerson CJ, Corte TJ, Jenkins
G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM,
et al: Acute exacerbation of idiopathic pulmonary fibrosis an
international working group report. Am J Respir Crit Care Med.
194:265–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kenmotsu H, Naito T, Mori K, Ko R, Ono A,
Wakuda K, Imai H, Taira T, Murakami H, Endo M and Takahashi T:
Effect of platinum-based chemotherapy for non-small cell lung
cancer patients with interstitial lung disease. Cancer Chemother
Pharmacol. 75:521–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimizu R, Fujimoto D, Kato R, Otoshi T,
Kawamura T, Tamai K, Matsumoto T, Nagata K, Otsuka K, Nakagawa A,
et al: The safety and efficacy of paclitaxel and carboplatin with
or without bevacizumab for treating patients with advanced
nonsquamous non-small cell lung cancer with interstitial lung
disease. Cancer Chemother Pharmacol. 74:1159–1166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shukuya T, Ishiwata T, Hara M, Muraki K,
Shibayama R, Koyama R and Takahashi K: Carboplatin plus weekly
paclitaxel treatment in non-small cell lung cancer patients with
interstitial lung disease. Anticancer Res. 30:4357–4361.
2010.PubMed/NCBI
|
|
29
|
Kakiuchi S, Hanibuchi M, Tezuka T, Saijo
A, Otsuka K, Sakaguchi S, Toyoda Y, Goto H, Kawano H, Azuma M, et
al: Analysis of acute exacerbation of interstitial lung disease
associated with chemotherapy in patients with lung cancer: A
feasibility of S-1. Respir Investig. 55:145–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kenmotsu H, Naito T, Kimura M, Ono A,
Shukuya T, Nakamura Y, Tsuya A, Kaira K, Murakami H, Takahashi T,
et al: The risk of cytotoxic chemotherapy-related exacerbation of
interstitial lung disease with lung cancer. J Thorac Oncol.
6:1242–1246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohshimo S, Ishikawa N, Horimasu Y, Hattori
N, Hirohashi N, Tanigawa K, Kohno N, Bonella F, Guzman J and
Costabel U: Baseline KL-6 predicts increased risk for acute
exacerbation of idiopathic pulmonary fibrosis. Respir Med.
108:1031–1039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Homma S, Sugino K and Sakamoto S: The
usefulness of a disease severity staging classification system for
IPF in Japan: 20 years of experience from empirical evidence to
randomized control trial enrollment. Respir Investig. 53:7–12.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ley B, Ryerson CJ, Vittinghoff E, Ryu JH,
Tomassetti S, Lee JS, Poletti V, Buccioli M, Elicker BM, Jones KD,
et al: A multidimensional index and staging system for idiopathic
pulmonary fibrosis. Ann Intern Med. 156:684–691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kobayashi H, Naito T, Omae K, Omori S,
Nakashima K, Wakuda K, Ono A, Kenmotsu H, Murakami H, Endo M and
Takahashi T: ILD-NSCLC-GAP index scoring and staging system for
patients with non-small cell lung cancer and interstitial lung
disease. Lung Cancer. 121:48–53. 2018. View Article : Google Scholar : PubMed/NCBI
|