Introduction
Colorectal cancer (CRC) was estimated to account for
881,000 deaths worldwide in 2018 and it remains one of the major
causes of death globally (1). The
incidence of distant metastasis is as high as 25% at initial
diagnosis, and approximately half of the patients with CRC will
develop metastatic disease (2).
Cetuximab (CTX; Erbitux, Merck KGaA, Darmstadt,
Germany) is a monoclonal antibody that inhibits ligand binding and
ligand-dependent downstream signaling by specifically targeting the
epidermal growth factor receptor (EGFR) (3,4).
Accumulation of data from several clinical studies revealed the
efficacy of CTX combined with the standard therapy arm
(FOLFOX/FOLFIRI) in treating patients with metastatic CRC (mCRC)
with wild-type KRAS (5,6). Therefore, in the clinical setting, the
use of CTX is currently restricted to patients with wild-type
mCRC.
Zoledronic acid (ZOL) is a member of the
bisphosphonate molecular class and is used in the clinical setting
to reduce skeletal-related events in patients with bone metastasis.
Previous studies reported on the antitumor activity of ZOL against
several human cancers, such as breast, prostate and colorectal
cancers, in vitro or in vivo (7,8).
We herein report the clinical course and computed
tomography (CT) imaging findings in a patient with mCRC treated
with ZOL and CTX.
Case report
A 58-year-old male patient presented to Gifu
University Hospital (Gifu, Japan) with abdominal pain, symptoms of
colonic penetration due to stenosis caused by rectal cancer in
January 2014, and initially underwent an ileostomy. Peritoneal
dissemination was detected during the ileostomy procedure, and CTX
+ mFOLFOX was started to treat the unresectable wild-type KRAS CRC.
Analysis of RAS and EGFR status performed by SRL, Inc. (Tokyo,
Japan) revealed wild-type RAS and high expression of EGFR in this
patient. The patient underwent 6 courses of CTX + mFOLFOX before
undergoing low anterior resection and D3 lymphadenectomy as the
second surgery. Since R0 resection could be performed, 6 courses of
mFOLFOX were administered as postoperative chemotherapy, after
which time the patient was observed. A follow-up CT scan 6 months
after the second surgery revealed a metastasis to the thoracic
spine that was treated with X-ray radiation therapy 20 Gy/5 fr.
Approximately 9 months after the second surgery, the
patient complained of gradual onset of fatigue and shortness of
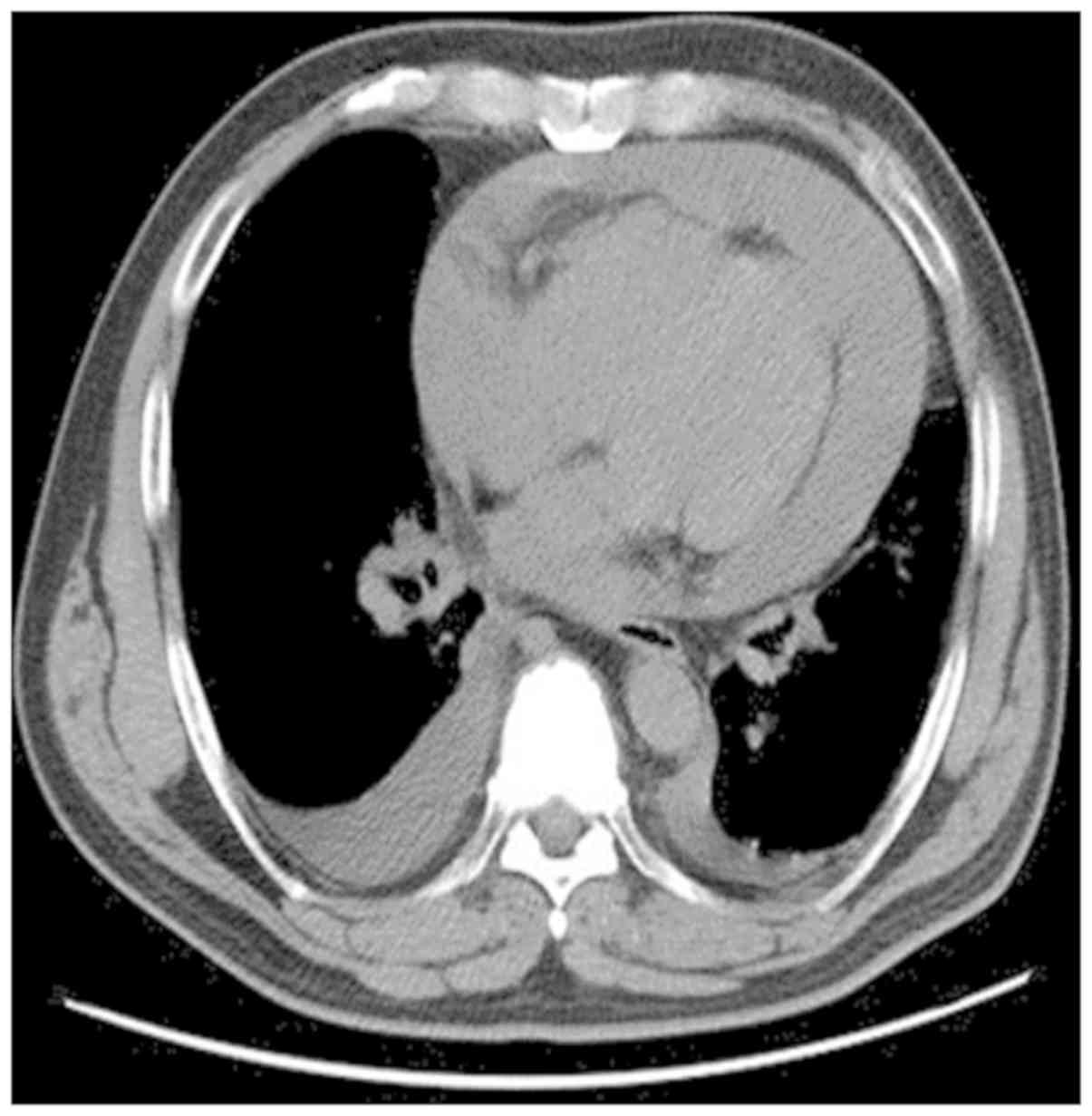
breath; a chest CT scan revealed pericardial effusion and cardiac
tamponade, which were managed by pericardial drainage (Fig. 1).
At 1 year after the second surgery, the patient
began to have difficulty walking, which was caused by the thoracic
spine metastasis and compression of the spinal cord. At that time,
combination therapy with CTX and ZOL (CTX: 400 mg weekly, ZOL: 4
mg/body tri-weekly) was initiated. There were several reasons for
selecting CTX and ZOL in this patient: First, the idea of using
irinotecan (CPT-11) was abandoned, as the patient had cardiac and
pleural effusion. Second, we hypothesized that the tumor was
refractory to oxaliplatin, as recurrence occurred within 3 months
after chemotherapy. An alternative treatment for this patient would
be the administration of regorafenib or TAS-102; however, these
drugs are associated with increased risks in patients with renal
dysfunction, such as in the present case.
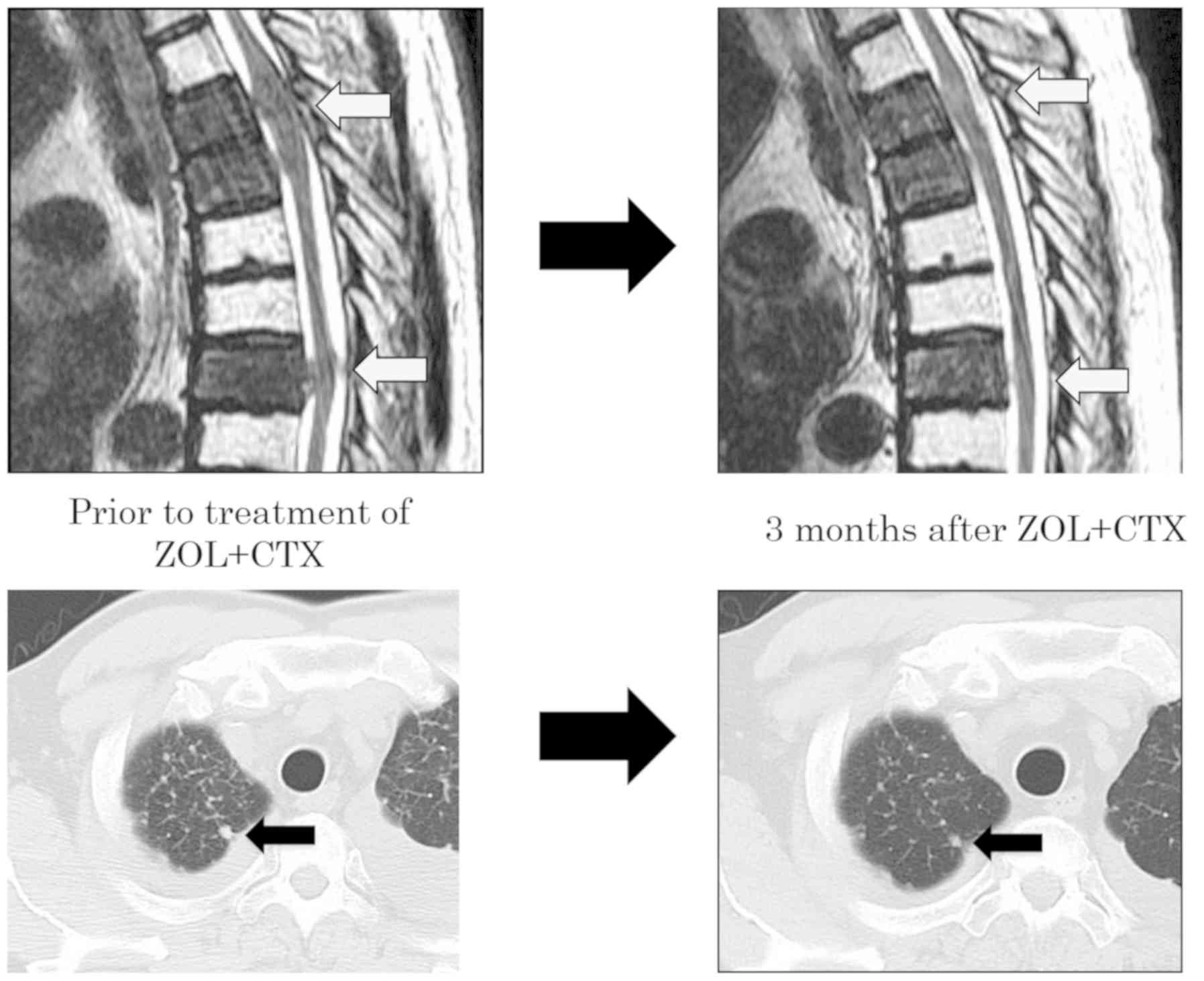
Following treatment with CTX and ZOL, the size of
the thoracic and lung metastasis was found to be decreased on CT
imaging ~5 months following the initiation of the combination
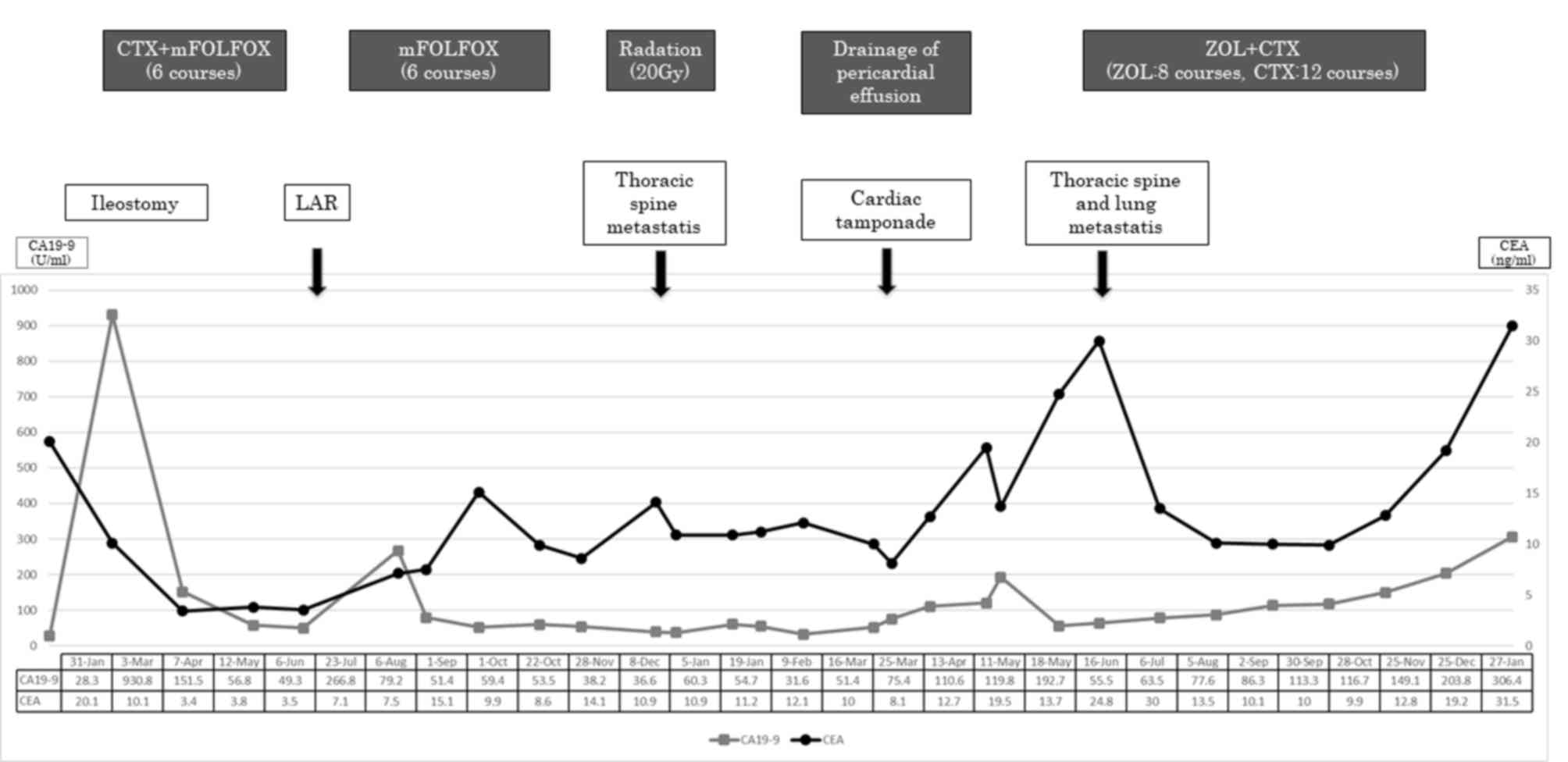
therapy. In parallel with these findings, the levels of the tumor
markers carcinoembryonic antigen (normal <5.0 ng/ml) and
carbohydrate antigen 19-9 (normal <37.0 U/ml) also decreased
from 15.1 to 8.6 and 266.8 to 38.2 respectively (Figs. 2 and 3). However, the disease gradually
progressed 3 months after the initiation of the ZOL + CTX
combination therapy. In total, the patient was administered 28
courses of CTX and 8 courses of ZOL, but eventually succumbed to
the disease 2 years and 3 months after the first surgery.
Discussion
Following its approval in 2004, bevacizumab, a
humanized recombinant monoclonal antibody against vascular
endothelial growth factor-A, became the first biological therapy
for the initial treatment of mCRC. Bevacizumab achieved
improvements in progression-free and overall when combined with
chemotherapy, such as FOLFIRI and FOLFOX, in several studies
(9,10). In the same year, a randomized trial
of CTX alone or in combination with CPT-11 exhibited clinical
effectiveness in CPT-11-refractory CRC, confirming the results of
phase 2 studies of third-line therapy. CPT-11 and combination
therapy achieved markedly higher response rates compared with CTX
monotherapy alone. However, CTX monotherapy was effective compared
with placebo and was associated with only mild toxicity, including
skin toxicity; thus, it may be a viable option for patients who are
not considered candidates for further treatment or who choose not
to receive such combination therapy (11). In addition, in 2006, CTX, which is a
monoclonal antibody against EGFR, was introduced as treatment for
CRC and these drugs are currently considered to largely contribute
to the better prognosis of patients with CRC.
Molecules of the EGFR family constitute a signaling
pathway that plays an important role in intracellular reactions.
Three major pathways are involved in EGFR-mediated signaling. The
first pathway is that of the mitogen-activated protein kinase
(MAPK) cascades. EGFR tyrosine autophosphorylation activates Ras,
which leads to a multistep phosphorylation event and subsequently
activates MAPKs and extracellular signal-regulated protein kinase
(ERK)1 and ERK2. These kinases regulate a large number of molecules
that are involved in cell transformation, proliferation and
survival (12). Another important
pathway secondary to the EGFR activation is
phosphatidylinositol-3-kinase and the downstream protein
serine/threonine kinase Akt. Akt transduces intracellular signals
linked to cell survival, proliferation and motility (13). The third pathway is represented by
Jak2/STAT3. EGF, the mitogenic hormone that activates EGFR, plays
an important role in regulating the proliferation and
differentiation of normal and neoplastic cells in vitro and
in vivo.
As the newest nitrogen-containing bisphosphonate,
the suppression of bone resorption of ZOL increases by 2,000 times
in comparison with that of flexor chloride sodium phosphate, and it
is 200 times that of pamidronate, which is widely used to prevent
and treat bone metastases from solid tumors, and to delay the
development of cancer-related bone damage. Clinical experience has
proven that ZOL does not only prevent bone diseases, but also
affects cancer activity by inhibiting the proliferation and
reducing the activity of cancer cell0s (14). The efficacy of combination therapy
with ZOL and CTX on CRC cells was previously reported in a
preclinical study (8). The
administration of ZOL carries the risk of osteonecrosis of jaw.
However, this side effect did not occur for this patient.
There are two mechanisms underlying the antitumor
effect of the ZOL and CTX combination therapy in our patient: One
mechanism includes the inhibition of RAS signaling by both agents.
In KRAS wild-type tumors, CTX inhibits the intracellular signaling
cascades, such as the RAS signaling pathways that promote cell
growth, by competing with the ligand for binding to the EGFR
receptor (3,15). In a preclinical study, ZOL exerted an
antitumor effect on CRC by inhibiting prenylation of RAS (8). The other mechanism is the antitumor
activity occurring via targeting pathways unrelated to EGFR, such
as antibody-dependent cytotoxicity (ADCC) (16). A previous report suggested that ZOL
enhances ADCC by increasing the number of δγT cells (17). This evidence suggests that
combination therapy with ZOL and CTX may synergistically enhance
ADCC activity and exert an antitumor effect.
In our patient, radiotherapy alone was selected
after the recurrence to the thoracic spine, as there are currently
no reports confirming the effectiveness of an anti-EGFR agent
against the bone metastases of CRC in the English literature;
furthermore, the recurrence was limited to the thoracic spine
(18).
Our patient had difficulty walking due to the
thoracic spine metastasis and compression of the spinal cord.
Combination therapy with ZOL and CTX for unresectable rectal cancer
with bone metastases was very effective in improving the patient's
ability to walk. The compression of the spinal cord diminished
after treatment and the patient did not complain of any more
difficulty walking while he was alive.
It is not possible to determine whether CTX and ZOL,
alone or in combination, had antitumor effects. Their effectiveness
against the lung metastasis may be due to the re-administration of
CTX. However, as mentioned above, the effectiveness of anti-EGFR
therapy for bone metastases is uncertain and the administration of
ZOL may have contributed to the reduction in tumor size. These
findings suggest that ZOL may play an important role as an effector
for cancer therapy. To the best of our knowledge, this is the first
report of the clinical course of a patient while under treatment
with the combination of ZOL and CTX.
In conclusion, our results demonstrated that
combination therapy with ZOL and CTX inhibited the growth of
metastatic rectal cancer. Therefore, the efficacy of this
combination therapy should be proven in future clinical trials.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and accompanying
images.
Authors' contributions
The supervision of the current study was by KY; YT,
NM, TaT, ToT, SM, HI, YT, KY interpreted the clinical data and YT
and NM wrote, reviewed and/or revised the manuscript.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADCC
|
antibody-dependent cytotoxicity
|
|
CRC
|
colorectal cancer
|
|
CT
|
computed tomography
|
|
CTX
|
cetuximab
|
|
EGFR
|
epidermal growth factor receptor
|
|
ERK
|
extracellular signal-regulated protein
kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
mCRC
|
metastatic CRC
|
|
ZOL
|
zoledronic acid
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram J, Siegel
RL, Torre LA and Jemal A: Global cancer statistics, 2018 GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cance J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
2
|
Van Cutsem E and Oliveira J; ESMO
Guidelines Working Group, : Advanced colorectal cancer: ESMO
clinical recommendations for diagnosis, treatment and follow-up.
Ann Oncol. 4 (Suppl):S61–S63. 2009.
|
|
3
|
Goldstein NI, Prewett M, Zuklys K,
Rockwell P and Mendelsohn J: Biological efficacy of a chimeric
antibody to the epidermal growth factor receptor in a human tumor
xenograft model. Clin Cancer Res. 1:1311–1318. 1995.PubMed/NCBI
|
|
4
|
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ,
Kussie P and Ferguson KM: Structural basis for inhibition of the
epidermal growth factor receptor by cetuximab. Cancer Cell.
7:301–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bokemeyer C, Bondarenko I, Hartmann JT, de
Braud F, Schuch G, Zubel A, Celik I, Schlichting M and Koralewski
P: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
The OPUS study. Ann Oncol. 22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Kohne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Corey E, Brown LG, Quinn JE, Poot M,
Roudier MP, Higano CS and Vessella RL: Zoledronic acid exhibits
inhibitory effects on osteoblastic and osteolytic metastases of
prostate cancer. Clin Cancer Res. 9:295–306. 2003.PubMed/NCBI
|
|
8
|
Kato J, Futamura M, Kanematsu M, Gaowa S,
Mori R, Tanahashi T, Matsuhashi N and Yoshida K: Combination
therapy with zoledronic acid and cetuximab effectively suppresses
growth of colorectal cancer cells regardless of KRAS status. Int J
Cancer. 138:1516–1527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tournigand C, Andre T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lewis TS, Shapiro PS and Ahn NG: Signal
transduction through MAP kinase cascades. Adv Cancer Res.
74:49–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han FS, Lin MB, Zhu HY, Chen YQ, Shui W
and Xu JM: Anti-proliferation effect of zoledronic acid on human
colon cancer line SW480. Asian Pac J Trop Med. 9:168–171. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schubbert S, Shannon K and Bollag G:
Hyperactive Ras in developmental disorders and cancer. Nat Rev
Cancer. 7:295–308. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Correale P, Marra M, Remondo C, Migali C,
Misso G, Arcuri FP, Del Vecchio MT, Carducci A, Loiacono L, Tassone
P, et al: Cytotoxic drugs up-regulate epidermal growth factor
receptor (EGFR) expression in colon cancer cells and enhance their
susceptibility to EGFR-targeted antibody-dependent
cell-mediated-cytotoxicity (ADCC). Eur J Cancer. 46:1703–1711.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maniar A, Zhang X, Lin W, Gastman BR,
Pauza CD, Strome SE and Chapoval AI: Human gammadelta T lymphocytes
induce robust NK cell-mediated antitumor cytotoxicity through CD137
engagement. Blood. 116:1726–1733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura S, Fukui T, Suzuki S, Takeda H,
Watanabe K and Yoshioka T: Long-term survival after a favorable
response to anti-EGFR antibody plus chemotherapy to treat bone
marrow metastasis: A case report of KRAS-wildtype rectal cancer.
Onco Targets Ther. 10:1143–1147. 2017. View Article : Google Scholar : PubMed/NCBI
|