Introduction
Chemotherapy with fluorouracil-epirubicin
(EPI)-cyclophosphamide, or the FEC regimen, and
EPI-cyclophos-phamide, or EC regimen, is widely used in breast
cancer treatment. These regimens contain EPI, an
anthracycline-based anti-malignant tumor drug, and are recommended
to be used in combination with oral aprepitant (AP), an antiemetic
drug that is a selective neurokinin-1 receptor antagonist, or
intravenous fosaprepitant (FAP), a phosphorylated prodrug of AP, in
clinical practice guidelines such as ASCO, MASCC/ESMO, and NCCN
(1–3). AP is taken orally and FAP is
administered intravenously to prevent systemic adverse events such
as nausea and vomiting. Oral AP is ingested once before and twice
after EPI treatment, once/day for 3 days mostly for inpatients, and
intravenous FAP is administered once by constant-rate infusion over
30 min just before EPI treatment mostly for outpatients (4). It has been reported that intravenous
FAP alone and an intravenous anthracycline such as doxorubicin
alone can cause infusion-site adverse events (4–9). In
addition, the combined use of FAP and an anthracycline is reported
to induce infusion-site adverse events such as vascular pain and
venous inflammation with high probability (10–12). In
our hospital (Chugoku Rosai Hospital), we have experienced such
infusion-site adverse events in breast cancer patients receiving
chemotherapy with the FEC regimen and intravenous FAP
(Proemend®, Ono Pharmaceutical Co., Ltd., Osaka, Japan)
infusion. Interaction between FAP (Proemend®) and EPI at
the infusion site was suspected, as the frequency of vascular pain
and venous inflammation seemed to increase after switching from
oral AP (Emend®, Ono Pharmaceutical Co., Ltd.) to
intravenous FAP (Proemend®). Although venous
inflammation is not a serious life-threatening reaction, it
disrupts the patient's quality of life (QOL) and is a major
disadvantage to patients, depending on the symptoms.
In the present study, infusion-site adverse events
in chemotherapy with EPI and FAP were studied by comparing the
vascular tissue concentrations of EPI and by histological
observation at the EPI infusion and non-infusion sites in rats to
consider a safer method in order to avoid local adverse events at
the EPI infusion site.
Materials and methods
Materials
AP or Emend®, and FAP dimeglumine or
Proemend® Intravenous Infusion 150 mg, were obtained
from Ono Pharmaceutical Co., Ltd. EPI was obtained from Nippon
Kayaku Co., Ltd., (Tokyo, Japan). Other antitumor agents used were
fluorouracil or Fluorouracil Injection 1,000 mg ‘Towa’, obtained
from Towa Pharmaceutical Co., Ltd. (Osaka, Japan), and
cyclophosphamide or Endoxan® 500 mg for injection
obtained from Shionogi & Co., Ltd. (Osaka, Japan). All other
chemicals used were of the highest purity available.
Patients and treatments
In total, eight breast cancer patients were
hospitalised for chemotherapy with the FEC regimen (six advanced
cancer patients) or EC regimen (two recurrent cancer patients) to
treat breast cancer. Ages of the female patients ranged from 32 to
74 years and the body weights ranged from 47 to 70 kg. AP (or
Emend®) was taken orally at a dose of 125 mg (1st day),
and 1 h after oral AP, the first course of chemotherapy was started
by infusing EPI (100 mg/m2) in 5–10 min,
cyclophosphamide (500 mg/m2) in 30 min, and fluorouracil
(500 mg/m2) in 30 min for the FEC regimen, or EPI (90
mg/m2) infusion in 5–10 min and cyclophosphamide (600
mg/m2) infusion in 30 min for the EC regimen,
respectively. On the 2nd and 3rd days, AP (or Emend®, 80
mg) alone was taken orally in both the FEC and EC regimens.
Thereafter, patients were discharged from the hospital and received
the following courses of the FEC or EC regimen as outpatients every
3 weeks. Each time, they received FAP dimeglumine (150 mg as FAP)
by a constant-rate infusion over 30 min just before chemotherapy,
in which FAP dimeglumine, or Proemend® Intravenous
Infusion 150 mg, in a vial was dissolved with 100 ml saline.
Pre-operative and post-operative chemotherapy were administered for
up to 4 courses, and chemotherapy for advanced and recurrent cancer
was administered at up to <900 mg/m2 of EPI as the
cumulative amount while observing the effect.
Vascular distribution of EPI in
rats
Male Sprague-Dawley (SD) rats (7-weeks-old) weighing
approximately 250 g were obtained from Hiroshima Jikken Dobutsu
Kenkyujo (or Hiroshima Institute of Experimental Animals,
Hiroshima, Japan) and were maintained under a 12-h light/12-h dark
cycle for at least 1 week before the experiments. The rats were
anaesthetised with pentobarbital (50 mg/kg) via intraperitoneal
injection and randomly divided into the following 3 groups: FAP-S
Group, 10-min FAP dimeglumine infusion (3 mg as FAP/kg) from the
left jugular vein and then 5-min EPI infusion (1 mg/kg) from the
left jugular vein; FAP-D Group, 10-min FAP dimeglumine infusion (3
mg as FAP/kg) from the right jugular vein and then 5-min EPI
infusion (1 mg/kg) from the left jugular vein; and AP Group, AP was
administered orally (3 mg/kg) followed by 5-min EPI infusion (1
mg/kg) from the left jugular vein after 60 min. FAP dimeglumine
(Proemend®) and EPI were dissolved in saline at
concentrations of 1.5 mg as FAP/ml and 1 mg/ml, respectively. The
constant-rate infusion of the FAP dimeglumine solution (3 mg as
FAP/2 ml saline/kg) for 10 min followed by the EPI solution (1
mg/ml/kg) for 5 min was performed using the STC-525 Terumo infusion
pump (Terumo Corporation Tokyo, Japan). A 26 G needle connected to
polyethylene tubing from the infusion pump was inserted into the
jugular vein at approximately 7-mm length in the upward direction
from a position just above the intersection of the subclavian vein
and jugular vein. At 30 min or 24 h after EPI infusion, the rat
abdomen was incised, and the portal vein was cut to collect a blood
sample under anaesthesia. After the rat died due to blood loss,
approximately 2 cm samples of the jugular vein at the infusion site
and on the opposite site of the neck were excised. The isolated
vascular tissue samples were washed gently with a small amount of
ice-cold saline to remove blood and were then wiped with paper to
remove water. Blood samples collected were centrifuged at 3,000 ×
g, 4°C for 5 min to obtain plasma samples. The obtained plasma and
vascular tissue samples were frozen at −80°C until analysis.
Histological analysis
In separate experiments, rats were treated with a
two-times higher dose of each drug (EPI 2 mg/kg, FAP 6 mg/kg, AP 6
mg/kg) for histological analysis than those used for the vascular
tissue distribution study to increase drug pharmacological action.
Rats were sacrificed at 24 h after EPI infusion and vascular tissue
along with the surrounding tissue at the EPI infusion site and its
opposite site was isolated. Vascular tissue samples were washed
gently with a small amount of ice-cold saline, fixed in 15%
formalin, and then embedded in paraffin. After haematoxylin-eosin
staining of tissue sections of approximately 2–4-µm thickness, the
tissue was examined under an optical microscope (Nikon Eclipse
E1000, Nikon Instech Co., Ltd.) at magnification, ×200.
Analysis of EPI
Concentrations of EPI in plasma samples and vascular
tissue samples were determined by high performance liquid
chromatography (HPLC). The plasma sample (200 µl) was mixed with an
equal volume of acetonitrile (200 µl), centrifuged at 10,000 × g
(4°C) for 5 min, and 1% acetic acid (80 µl) was added to the
supernatant (120 µl). The vascular tissue sample (about 20 mg) was
weighed and homogenised in a 19-fold volume of 50% acetonitrile by
ultrasonic treatment (Tomy UD-201, Tomy Seiko Co., Ltd., Tokyo,
Japan) for approximately 2 min. The suspension was centrifuged at
10,000 × g (4°C) for 5 min, and a mixture of 1% acetic acid (40 µl)
and HPLC mobile phase (100 µl of a mixture of 0.1% acetic acid and
acetonitrile, 7:3) for EPI analysis was added to the supernatant
(60 µl). The suspension was filtered with a 0.45 µm-syringe filter,
and the filtrate was subjected to EPI analysis by HPLC. The HPLC
column used was a YMC-Triart C18 column (150×4.6 mm I.D.; YMC Inc.,
Kyoto, Japan). The mobile phase was a mixture of 1% acetic acid and
acetonitrile in a ratio of 7:3 (v/v), and the flow rate was set at
1.0 ml/min. EPI was detected fluorometrically at an excitation
wavelength of 470 nm and an emission wavelength of 585 nm. The
obtained calibration curve was linear in a concentration range from
2 ng to 500 ng/ml and from 1 to 10 µg/ml.
Statistical analysis
The data are presented as the mean ± S.D. (n=4), and
statistical analysis was performed by one-way ANOVA, followed by
the Tukey-Kramer method for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Chemotherapy for breast cancer
patients
After single oral administration of AP, chemotherapy
with the FEC regimen or EC regimen was started in 8 breast cancer
patients. After the first course of chemotherapy, AP was
administered for 2 more days. The patients left the hospital and
the following course of chemotherapy was performed as outpatients
by intravenous infusion of FAP dimeglumine just before the
following course of chemotherapy. Before starting the 2nd course of
chemotherapy, none of the patients admitted any side effects on the
skin. After starting the 2nd or 3rd course of chemotherapy, 5
patients out of 8 complained of vascular pain and 3 patients among
them showed angitis at the EPI infusion site. In addition, 2
patients of expressed grade 2 injection site reactions that
corresponded to the common terminology criteria for the adverse
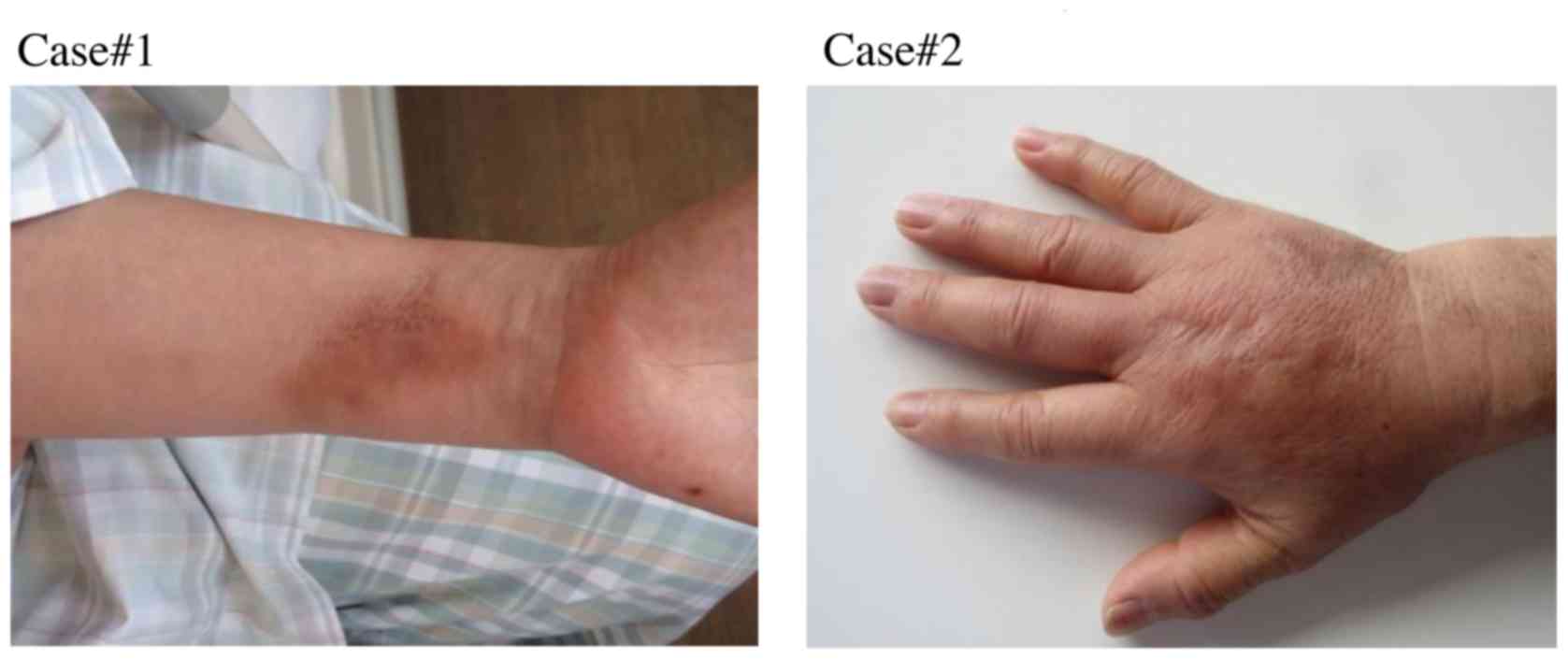
events version 4.0 as follows (Cases #1 and #2):
Case #1
A female breast cancer patient (45 years old
weighing 59.8 kg) received FEC chemotherapy. Although vascular pain
appeared after the 3rd course of treatment, the 4th course of
treatment was performed according to the initial schedule plan.
Thereafter, grade 2 induration appeared at the infusion site
(inside the wrist) (Fig. 1).
Case #2
A female breast cancer patient (74 years old
weighing 55.5 kg) received FEC chemotherapy. After the 2nd course
of chemotherapy, vascular pain, swelling, and induration appeared
at the infusion site (back of the hand). After the 3rd course,
phlebitis (grade 2) appeared at the infusion site, and FEC
chemotherapy was discontinued and changed to other drugs
(trastuzumab and docetaxel) (Fig.
1).
These results suggested the incidence of an
interaction between FAP dimeglumine and EPI, since the frequency of
induction of adverse events such as vascular pain and phlebitis
seemed to be increased after switching to intravenous FAP
dimeglumine from oral AP in clinical practice.
Vascular tissue distribution of EPI in
rats
At 30 min and 24 h after the 5-min constant-rate EPI
infusion, concentrations of EPI in plasma and vascular tissue were
compared among the FAP-S, FAP-D, and AP groups. There was no
significant difference in the plasma EPI concentrations at 30 min
among the three different groups, and EPI was not detected in the
plasma at 24 h after EPI infusion (Table
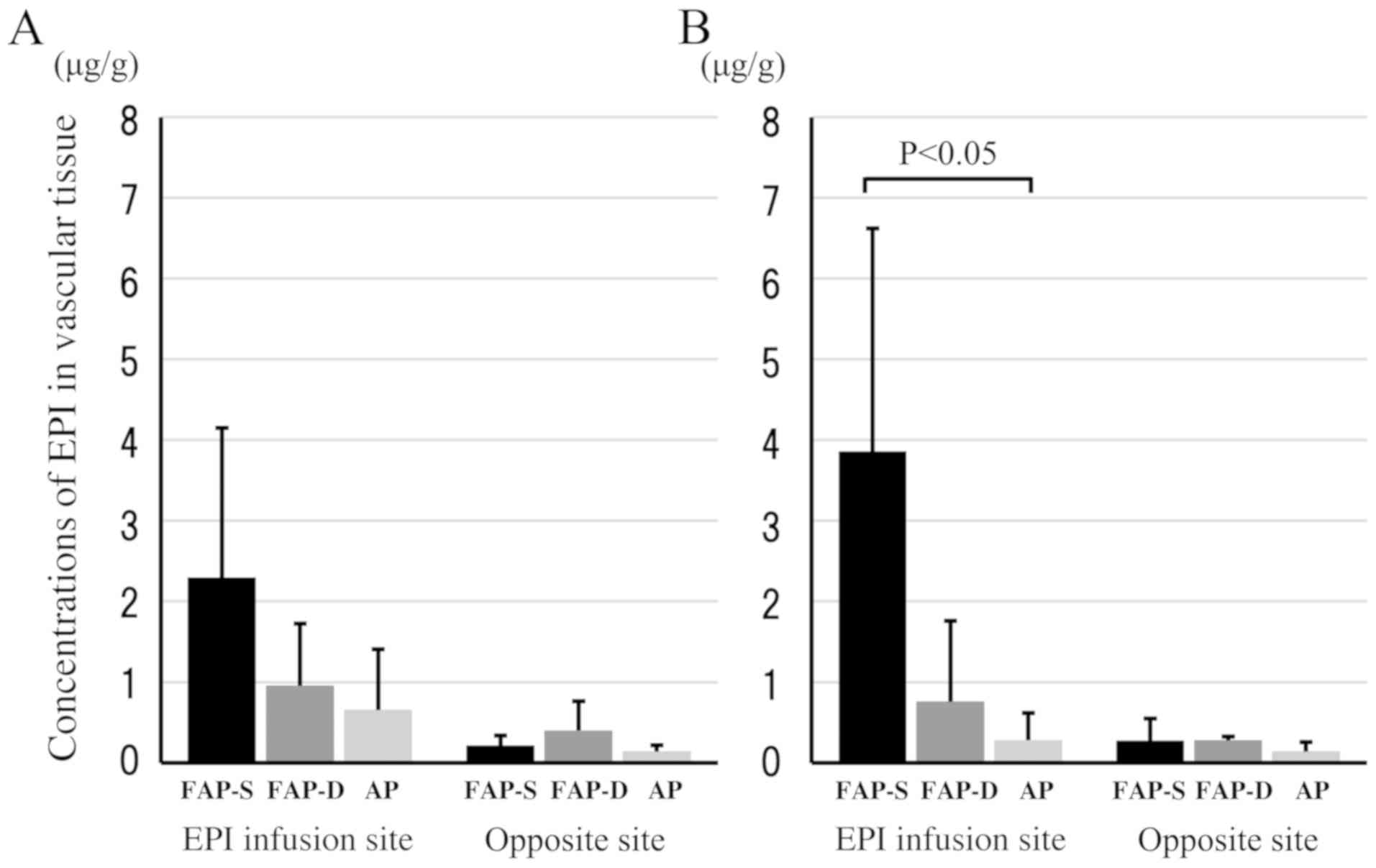
I). The average concentrations of EPI in vascular tissue at the
EPI infusion site at 30 min and 24 h were in the following order:
FAP-S group > FAP-D group > AP group (Fig. 2A and B). In particular, the vascular
tissue concentrations of EPI at 24 h were almost the same as those
at 30 min in each group, irrespective of the disappearance of EPI
from plasma. This indicates that EPI can accumulate in vascular
tissue. A significant difference was detected in vascular tissue
EPI concentrations between the FAP-S and AP groups at 24 h
(P<0.05). Infusion-site vascular tissue of EPI showed higher EPI
concentrations than those in the opposite-site (or
non-infusion-site) vascular tissue in all three groups.
Collectively, the FAP-S group showed the highest vascular tissue
concentrations of EPI at the EPI infusion site compared to those of
the FAP-D group, AP group, and the non-infusion sites of all three
groups at 30 min and 24 h after EPI infusion (Fig. 2).
 | Table I.Concentrations of EPI in plasma and
vascular tissue at the infusion-site after constant-rate EPI
infusion over 5 min in rats. |
Table I.
Concentrations of EPI in plasma and
vascular tissue at the infusion-site after constant-rate EPI
infusion over 5 min in rats.
| Sample (sampling
time) | FAP-S group (ng/ml or
µg/g) | FAP-D group (ng/ml or
µg/g) | AP group (ng/ml or
µg/g) |
|---|
| Plasma (30 min) | 9.10±2.40 | 10.8±2.88 | 6.47±3.06 |
| Plasma (24 h) | ND | ND | ND |
| Vascular tissue (30
min) | 2.30±1.85 | 0.958±0.767 | 0.659±0.751 |
| Vascular tissue (24
h) |
3.86±2.76a | 0.762±0.997 | 0.281±0.339 |
Histological examination of vascular
tissue in rats
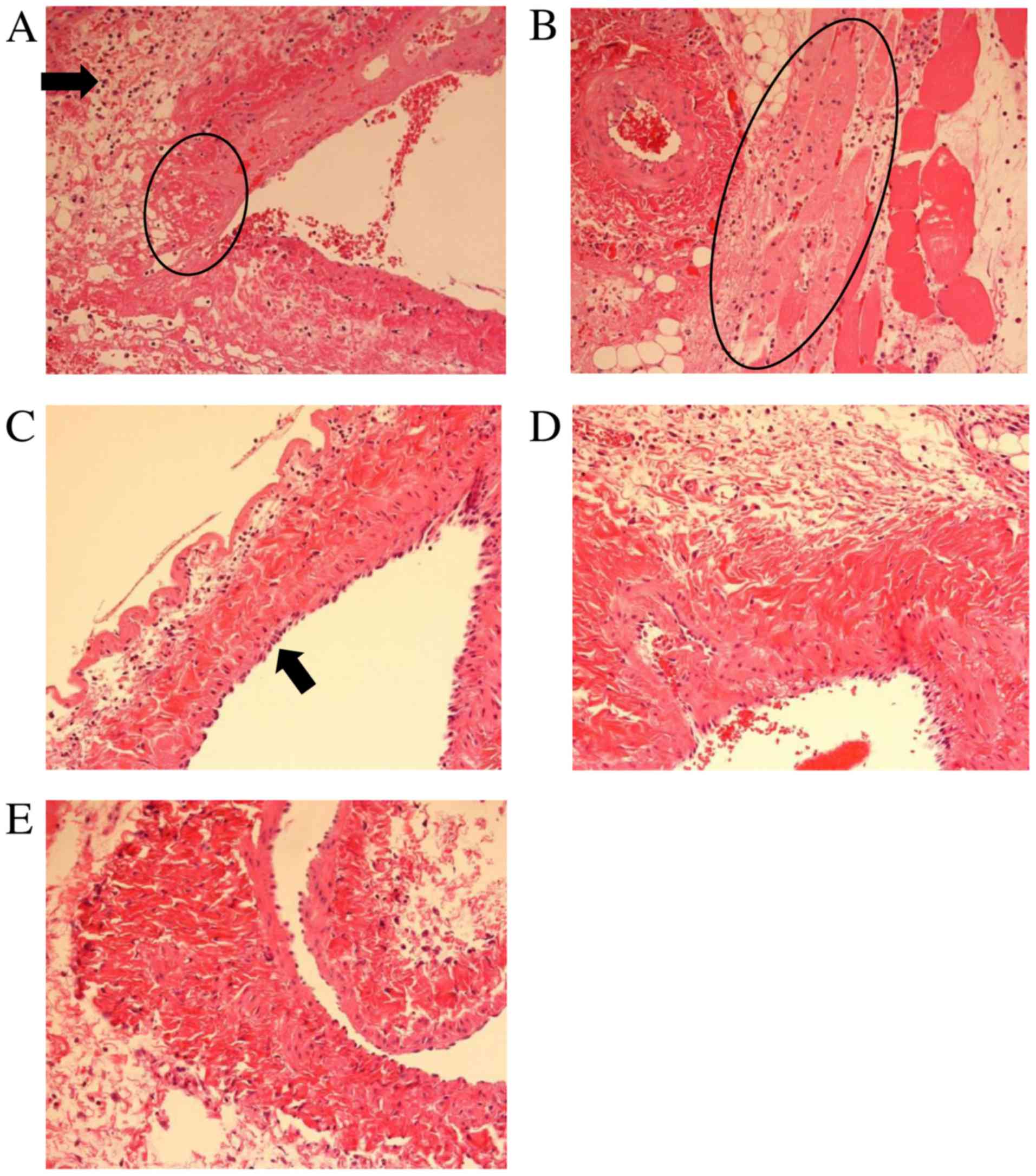
At 24 h after EPI infusion, the vascular tissue at
the infusion site was examined histologically by microscopic
observation. The vascular endothelial cells at the EPI infusion
site of the FAP-S group showed infiltration of neutrophils and
necrosis, and the necrosis spread to the surrounding tissue of the
EPI infusion-site jugular vein (Fig. 3A
and B). The FAP-D group showed swelling of vascular endothelial
cells at the EPI infusion site, but neither necrosis nor
inflammation was observed (Fig. 3C).
The infusion site of FAP in the FAP-D group showed no significant
histological alteration in the vascular tissue (Fig. 3D). The AP group also showed no
significant alteration in the vascular tissue even at the EPI
infusion site (Fig. 3E).
Collectively, the magnitude of histological damage at infusion-site
adverse events was in the following order: EPI-infusion site of the
FAP-S group >> EPI-infusion site of the FAP-D group >>
EPI-infusion site of the AP group and FAP-infusion site of the
FAP-D group (no damage).
Discussion
When cancer cells are found in patients, cancer
cells are thought to have already spread to other parts of the body
through blood and lymphatic vessels in the case of invasive breast
cancer. It is not easy to detect micro-metastasis of cancer cells
in other tissues by diagnostic imaging and to remove them by
operation. Chemotherapy is essential in addition to operation in
the treatment of invasive breast cancer, and chemotherapy for
breast cancer has been proven equivalent in terms of survival and
overall disease progression in both pre-operative and
post-operative stages (13).
However, in the case of postoperative chemotherapy, there is a
possibility of causing lymphedema in patients who received lymph
node dissection or sentinel lymph node biopsy. Intravenous
injection of anticancer drugs from the affected lymph node side
should be avoided in such patients. In cancer chemotherapy,
incidence of adverse events such as nausea and vomiting that
decrease the patient's QOL is common and has significant effects on
subsequent chemotherapy. Antiemetic guidelines such as ASCO,
MASCC/ESMO, and NCCN were set up in response to the risk of cancer
chemotherapy (1–3).
Chemotherapies with FEC and EC regimens containing
EPI are widely performed in breast cancer treatment, and the use of
dexamethasone, 5-HT3 antagonists, and AP or FAP is recommended in
any of the guidelines to decrease the incidence of systemic adverse
events. However, intravenous FAP infusion may be associated with a
higher risk of infusion-site adverse events than AP when combined
with an anthracycline in chemotherapy (14,15). In
contrast, the incidence of infusion-site adverse events is
reportedly low when FAP infusion is combined with cisplatin-based
chemotherapy, suggesting the contribution of some interaction
between FAP (or Proemend®) and anthracyclines such as
doxorubicin and EPI (15).
We also experienced the incidence of infusion-site
adverse events such as grade-2 induration and grade-2 phlebitis in
addition to vascular pain in 2 breast cancer patients who received
FEC chemotherapy, in which they received intravenous infusion of
FAP (or Proemend®) and 3 anticancer drugs (EPI,
cyclophosphamide, and fluorouracil) serially from the same site on
the vein (Fig. 1). The most common
FAP infusion-site adverse events in doxorubicin/cyclophosphamide
(AC) chemotherapy for 98 breast cancer patients were reported to be
infusion site pain (n=26), erythema (n=22), swelling (n=12),
superficial thrombosis (n=8), infusion site hives (n=5), and
phlebitis/thrombophlebitis (n=5), where 26 patients experienced
more than one type of infusion-site adverse events (11). The mechanism of the interaction
between FAP (or Proemend®) and anthracyclines at the
infusion site is not yet clarified. The injection site reaction was
reported to be significantly reduced from 28.7 to 5.74% when FAP
(or Proemend®) was diluted from 150/150 to 150 mg/250 ml
and infused over 30 min (9). Though
such a reaction caused by FAP alone or EPI alone was not observed
histologically in the present study (Fig. 3), the above report regarding the
reduction of infusion rate (9)
suggests that the distribution of anticancer drug(s) into the
infusion-site vascular tissue from blood (or plasma) circulation
could be involved in infusion site adverse events. EPI with
cytotoxicity is known to have a large distribution volume in rats
and human and distributes to various tissues with an inter-organ
variation (16–18). In contrast, cisplatin with a low
incidence of infusion-site adverse events has a relatively small
tissue distribution volume of approximately 20% body weight, which
corresponds to the volume of plasma and intercellular space not
including the intracellular aqueous space, in the body (19).
In the present study, therefore, we examined the
induction mechanism of infusion-site adverse events in chemotherapy
with FAP and EPI from the viewpoint of EPI distribution into
infusion-site vascular tissue using rats. The doses of intravenous
EPI (1 or 2 mg/kg) used for the vascular tissue distribution study
(Table I, Fig. 2) and histological analysis study
(Fig. 3), respectively, were lower
than those used in clinical FEC chemotherapy (approximately 3
mg/kg). The infusion period (5 min) of EPI performed in the present
study was close to that used in clinical chemotherapy (5–10 min).
In contrast, the infusion rate (10 min-infusion) of FAP conducted
in the present animal study was faster than that used in clinical
chemotherapy (over 30 min), though the actual drug concentration in
blood circulation at the infusion site varied depending on the
physiological blood stream velocity at anatomically different
positions. It is not easy to adjust the local concentrations of
infused drugs in the infusion-site blood stream appropriately
between animal studies and clinical practice.
In the FAP-S group, FAP and EPI were administered
intravenously from the same position on the jugular vein. In the
FAP-D group, FAP and EPI were administered intravenously from
different jugular veins, and in the AP group, AP was administered
orally, and EPI was administered intravenously into the jugular
vein. The EPI infusion site of the FAP-S group showed greater EPI
concentrations in vascular tissue at 30 min and 24 h after EPI
infusion than those in other cases including the FAP-D group, AP
group, and opposite non-infusion sites (Table I, Fig.
2), suggesting that FAP (or Proemend®) contains some
components that enhance the vascular distribution of EPI. In
addition, the FAP-S group alone showed inflammation and necrosis at
the infusion-site vascular tissue in which necrosis had spread to
the peripheral surrounding tissues (Fig.
3). In contrast, such inflammation at the vascular tissue was
not observed in the FAP-D and AP groups in the present study, even
though it was reported that FAP alone and EPI alone can cause
infusion site adverse events (4–9). In
clinical practice, complaints of vascular pain from patients also
appeared after switching from oral AP to intravenous FAP.
Collectively, higher vascular tissue concentrations of EPI were
thought to result in inflammation and necrosis at the infusion
site, and an increase in EPI distribution into vascular tissue was
thought to be induced by the combined use of intravenous FAP (or
Proemend®) (Fig. 2).
Regarding the tissue accumulation of EPI over a longer period (more
than 24 h as shown in Fig. 2),
irrespective of the disappearance of EPI from plasma at 24 h
(Table I), the presence of some
component(s) affecting the accumulation of EPI, a weakly basic drug
with pKa =7.77, in tissue was suspected as observed with some other
weakly basic drugs (20). Further
studies are necessary regarding the tissue distribution mechanism
of EPI, in the presence and absence of FAP (or
Proemend®), including the tissue binding components and
interorgan variation as reported (16–18).
Proemend® Intravenous Infusion 150 mg
contains FAP dimeglumine 245.3 mg (or 150 mg as FAP), 5.7 mg sodium
edetate hydrate, 78.8 mg polysorbate 80, and so on as additives in
a single vial, and its contents are dissolved carefully by adding
100–150 ml saline for clinical use (21). Recently, considering that polysorbate
80, a synthetic non-ionic surfactant, is associated with
treatment-emergent adverse events, a polysorbate 80-free aprepitant
IV formulation (HTX-019) was developed. HTX-019 showed
bioequivalence to commercially available FAP infusion solution
(Proemend®) with a low risk of polysorbate 80
surfactant-associated systemic hypersensitivity and infusion-site
adverse events (22–24). For example, the incidence of
infusion-site pain caused by HTX-019 (n=99) and
Proemend® (n=100) was 1 and 9%, respectively (22). In our study, the EPI concentration in
vascular tissue was relatively low, and no significant histological
damage was observed in vascular tissue when EPI was infused from
different jugular veins from FAP (Proemend®). Taken
together, these results imply the contribution of polysorbate 80 in
increased vascular tissue distribution of EPI and infusion-site
adverse events when FAP (Proemend®) and EPI are infused
from the same position of the blood vessel (FAP-S group). The
enhancing tendency of Proemend® on the vascular tissue
distribution of EPI was also observed in the FAP-D group, in which
the vascular concentration of FAP (or Proemend®) in the
opposite jugular vein from the EPI infusion site was the same as
that in the EPI infusion site of the FAP-S group. The average
vascular tissue concentration of EPI 30 min after administration
was in the following order: FAP-D > FAP-S > oral AP, although
there was no significant difference among the 3 groups (Fig. 2A). Further studies are necessary to
clarify the induction mechanism of EPI infusion-site adverse events
in detail, including the enhancing mechanism of FAP (or
Proemend®) on tissue distribution of EPI in vascular
tissue.
In conclusion, EPI infusion-site adverse events
induced by FAP (or Proemend®) infusion were analysed
from the viewpoint of vascular tissue distribution of EPI and
histological observation in the present study. The higher EPI
concentrations in infusion-site vascular tissue and induction of
severe adverse events such as inflammation and necrosis were
observed when EPI was infused from the same site with FAP infusion.
In contrast, infusion of EPI from different veins other than those
used for FAP infusion resulted in lower vascular tissue
concentrations of EPI and prevented the induction of inflammation
and necrosis at the EPI infusion site in rats. In clinical
practice, venous infusion of drugs should be performed from the
other half of the body where breast cancer surgery is performed;
the above findings suggest that the following administration
methods are preferable: Infusion of EPI from a vein different from
that used for FAP infusion (only for neoadjuvant chemotherapy),
central venous catheter infusion of FAP and EPI, or oral AP and
venous infusion of EPI.
Acknowledgements
Not applicable.
Funding
The present study was supported by research funds to
promote the hospital functions of Japan Organization of
Occupational Health and Safety.
Availability of data and materials
The dataset supporting the conclusions of the
present study is included within the article.
Authors' contributions
MY, YM, KO and TM designed the study and analyzed
the data. MY and KO performed experiments using rats. TN performed
histological analysis. RK, SM, and MT participated in performing
chemotherapy regimens. MY and TM wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The chemotherapy for breast cancer patients with FAP
infusion and FEC regimen or EC regimen was carried out at Chugoku
Rosai Hospital (Kure, Hiroshima, Japan) according to the principles
of the Declaration of Helsinki. This study protocol was approved by
the ethics committee of Chugoku Rosai Hospital (approval no.
2016-01). Eight Japanese breast cancer patients (2 for EC regimen
and 6 for FEC regimen) participated after providing written
informed consent. The protocol of experiments using rats was
reviewed and was approved by the Committee of Research Facilities
for Laboratory Animal Sciences, Hiroshima International University
(AE17-027).
Patient consent for publication
Patients provided written informed consent and
agreed to the publication of their results.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AP
|
aprepitant
|
|
EPI
|
epirubicin
|
|
FAP
|
fosaprepitant
|
|
FEC
|
fluorouracil-epirubicin-cyclophosphamide
|
|
HPLC
|
high performance liquid
chromatography
|
|
S.D.
|
standard deviation
|
References
|
1
|
Hesketh PJ, Kris MG, Basch E, Bohlke K,
Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis L, Dusetzina
SB, et al: Antiemetics: American society of clinical oncology
clinical practice guideline update. J Clin Oncol. 35:3240–3261.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rolia F, Molassiotis A, Herrstedt J, Aapro
M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer
P, et al: 2016 MASCC and ESMO guideline update for the prevention
of chemotherapy- and radiotherapy-induced nausea and vomiting and
of nausea and vomiting in advanced cancer patients. Ann Oncol. 27
(Suppl 5):v119–v133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berger MJ, Ettinger DS, Aston J, Barbour
S, Bergsbaken J, Bierman PJ, Brandt D, Dolan DE, Ellis G, Kim EJ,
et al: NCCN guidelines insights: Antiemesis, version 2.2017. J Natl
Compr Canc Netw. 15:883–893. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pritchett W and Kinsley K: Benefits and
risks of fosaprepitant in patients receiving emetogenic regimens.
Clin J Oncol Nurs. 20:555–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow AY, Chin C, Dahl G and Rosenthal DN:
Anthracyclines cause endothelial injury in pediatric cancer
patients: A pilot study. J Clin Oncol. 24:925–928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ben Aharon I, Bar Joseph H, Tzabari M,
Shenkman B, Farzam N, Levi M, Shalgi R, Stemmer SM and Savion N:
Doxorubicin-induced vascular toxicity-targeting potential pathways
may reduce procoagulant activity. PLoS One. 8:e751572013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bar-Joseph H, Stemmer SM, Tsarfaty I,
Shalgi R and Ben-Aharon I: Chemotherapy-induced vascular
toxicity-real-time in vivo imaging of vessel impairment. J Vis Exp.
e516502015.PubMed/NCBI
|
|
8
|
Lundberg JD, Crawford BS, Phillips G,
Berger MJ and Wesolowski R: Incidence of infusion-site reactions
associated with peripheral intravenous administration of
fosaprepitant. Support Care Cancer. 22:1461–1466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chau E, Lundberg J, Phillips G, Berger M
and Wesolowski R: Updated report on incidence of infusion-site
reactions associated with peripheral intravenous administration of
fosaprepitant. J Oncol Pharm Pract. 10781552187693472018.PubMed/NCBI
|
|
10
|
Sato Y, Kondo M, Inagaki A, Komatsu H,
Okada C, Naruse K, Sahashi T, Kuroda J, Ogura H, Uegaki S, et al:
Highly frequent and enhanced injection site reaction induced by
peripheral venous injection of fosaprepitant in
anthracycline-treated patients. J Cancer. 5:390–397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lead AD, Kadakia KC, Looker S, Hilger C,
Sorgatz K, Anderson K, Jacobson A, Grendahl D, Seisler D, Hobday T
and Loprinzi CL: Fosaprepitant-induced phlebitis: A focus on
patients receiving doxorubicin/cyclophosphamide therapy. Support
Care Cancer. 22:1313–1317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel P, Leeder JS, Piquette-Miller M and
Dupuis LL: Aprepitant and fosaprepitant drug interactions: A
systematic review. Br J Clin Pharmacol. 83:2148–2162. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mauri D, Pavlidis N and Ioannidis JPA:
Neoadjuvant versus adjuvant systemic treatment in breast cancer: A
meta-analysis. J Natl Cancer Inst. 97:188–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsuda T, Kyomori C, Mizukami T, Taniyama
T, Izawa N, Horie Y, Hirakawa M, Ogura T, Nakajima T, Tsugawa K and
Boku N: Infusion site adverse events in breast cancer patients
receiving highly emetic chemotherapy with prophylactic anti-emetic
treatment with aprepitant and fosaprepitant: A retrospective
comparison. Mol Clin Oncol. 4:603–606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujii T, Nishimura N, Urayama KY, Kanai H,
Ishimaru H, Kawano J, Takahashi O, Yamauchi H and Yamauchi T:
Differential impact of fosaprepitant on infusion site adverse
events between cisplatin- and anthracycline-based chemotherapy
regimens. Anticancer Res. 35:379–383. 2015.PubMed/NCBI
|
|
16
|
Italia C, Paglia L, Trabattoni A, Luchini
S, Villas F, Beretta L, Marelli G and Natale N: Distribution of
4′Epi-doxorubicin in human tissues. Br J Cancer. 47:545–547. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camaggi CM, Strocchi E, Carisi P, Martoni
A, Melotti B and Pannuti F: Epirubicin metabolism and
pharmacokinetics after conventional- and high-dose intravenous
administration: A cross-over study. Cancer Chemother Pharmacol.
32:301–309. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robert J: Clinical pharmacokinetics of
epirubicin. Clin Pharmacokinet. 26:428–438. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen R, Li J, Hu WW, Wang ML, Zou SL and
Miao LY: Circadian variability of pharmacokinetics of cisplatin in
patients with non-small-cell lung carcinoma: Analysis with the
NONMEM program. Cancer Chemother Pharmacol. 72:1111–1123. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murakami T and Yumoto R: Role of
phosphatidylserine binding in tissue distribution of
amine-containing basic compounds. Expert Opin Drug Metab Toxicol.
7:353–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Interview Form ‘PROEMEND for intravenous
infusion 150 mg’. March. 2016, http://www.pmda.go.jp/
|
|
22
|
Ottoboni T, Keller MR, Cravets M,
Clendeninn N and Quart B: Bioequivalence of HTX-019 (aprepitant IV)
and fosaprepitant in healthy subjects: A phase I, open-label,
randomized, two-way crossover evaluation. Drug Des Devel Ther.
12:429–435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ottoboni T, Lauw M, Keller MR, Cravets M,
Manhard K, Clendeninn N and Quart B: Safety of HTX-019 (intravenous
aprepitant) and fosaprepitant in healthy subjects. Future Oncol.
14:2849–2859. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwartzberg LS and Navari RM: Safety of
polysorbate 80 in the oncology setting. Adv Ther. 35:754–767. 2018.
View Article : Google Scholar : PubMed/NCBI
|