Introduction
Up to 40% of the patients diagnosed with non-small
cell lung cancer (NSCLC) develop brain metastases (BMs) over the
course of their disease (1,2). Patients with untreated BMs have an
extremely poor prognosis, with a median survival of 1–2 months
(3), although survival can be
improved to a median of 4–6 months with the introduction of whole
brain radiotherapy (WBRT) (4). WBRT
has long been recognized as the standard treatment for patients
with multiple BMs.
Recently, owing to concern regarding the side
effects and neurological dysfunction associated with WBRT,
increasing numbers of studies examining the effectiveness of
stereotactic radiosurgery (SRS) in patients with a low number of
BMs have been performed. A multi-institutional retrospective study
demonstrated that patients with fewer than four BMs from NSCLC
treated with SRS as the initial therapy experienced longer survival
than those treated with WBRT, even after propensity score
adjustment (5). In addition, a
prospective observational study showed that overall survival (OS)
following initial treatment with SRS alone was similar for patients
with 5–10 BMs and patients with 2–4 BMs (6). SRS has been regarded as a reasonable
treatment alternative for patients with as many as 10 BMs.
Additionally, some retrospective studies have shown that SRS is as
safe and effective for 10 or more lesions as it is for a smaller
number of lesions (7–11).
However, these previous studies included various
types of primary tumors, in addition to NSCLC, and none directly
compared survival outcomes for SRS and WBRT as initial radiological
treatments for BMs. Therefore, we conducted this retrospective
study to compare the survival benefit and prevention of
neurological death for SRS and WBRT in patients with advanced NSCLC
with 10–20 BMs.
Materials and methods
Patient population
The present study included all patients with 10–20
BMs from NSCLC who were treated with either SRS or WBRT as the
initial treatment for brain lesions at Komaki City Hospital
(Komaki, Japan) between January 2009 and December 2016. All data
were retrospectively obtained from electronic medical records. All
patients were diagnosed with BMs by gadolinium enhanced T1-weighed
magnetic resonance imaging (MRI). Patients were included in this
study if they had: i) pathologically proven NSCLC; ii) 10–20 BMs
treated with SRS or WBRT; iii) a life expectancy of more than 2
months according to the attending physicians or neurologists; iv)
no history of prior treatment with either SRS or WBRT or surgery
for BMs; v) lesions with a maximum diameter of 4 cm or less per
metastasis; and vi) no apparent leptomeningeal disease. The life
expectancy was mainly predicted by diagnosis-specific graded
prognostic assessment (DS-GPA) score as well as Eastern Cooperative
Oncology Group Performance Status (ECOG-PS) or Karnofsky
Performance Status (KPS) (12) As
patients DS-GPA and Lung-molGPA scores of 0–1 showed, respectively,
median OS of 3.0 and 6.9 months, some patients were included even
with low performance status (12,13). Due
to the lack of relevant data, pulmonologists or neurosurgeons help
patients make informed decisions to select their treatment modality
depending on the patients' preference. For patients with relatively
larger lesion (≥10 cm3) who are not candidate for
surgery, the two-session SRS would be offered as an option
(14). For patients with the desire
for home care, SRS would also be an option because SRS is a one-day
treatment while WBRT takes over 2 weeks. Follow-up MRI to detect
brain lesions or enhanced computed tomography (CT) scanning for
systemic lesions was performed every 3–4 months or when clinically
indicated after SRS or WBRT. Study approval was obtained from the
Institutional Review Board of Komaki City Hospital (no.
171013).
SRS and WBRT techniques and
treatment
SRS was performed using Gamma Knife model C or
Perfexion (Elekta AB, Stockholm, Sweden). Gamma Knife surgery was
performed with the aid of the Leksell Model G stereotactic frame
(Elekta AB). After administration of a mild sedative and local
anesthesia, the stereotactic frame was applied. Patient treatment
was planned with GammaPlan software (Elekta AB). Thin-slice axial
spoiled gradient echo images with gadolinium enhancement were used
for tumor delineation. Most BMs were treated at a tumor margin dose
of 18–20 Gy with an isodose line of 50–95%, depending on tumor
volume; treatment occurred in a single session when the tumor
volume was less than 10 cm3. Some lesions were treated
at a reduced margin dose of 12–16 Gy when the tumors were
relatively large (≥10 cm3) or proximal to critical
structures, such as the brainstem or the optic apparatus. In one
patient, two lesions were treated with two-session Gamma Knife
surgery within two-week interval (15), during which a margin dose of 13 Gy
per session was delivered because of the multiple large BMs. All
the other patients received a single session SRS as one-day
treatment. We administered WBRT with 30 Gy in 10 fractions over 2
weeks using a linear accelerator. Providing that WBRT had been
refused by the patient, SRS was applied for multiple BM when the
patients' systemic condition was such that SRS intervention would
be tolerable and fully informed consent for treatment had been
obtained. In cases in which the intracranial tumor burden increased
as a result of tumor growth or new metastases, repeat SRS or
subsequent WBRT was recommended.
Statistical analysis
Study outcomes were comparison of OS, time to
intracranial progression (TTIP), and neurological survival (NS)
between patients treated with SRS and those treated with WBRT, and
identification of the prognostic factors that contributed to OS. OS
was defined as the time from the date of diagnosis of BMs to death
from any cause. TTIP was defined as the time from the date of
diagnosis of BMs to detection of any recurrence in the brain;
detection of carcinomatous meningitis, which was verified by
examination of cerebrospinal fluid; or the date of the last
follow-up imaging. NS was defined as the time from the date of
diagnosis of BMs to neurological death. In NS analysis, only deaths
from neurological causes were used as endpoints, and patients who
died from non-neurological causes were censored. Neurological death
was defined as death from any form of progression of neurologic
dysfunction caused by intracranial disease, including intracranial
recurrence or carcinomatous meningitis, and other illnesses with
severe neurologic dysfunction, as described by Patchell et
al (16). We also conducted
subgroup analysis for epidermal growth factor receptor/anaplastic
lymphoma kinase (EGFR/ALK) mutation-positive patients, comparing OS
between the two treatment modalities.
We used t-tests or Mann-Whitney U tests for
continuous variables, and Chi-square tests or Fisher's exact tests
for categorical variables to detect differences between the groups.
Estimated survival was calculated using the Kaplan-Meier method,
with 95% confidence intervals (CIs); comparisons between the groups
were performed using log-rank tests. To detect the independent
prognostic factors for survival, Cox proportional hazards modeling
was performed for all patients. The following parameters at the
time of diagnosis of BMs were included in univariate and
multivariate analysis: sex, age, smoking status, ECOG performance
status score (PS), initial clinical stage of lung cancer, EGFR/ALK
mutation status, symptoms from the brain lesions (yes or no),
extracranial metastases (yes or no), maximal diameter of the brain
lesions, chemotherapy or EGFR/ALK-tyrosine kinase inhibitor (TKI)
administration prior to brain radiotherapy (yes or no),
chemotherapy or EGFR/ALK-TKI administration subsequent to brain
radiotherapy (yes or no), DS-GPA class for NSCLC (12), Lung-molGPA score (13), and salvage treatment for recurrence
of BMs such as SRS or WBRT (yes or no). All analyses were performed
using the Statistical Package for the Social Sciences (SPSS v.21;
SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient characteristics
The patient characteristics are summarized in
Table I. We identified 44 patients
for the survival analysis. Twenty-four patients (55%) were treated
with SRS and 20 patients (45%) were treated with WBRT. The median
follow-up periods were 29 weeks (range: 7–233 weeks) in the SRS
group and 28 weeks (range: 3–164 weeks) in the WBRT group. No
significant differences were observed between the two groups in
terms of patient characteristics, including age, sex, histology,
smoking status. EGFR/ALK status, clinical stage, PS, systemic
treatment, symptoms from BMs, DS-GPA score, Lung-molGPA score, or
subsequent systemic treatment. However, patients treated with SRS
had fewer lesions (median 11 vs. 15, P=0.008) and greater lesion
diameters (median 17 vs. 12.5 mm, P=0.069) than patients treated
with WBRT. Patients with fewer and larger lesions tended to receive
SRS while patients with more and smaller lesions to receive WBRT.
With regard to salvage treatment for BMs, five of 10 patients in
the SRS group received repeat SRS, one patient received WBRT, and
the remaining four patients received both. In contrast, one patient
in the WBRT group received SRS.
 | Table I.Patient characteristics (n=44). |
Table I.
Patient characteristics (n=44).
| Characteristics | SRS group (n=24) | WBRT group
(n=20) | P-value |
|---|
| Median age, years
(range) | 67.6
(46–80) | 67.5
(49–84) | 0.519 |
| Sex
(male/female) | 17/7 | 10/10 | 0.158 |
| Histology
(Ad/Sq/others) | 23/1/0 | 17/1/2 | 0.278 |
| Smoking status
(current/former/never) | 7/11/6 | 4/8/8 | 0.542 |
| EGFR/ALK mutation
status (positive/negative/unknown) | 13/9/2 | 11/4/5 | 0.219 |
| Clinical stage at
diagnosis (I–II/III/IV) | 1/6/17 | 3/1/16 | 0.118 |
| ECOG-PS
(0/1/2/3/4) | 7/12/1/3/1 | 3/9/3/3/2 | 0.554 |
| Prior TKI
(yes/no) | 4/20 | 5/15 | 0.710 |
| Prior chemotherapy
(yes/no) | 7/17 | 8/12 | 0.450 |
| Symptoms from BMs
(yes/no) | 7/17 | 8/12 | 0.450 |
| Number of BMs, median
(range) | 11
(10–19) | 15
(10–20) | 0.008 |
| Maximum diameter of
BMs median, mm (range) | 17.0 (8–40) | 13.5 (5–32) | 0.069 |
| Extracranial
metastases (present/absent) | 24/0 | 17/3 | 0.086 |
| DS-GPA score
(0–1.0/1.5–2.0/2.5-) | 17/7/0 | 14/6/0 | 0.952 |
| Lung-molGPA score
(0–1.0/1.5–2.0/2.5-) | 8/9/7 | 6/9/5 | 0.879 |
| Subsequent
chemotherapy (yes/no) | 14/10 | 12/8 | 0.911 |
| Subsequent TKI
(yes/no) | 11/13 | 10/10 | 0.783 |
| Salvage treatment
(yes/no) | 10/14 | 1/19 | 0.005 |
Response to WBRT and SRS
Local control was achieved at 6 and 12 months in 100
and 90%, respectively, of both the SRS and WBRT groups (P=0.764).
Distant brain failure was seen at 6 and 12 months in 29.9 and
69.9%, respectively, of the SRS group vs. 0 and 10.0%,
respectively, of the WBRT group (P=0.005).
Survival data in WBRT and SRS
groups
The overall group survival rates were 84.1, 59.1 and
27.3% at 3 and 6 months, and 1 year, respectively, after WBRT or
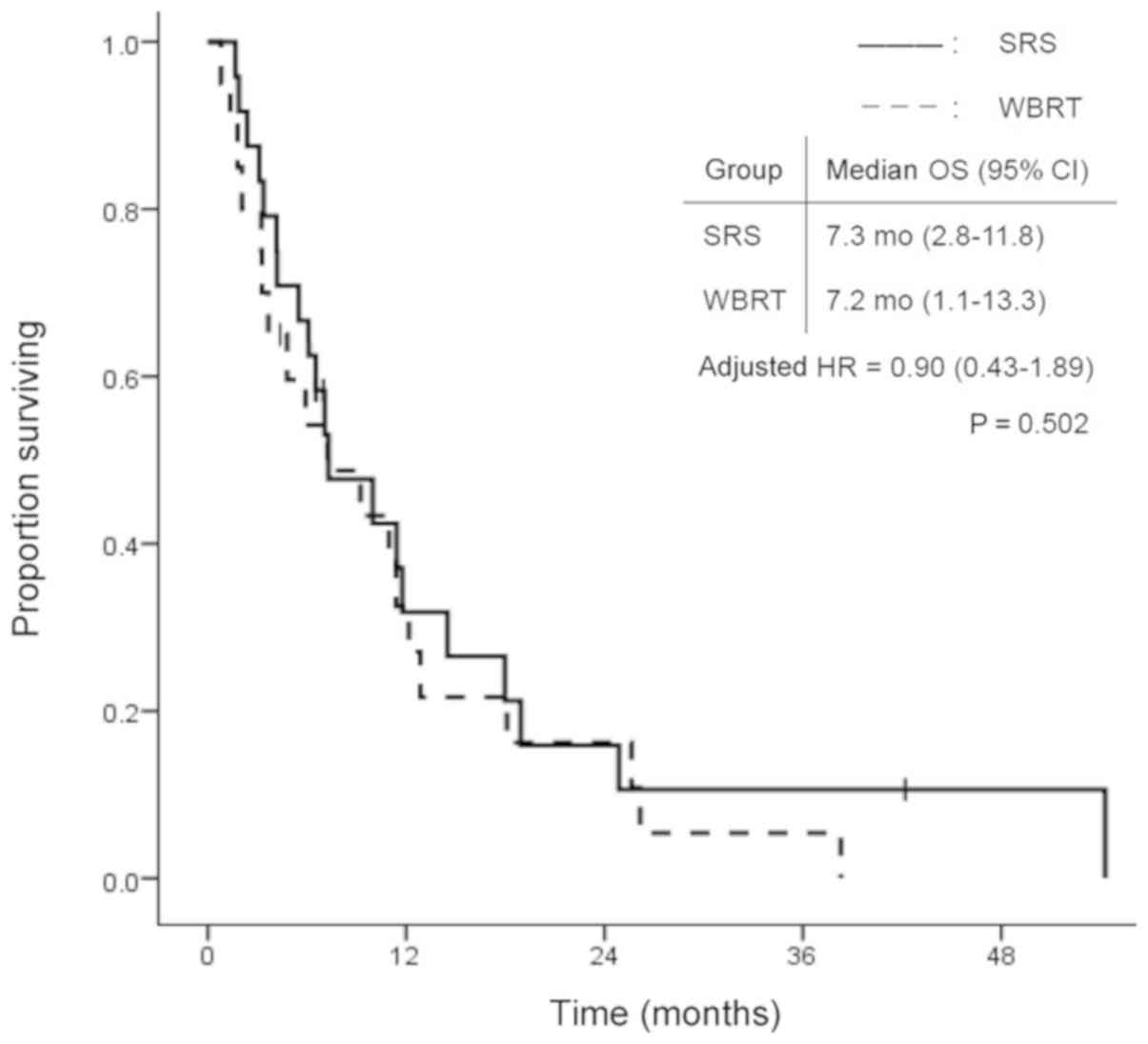
SRS treatment. Fig. 1 shows the
survival curves in the SRS and WBRT groups. The median survival
time was 7.3 months (95% CI: 2.8–11.8) in the SRS group and 7.2
months (95% CI: 1.1–13.3) in the WBRT group. There was no
statistically significant difference in OS between the two groups
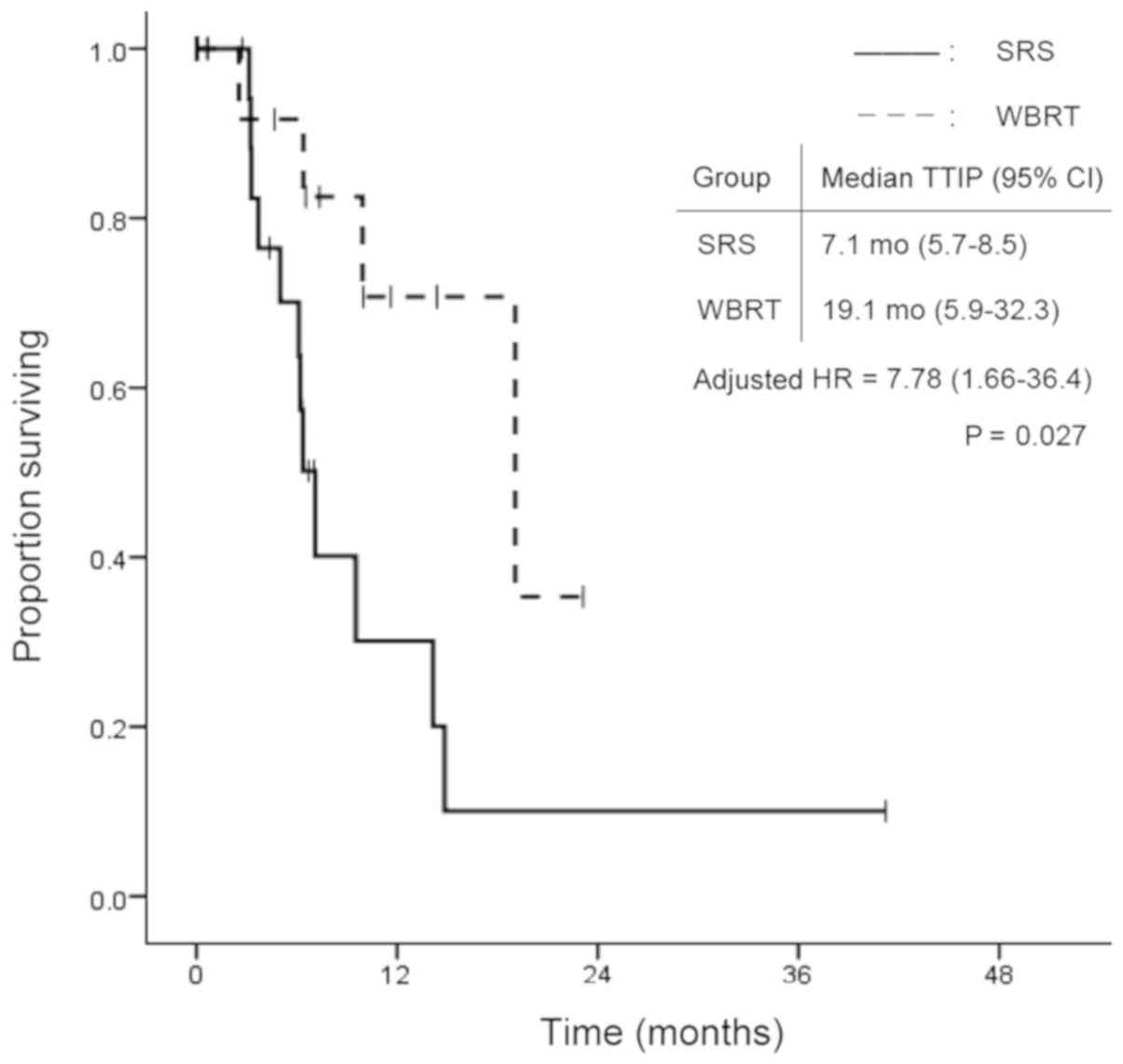
(P=0.502). In contrast, TTIP was significantly longer in the WBRT
group. Median TTIP was 7.1 months (95% CI: 5.7–8.5) in the SRS
group and 19.1 months (95% CI: 5.9–32.3) in the WBRT group
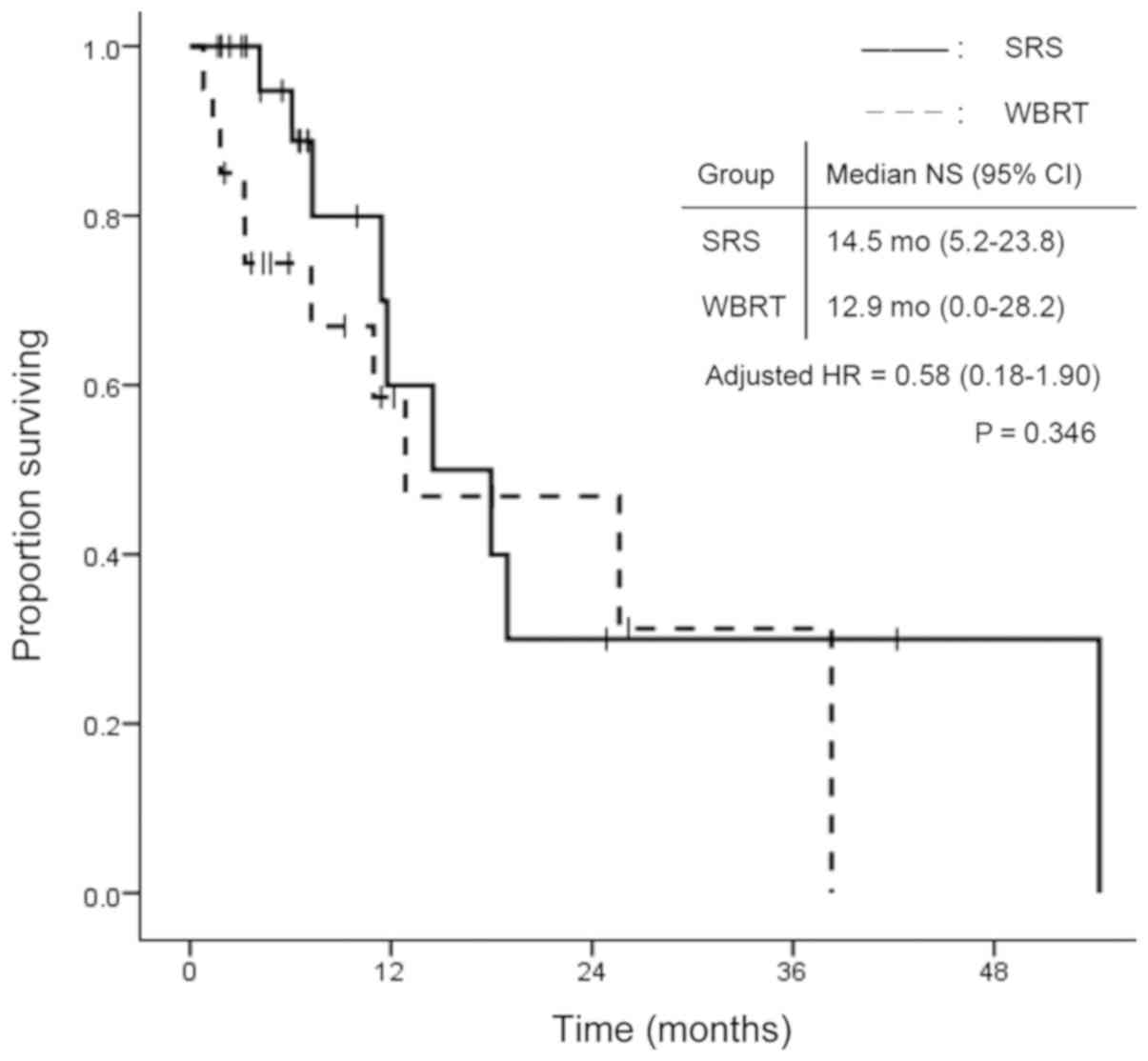
(P=0.009; Fig. 2). Fig. 3 shows the time to death from
neurological causes for the two treatment modalities. There was no
significant difference between the two groups: 14.5 months (95% CI:
5.2–23.8) in the SRS group and 12.9 months (95% CI: 0.0–28.2) in
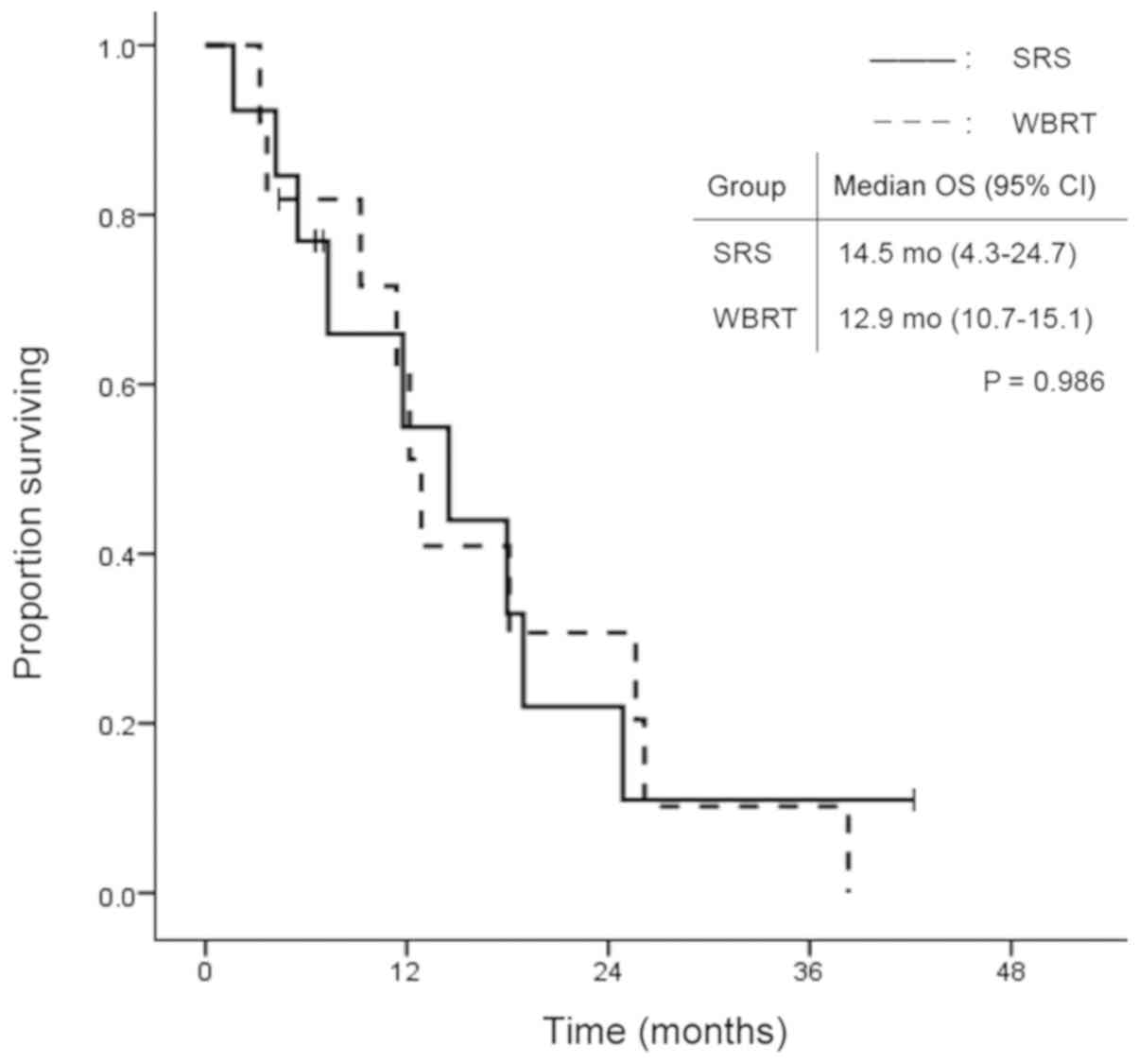
the WBRT group (P=0.346). In the subgroup analysis for EGFR/ALK
mutation-positive patients, OS did not differ significantly between
the two treatment modalities (Fig.
4).
Prognostic factors for survival in
univariate and multivariate analyses
The prognostic factors for survival in univariate
and multivariate analyses are shown in Table II. Multivariate analysis confirmed
that non-adenocarcinomatous histology [hazard ratio (HR)=7.39; 95%
CI, 1.88–28.99; P=0.004], lower PS (HR=1.38; 95% CI, 1.01–1.90;
P=0.045), subsequent EGFR/ALK-TKI administration (HR=0.21; 95% CI,
0.09–0.47; P<0.001), subsequent chemotherapy (HR=0.36; 95% CI,
0.15–0.83; P=0.017) and salvage treatment (HR=0.33; 95% CI,
0.12–0.91; P=0.032) were independent prognostic factors, but there
were no significant differences between the SRS group and WBRT
group with respect to these factors.
 | Table II.Univariate and multivariate analyses
of covariates associated with overall survival. |
Table II.
Univariate and multivariate analyses
of covariates associated with overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Treatment
(SRS/WBRT) | 0.80 | 0.42–1.52 | 0.502 |
|
|
|
| Age | 1.00 | 0.97–1.04 | 0.900 |
|
|
|
| Sex
(male/female) | 1.21 | 0.62–2.37 | 0.580 |
|
|
|
| Histology
(non-Ad/Ad) | 6.38 |
2.11–19.35 | 0.001 | 7.39 |
1.88–28.99 |
0.004 |
| EGFR/ALK mutation
status (positive/negative/unknown) | 0.34 | 0.18–0.67 | 0.002 |
|
|
|
| Clinical stage
(IV/I–III) | 1.43 | 0.67–3.06 | 0.358 |
|
|
|
| Prior TKI
(yes/no) | 0.88 | 0.40–1.95 | 0.754 |
|
|
|
| Prior chemotherapy
(yes/no) | 1.39 | 0.71–2.75 | 0.340 |
|
|
|
| Smoking status
(current/former/never) | 1.21 | 0.58–2.50 | 0.613 |
|
|
|
| Symptoms from BMs
(yes/no) | 1.17 | 0.59–2.32 | 0.660 |
|
|
|
| Number of BMs | 0.98 | 0.89–1.08 | 0.618 |
|
|
|
| Maximal
diameter | 1.04 | 1.00–1.08 | 0.055 |
|
|
|
| ECOG-PS | 1.48 | 1.12–1.97 | 0.006 | 1.38 | 1.01–1.90 |
0.045 |
| Extracranial
metastases (present/absent) | 0.89 | 0.21–3.75 | 0.878 |
|
|
|
| DS-GPA score | 0.62 | 0.28–1.40 | 0.248 |
|
|
|
| Lung-molGPA
score | 0.53 | 0.34–0.83 | 0.005 | 1.11 | 0.61–2.03 |
0.728 |
| Subsequent TKI
(yes/no) | 0.37 | 0.19–0.71 | 0.003 | 0.21 | 0.09–0.47 | <0.001 |
| Subsequent
chemotherapy (yes/no) | 0.48 | 0.24–0.95 | 0.035 | 0.36 | 0.15–0.83 |
0.017 |
| CNS-PD
(yes/no) | 0.59 | 0.30–1.15 | 0.123 |
|
|
|
| Salvage treatment
for BMs (yes/no) | 0.46 | 0.22–0.99 | 0.046 | 0.33 | 0.12–0.91 |
0.032 |
Adverse events
Based on Common Terminology Criteria for Adverse
Events (CTCAE) v4.0, the rate of radiation-induced
leukoencephalopathy on follow-up MRI were as follows: grade
1/2/3/4/not evaluated = 14/4/1/0/5 in the SRS group and 5/3/4/8 in
the WBRT group, respectively. Radiation induced changes in the SRS
group were relatively lower than in the WBRT group. The other
radiation-induced adverse events such as neuropathy, seizure,
symptomatic radiation necrosis, cerebral hemorrhage were not
detected in any patients included in our analysis.
Discussion
In this single-center retrospective study, we
compared SRS and WBRT as the initial treatment for patients with
10–20 BMs from NSCLC. We found that there were no significant
differences in OS between the treatment groups, although TTIP and
the local control rate were significantly better in the WBRT
group.
Conventionally, WBRT has been considered the
standard treatment for multiple BMs. However, there have been
numerous reports of survival time comparable to that of WBRT in
patients treated with SRS for 5–10 BMs, with similar side effects,
and the adoption of SRS has therefore been gradually increasing
(6,17–19).
Recently, some retrospective studies have evaluated the use of SRS
for 10 or more BMs (7–11). The findings of those studies have
suggested that treatment with SRS for 10 or more lesions is not
inferior to that in patients with smaller numbers of BMs in terms
of safety and efficacy. However, the studies included various
primary sites besides NSCLC, and none directly compared SRS with
WBRT with respect to survival. Therefore, the present study focused
on initial radiological treatment for more than 10 BMs from NSCLC
and compared the two treatment options.
SRS generally has advantages when compared with WBRT
in terms of safety, such as the late development of cognitive
dysfunction and alopecia, which could affect patients' quality of
life (20–25). Yamamoto et al reported the
safety and feasibility of SRS for ten or more BMs when compared to
the patients with less lesions (11)
In addition SRS can be applied repeatedly for BMs and can be
performed in a shorter time, resulting in a reduced burden on
patients. In fact, 10 patients in the SRS group in the present
study required re-irradiation for recurrent BMs; five of these
underwent SRS alone without WBRT, while the remainder needed WBRT.
Thus, if patients would be tolerable for the adverse events of SRS
such as stereotactic frame application for immobilization, SRS for
10 or more BMs may delay the administration of WBRT and the adverse
events associated with this treatment modality.
Patients treated with WBRT compared to SRS in the
present study showed superior intracranial control and TTIP, but
these outcomes did not lead to either fewer neurological deaths or
OS prolongation. While the risk of intracranial recurrence in the
SRS group was higher than WBRT group, the adverse events such as
leukoencephalopathy were fewer in the SRS group. Besides severe
neuropathy, seizure, symptomatic radiation necrosis, cerebral
hemorrhage were not observed in either group. These results were
similar to the findings of previous prospective studies conducted
for fewer than 10 BMs (21,22,26,27). On
the basis of those findings, upfront SRS is a good option for
patients with 10 or more BMs from NSCLC to shorten the duration of
the treatment and avoid the long-term adverse events from WBRT if
patients are appropriate for local anesthesia and mild
sedation.
With respect to EGFR and ALK mutational status,
patients with those mutations accounted for half of the patients in
this study. No statistical difference in survival was seen between
the WBRT group and the SRS group with regard to patients with these
mutations. In general, EGFR and ALK-TKIs are effective treatment
for BMs, leading to better prognosis for patients with such driver
mutations. As reported by Mangnuson et al, upfront SRS
followed by TKI administration is a reasonable treatment to avoid
late adverse events derived from WBRT, especially for patients with
these mutations (28).
The present study has several limitations. First,
this was a retrospective study with small sample size and lacked
evaluation of neurocognitive functions and detailed complications.
In a retrospective cohort study that compared 2–9 BMs with 10 or
more BMs treated with SRS, neurological deterioration and
SRS-related complications did not differ between the groups
(11). In addition, another
prospective observational study showed that patients with 5–10 BMs
did not experience more neurocognitive deterioration or post-SRS
complications compared to those with 2–4 BMs, whereas the addition
of WBRT generally resulted in worse effects on neurocognitive
function (21,22). These findings suggest that, even in
patients with 10 or more BMs, SRS may be safer and may better
maintain neurocognitive function than WBRT. In fact, in the SRS
group there were fewer leukoencephalopathy and may be fewer adverse
events related to WBRT described in other studies. We could not
show the detailed adverse events data because this was a
retrospective study and many of patients were outpatients. Second,
this study did not include details on control of primary tumor
which could affect the prognosis (29), because there were some patients
without any radiologic findings for primary lesions outside central
nervous system at the time of progression of BMs. Third, there were
differences in patient backgrounds between the two groups, such as
the number of BMs, diameter of BMs, PS, histology, and systemic
therapy. In fact, PS, histology, EGFR/ALK-TKI administration or
chemotherapy after diagnosis of BMs, and salvage treatment can
influence OS (30–32). Therefore, we conducted a multivariate
analysis that included those factors to adjust for bias with
respect to patient characteristics. Additionally, the absence of
the evaluation of the volume of BMs is also a limitation. Fourth,
we do not have the data of which sum of the volumes of BMs is
smaller or bigger between the two modalities because this study was
a retrospective study based on the electronic clinical records,
which did not include the information about the volume of BMs. We
also do not have the data or evidence if the high dose provided by
SRS alone would bring more benefit than WBRT alone especially in
patients with multiple BMs. Fifth, the assignment of patients to
treatment was based solely on the judgment of physicians. Thus,
there is a possibility that some bias not included in the analysis
was present. In general, patients with multiple small metastases
would generally be selected for WBRT, whereas those with fewer and
larger (≥10 cm3) lesions would be offered SRS in our
institution. Patients with long-term prognosis might be treated
with SRS repeatedly to prevent complications from WBRT. Further
study evaluating neurocognitive function, detailed complications,
control of the primary tumor, and the actual tumor volume or
planning tumor volume are warranted.
In conclusion, we compared SRS to WBRT as the
initial treatment for 10–20 BMs from NSCLC. The present study
demonstrated that there were no significant differences in OS and
NS between treatment with SRS and WBRT for BMs. Therefore, SRS may
be a useful alternative treatment for 10–20 BMs from NSCLC. Further
prospective randomized studies that evaluate neurocognitive
functions and complications are needed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TM drafted the manuscript and analyzed the data. TH,
HK, TS, YY, NA, EK contributed to study design and data collection.
TY helped to interpret the data and draft the manuscript. KM helped
to analyze the data in this study. KT conceived the study and
participated in its design and coordination. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Study approval was obtained from the Institutional
Review Board of Komaki City Hospital (approval no. 171013; Komaki,
Japan). Due to the anonymous and retrospective nature of the study,
the requirement for individual informed consent was waived.
Patient consent for publication
Not applicable.
Competing interests
TY has previously obtained research grants from
Nippon Boehringer Ingelheim; however, this had no bearing on the
design of the present study.
References
|
1
|
Barnholtz-Sloan JS, Sloan AE, Davis FG,
Vigneau FD, Lai P and Sawaya RE: Incidence proportions of brain
metastases in patients diagnosed (1973 to 2001) in the Metropolitan
Detroit Cancer Surveillance System. J Clin Oncol. 22:2865–2872.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shin DY, Na II, Kim CH, Park S, Baek H and
Yang SH: EGFR mutation and brain metastasis in pulmonary
adenocarcinomas. J Thoracic Oncol. 9:195–199. 2014. View Article : Google Scholar
|
|
3
|
Zimm S, Wampler GL, Stablein D, Hazra T
and Young HF: Intracerebral metastases in solid-tumor patients:
natural history and results of treatment. Cancer. 48:384–394. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borgelt B, Gelber R, Kramer S, Brady LW,
Chang CH, Davis LW, Perez CA and Hendrickson FR: The palliation of
brain metastases: final results of the first two studies by the
Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys.
6:1–9. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Halasz LM, Uno H, Hughes M, D'Amico T,
Dexter EU, Edge SB, Hayman JA, Niland JC, Otterson GA, Pisters KM,
et al: Comparative effectiveness of stereotactic radiosurgery
versus whole-brain radiation therapy for patients with brain
metastases from breast or non-small cell lung cancer. Cancer.
122:2091–2100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto M, Serizawa T, Shuto T, Akabane
A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, et
al: Stereotactic radiosurgery for patients with multiple brain
metastases (JLGK0901): a multi-institutional prospective
observational study. Lancet Oncol. 15:387–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang WS, Kim HY, Chang JW, Park YG and
Chang JH: Analysis of radiosurgical results in patients with brain
metastases according to the number of brain lesions: is
stereotactic radiosurgery effective for multiple brain metastases?
J Neurosurg. 113 (Suppl):73–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grandhi R, Kondziolka D, Panczykowski D,
Monaco EA III, Kano H, Niranjan A, Flickinger JC and Lunsford LD:
Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion
unit in the management of patients with 10 or more brain
metastases. J Neurosurg. 117:237–245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim CH, Im YS, Nam DH, Park K, Kim JH and
Lee JI: Gamma knife radiosurgery for ten or more brain metastases.
J Korean Neurosurg Soc. 44:358–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rava P, Leonard K, Sioshansi S, Curran B,
Wazer DE, Cosgrove GR, Norén G and Hepel JT: Survival among
patients with 10 or more brain metastases treated with stereotactic
radiosurgery. J Neurosurg. 119:457–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto M, Kawabe T, Sato Y, Higuchi Y,
Nariai T, Watanabe S and Kasuya H: Stereotactic radiosurgery for
patients with multiple brain metastases: a case-matched study
comparing treatment results for patients with 2–9 versus 10 or more
tumors. J Neurosurg. 121 Suppl:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sperduto PW, Kased N, Roberge D, Xu Z,
Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al: Summary
report on the graded prognostic assessment: an accurate and facile
diagnosis-specific tool to estimate survival for patients with
brain metastases. J Clin Oncol. 30:419–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sperduto PW, Yang TJ, Beal K, Pan H, Brown
PD, Bangdiwala A, Shanley R, Yeh N, Gaspar LE, Braunstein S, et al:
Estimating survival in patients with lung cancer and brain
metastases: an update of the Graded Prognostic Assessment for lung
cancer using molecular markers (Lung-molGPA). JAMA Oncol.
3:827–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Serizawa T, Higuchi Y, Yamamoto M,
Matsunaga S, Nagano O, Sato Y, Aoyagi K, Yomo S, Koiso T, Hasegawa
T, et al: Comparison of treatment results between 3- and 2-stage
Gamma Knife radiosurgery for large brain metastases: a
retrospective multi-institutional study. J Neurosurg. Sep
1–2018.(Epub ahead of print). View Article : Google Scholar
|
|
15
|
Hasegawa T, Kato T, Yamamoto T, Iizuka H,
Nishikawa T, Ito H and Kato N: Multisession gamma knife surgery for
large brain metastases. J Neurooncol. 131:517–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patchell RA, Tibbs PA, Walsh JW, Dempsey
RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS and Young
B: A randomized trial of surgery in the treatment of single
metastases to the brain. N Engl J Med. 322:494–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hunter GK, Suh JH, Reuther AM, Vogelbaum
MA, Barnett GH, Angelov L, Weil RJ, Neyman G and Chao ST: Treatment
of five or more brain metastases with stereotactic radiosurgery.
Int J Radiat Oncol Biol Phys. 83:1394–1398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raldow AC, Chiang VL, Knisely JP and Yu
JB: Survival and intracranial control of patients with 5 or more
brain metastases treated with gamma knife stereotactic
radiosurgery. Am J Clin Oncol. 36:486–490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto M, Kawabe T, Sato Y, Higuchi Y,
Nariai T, Barfod BE, Kasuya H and Urakawa Y: A case-matched study
of stereotactic radiosurgery for patients with multiple brain
metastases: comparing treatment results for 1–4 vs ≥ 5 tumors:
clinical article. J Neurosurg. 118:1258–1268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sahgal A, Ruschin M, Ma L, Verbakel W,
Larson D and Brown PD: Stereotactic radiosurgery alone for multiple
brain metastases? A review of clinical and technical issues. Neuro
Oncol. 19 Suppl 2:ii2–ii15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown PD, Jaeckle K, Ballman KV, Farace E,
Cerhan JH, Anderson SK, Carrero XW, Barker FG II, Deming R, Burri
SH, et al: Effect of radiosurgery alone vs radiosurgery with whole
brain radiation therapy on cognitive function in patients with 1 to
3 brain metastases: a randomized clinical trial. JAMA. 316:401–409.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang EL, Wefel JS, Hess KR, Allen PK,
Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, et
al: Neurocognition in patients with brain metastases treated with
radiosurgery or radiosurgery plus whole-brain irradiation: A
randomised controlled trial. Lancet Oncol. 10:1037–1044. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crossen JR, Garwood D, Glatstein E and
Neuwelt EA: Neurobehavioral sequelae of cranial irradiation in
adults: a review of radiation-induced encephalopathy. J Clin Oncol.
12:627–642. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Habets EJ, Dirven L, Wiggenraad RG,
Verbeek-de Kanter A, Lycklama À, Nijeholt GJ, Zwinkels H, Klein M
and Taphoorn MJ: Neurocognitive functioning and health-related
quality of life in patients treated with stereotactic radiotherapy
for brain metastases: a prospective study. Neuro Oncol. 18:435–444.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soffietti R, Kocher M, Abacioglu UM, Villa
S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD,
Carrie C, et al: A European Organisation for Research and Treatment
of Cancer phase III trial of adjuvant whole-brain radiotherapy
versus observation in patients with one to three brain metastases
from solid tumors after surgical resection or radiosurgery:
quality-of-life results. J Clin Oncol. 31:65–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aoyama H, Shirato H, Tago M, Nakagawa K,
Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, et al:
Stereotactic radiosurgery plus whole-brain radiation therapy vs
stereotactic radiosurgery alone for treatment of brain metastases:
a randomized controlled trial. JAMA. 295:2483–2491. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kocher M, Soffietti R, Abacioglu U, Villà
S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD,
Carrie C, et al: Adjuvant whole-brain radiotherapy versus
observation after radiosurgery or surgical resection of one to
three cerebral metastases: Results of the EORTC 22952–26001 study.
J Clin Oncol. 29:134–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Magnuson WJ, Lester-Coll NH, Wu AJ, Yang
TJ, Lockney NA, Gerber NK, Beal K, Amini A, Patil T, Kavanagh BD,
et al: Management of brain metastases in tyrosine kinase
inhibitor-naïve epidermal growth factor receptor-mutant
non-small-cell lung cancer: a retrospective multi-institutional
analysis. J Clin Oncol. 35:1070–1077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lorenzoni J, Devriendt D, Massager N,
David P, Ruíz S, Vanderlinden B, Van Houtte P, Brotchi J and
Levivier M: Radiosurgery for treatment of brain metastases:
estimation of patient eligibility using three stratification
systems. Int J Radiat Oncol Biol Phys. 60:218–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang T, Min W, Li Y, Yue Z, Wu C and Zhou
C: Radiotherapy plus EGFR TKIs in non-small cell lung cancer
patients with brain metastases: An update meta-analysis. Cancer
Med. 5:1055–1065. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo S, Chen L, Chen X and Xie X:
Evaluation on efficacy and safety of tyrosine kinase inhibitors
plus radiotherapy in NSCLC patients with brain metastases.
Oncotarget. 6:16725–16734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim DY, Lee KW, Yun T, Kim DW, Kim TY, Heo
DS, Bang YJ and Kim NK: Efficacy of platinum-based chemotherapy
after cranial radiation in patients with brain metastasis from
non-small cell lung cancer. Oncol Rep. 14:207–211. 2005.PubMed/NCBI
|