Introduction
Breast cancer is a heterogeneous complex of
diseases, comprising a spectrum of numerous subtypes with distinct
biological features. These biological subtypes lead to differences
in treatment responses and clinical outcomes (1). Traditional classification systems
include biological characteristics, including tumor size, lymph
node involvement, histological grade, patient age, estrogen
receptors (ER), progesterone receptors (PR) and human epidermal
growth factor receptor 2 (HER2 or c-erbB2) status (2). The diagnosis of breast cancer is based
on clinical examination in combination with imaging and is
confirmed by pathological assessment. Clinical examination includes
bimanual palpation of the breasts and locoregional lymph nodes.
Imaging diagnostics facilitate the assessment of the presence of
distant metastases (bones, liver and lungs). A neurological
examination is only required when symptoms are present (3).
The pathological report should include the
histological type, grade, immunohistochemical (IHC) evaluation of
estrogen receptor (ER) status (using a standardized assessment
methodology, e.g., Allred or H-score), and progesterone receptor
(PR) and human epidermal growth factor 2 receptor (HER2) gene
expression. HER2 gene amplification status may be determined
directly from all invasive tumors using in situ
hybridization (fluorescent, chromogenic or silver), either as a
replacement for IHC or for tumors with an ambiguous (2+) IHC score
(4). Proliferation markers such as
Ki67 should also be assessed (5).
Breast cancer tumors are divided into subtypes
according to aforementioned factors, defined by routine histology
and IHC (the 2015 St Gallen Consensus Conference). This
classification is very important for prognosis and treatment
decisions (1,6). Luminal-A is the most common subtype and
represents 50–60% of all breast cancer cases. This subtype is
defined as ER-positive and/or PR-positive tumors with a negative
HER2 and low Ki67 (proliferating cell nuclear antigen) index,
assessed by immunohistochemistry. Patients with luminal-A breast
cancer have a good prognosis and the relapse rate is significantly
lower compared with that for other subtypes (7). Luminal-B tumors comprise 15–20% of
breast cancer cases and have a more aggressive phenotype, higher
histological grade, increased proliferative index and a worse
prognosis. There are distinct HER2-negative (ER-positive;
HER2-negative; Ki67% high; PR low) and HER2-positive (ER-positive;
HER2-positive; any Ki67; any PR) luminal B subtypes. HER2-positive
cancer accounts for 15–20% of breast cancer subtypes. These tumors
are characterized by high expression of the HER2 gene and other
genes associated with the HER2 pathway and/or HER2 amplicon located
on the 17q12 chromosome (8). The
HER2-positive non-luminal subtype is highly proliferative, with
negative steroid receptor status. The other group are basal-like
tumors (triple negative, HER2-negative, ER and PR absent) (9). The progress in genetic diagnostics has
led to the identification of novel molecular factors, including the
BRCA1/2, CHEK2, TP53 and PALB2 genes (10–13).
Additionally, the role of tumor-infiltrating lymphocytes (TILs) in
carcinogenesis and cancer progression has been confirmed (14,15). The
development of novel specific molecular targets within cancer cells
is currently an important goal of oncology and part of treatment
individualization. In recent years there has been a significant
increase in the influence of genetic factors on the diagnostic
process and the therapeutic decisions of patients with cancer,
particularly breast cancer.
The purpose of the present study was to evaluate the
correlation between molecular factors, including BRCA1,
CHEK2 and NOD2 gene mutations, and well known
clinicopathological factors in patients with breast cancer. As a
follow-up study, it sought to assess the usefulness of molecular
factors in the traditional classification systems of breast
cancer.
Patients and methods
Patients
The present study retrospectively analyzed a data
from a previous study conducted between the years 2007 and 2016 in
the MSC Memorial Cancer Centre and Institute of Oncology, Gliwice
Branch (COI; Poland), clinicopathological prognostic factors were
analyzed in patients with breast cancer with confirmed BRCA1
(n=73), CHEK2 (n=51) and NOD2 (n=31) mutations. The
control group was selected from breast cancer patients without
mutations (n=392). The patients in all groups (BRCA1, CHEK2
and NOD2 mutation carriers, and the control group) were
treated according to the same protocol. All patients provided
written informed consent allowing their biological material to be
used to clinical research.
All patients were women diagnosed, treated and
followed-up at the COI in Gliwice. Patient underwent clinical
follow-up examinations every 3 months in the first 2 years, every 6
months thereafter until the 5th year following diagnosis, and every
year subsequently. Inclusion criteria were as follows: Breast
cancer confirmed by microscopic examination; performance status
ZUBROD 0–1; age >18 years; and normal values of renal and liver
function, bone marrow. Data for age at onset, menopausal status,
surgical procedures, disease stage according to TNM classification
(T-the size of the tumor; N-spread of cancer to nearby lymph nodes;
and M-metastasis), histology, estrogen and progesterone receptor
status, HER2 status and contralateral breast cancer were gathered
from hospital records and pathology reports. The analysis of
patient medical records was performed according to national legal
regulation. The complete characteristics of patients with regard to
demographic and clinicopathological features are presented in
Tables I and II. Treatment strategies are illustrated in
Table III. Preliminary results of
this study for BRCA, CHEK2 and NOD2 mutations have
been presented in our previous publications (16–18).
 | Table I.Clinicopathological characteristics
of the patient according to mutation. |
Table I.
Clinicopathological characteristics
of the patient according to mutation.
| Clincopathological
criteria | BRCA1,
n=73 | P-value
(BRCA1 vs. Control group) | CHEK2,
n=52 | P-value
(CHEK2 vs. Control group) | NOD2,
n=31 | P-value
(NOD2 vs. Control group) | Control group,
n=392 |
|---|
| Age (median 52
years) | 43 (24–74) | 0.0001 | 50 (26–71) | 0.081 | 47 (27–68) | 0.005 | 53 (26–78) |
| Menopausal
status |
| 0.0005 |
| 0.656 |
| 0.853 |
|
|
Postmenopausal | 21 (29%) |
| 24 (47%) |
| 15 (48%) |
| 200 (51%) |
|
Premenopausal | 52 (71%) |
| 27 (53%) |
| 16 (52%) |
| 192 (49%) |
| Cardiovascular
diseases |
| 1.00 |
| 0.513 |
| 0.681 |
|
|
Yes | 4 (5%) |
| 4 (8%) |
| 2 (6%) |
| 21 (5%) |
| No | 69 (95%) |
| 47 (92%) |
| 29 (94%) |
| 371 (95%) |
| Infectious
diseases |
| 1.00 |
| 0.412 |
| 0.302 |
|
|
Yes | 2 (3%) |
| 3 (6%) |
| 2 (6%) |
| 13 (3%) |
| No | 71 (97%) |
| 48 (94%) |
| 29 (94%) |
| 379 (97%) |
| Diabetes |
| 0.661 |
| 0.605 |
| 0.499 |
|
|
Yes | 2 (3%) |
| 0 (0%) |
| 1 (3%) |
| 8 (2%) |
| No | 71 (97%) |
| 51 (100%) |
| 30 (97%) |
| 384 (98%) |
| Hypertension |
| 0.159 |
| 0.116 |
| 0.620 |
|
|
Yes | 7 (10%) |
| 13 (25%) |
| 6 (19%) |
| 64 (16%) |
| No | 66 (90%) |
| 38 (75%) |
| 25 (81%) |
| 328 (84%) |
| History of cancer
in family |
|
|
|
|
|
|
|
|
Cancer | 31 (42%) | 0.065 | 31 (61%) | 0.0001 | 18 (58%) | 0.005 | 123 (31%) |
| Breast
cancer | 18 (25%) | 0.0009 | 14 (27%) | 0.001 | 11 (35%) | 0.0003 | 39 (10%) |
|
Colorectal cancer | 1 (1%) | 0.336 | 7 (14%) | 0.017 | 3 (10%) | 0.193 | 18 (5%) |
| Gastric
cancer | 3 (4%) | 0.726 | 5 (10%) | 0.044 | 2 (6%) | 0.302 | 13 (3%) |
| Lung
cancer | 5 (7%) | 0.579 | 4 (8%) | 0.505 | 1 (3%) | 1.00 | 20 (5%) |
| Larynx
cancer | 1 (1%) | 1.00 | 3 (6%) | 0.096 | 0 (0%) | 1.00 | 7 (2%) |
|
CNS | 1 (1%) | 1.00 | 4 (8%) | 0.013 | 0 (0%) | 1.00 | 5 (1%) |
 | Table II.Pathological characteristics of the
patients. |
Table II.
Pathological characteristics of the
patients.
| Pathological
features | BRCA1, n=73
(%) | P-value
(BRCA1 vs. Control group) | CHEK2, n=51
(%) | P-value
CHEK2 vs. Control group) | NOD2, n=31
(%) | P-value NOD2
vs. Control group) | Control group,
n=392 (%) |
|---|
| HER2
overexpression |
| 0.0001 |
| 0.0001 |
| 0.0001 |
|
|
Positive | 5 (7) |
| 9 (18) |
| 3 (10) |
| 199 (51) |
|
Negative | 68 (93) |
| 42 (82) |
| 28 (90) |
| 193 (49) |
| Tumor grade |
| 0.0001 |
| 0.003 |
| 0.687 |
|
|
G1-G2 | 33 (45) |
| 45 (88) |
| 23 (74) |
| 269 (69) |
| G3 | 40 (55) |
| 6 (12) |
| 8 (26) |
| 123 (31) |
| Estrogen
status |
| 0.0001 |
| 0.001 |
| 0.253 |
|
|
Positive | 25 (34) |
| 42 (82) |
| 22 (71) |
| 258 (66) |
|
Negative | 48 (66) |
| 9 (18) |
| 9 (29) |
| 134 (34) |
| Progesterone
status |
| 0.0001 |
| 0.009 |
| 0.447 |
|
|
Positive | 21 (29) |
| 40 (78) |
| 21 (68) |
| 231 (59) |
|
Negative | 52 (71) |
| 11 (22) |
| 10 (32) |
| 161 (41) |
| Clinical staging
nodes |
| 0.004 |
| 0.071 |
| 0.038 |
|
|
Positive | 20 (27) |
| 16 (31) |
| 8 (26) |
| 179 (46) |
|
Negative | 53 (73) |
| 35 (69) |
| 23 (74) |
| 213 (54) |
| Tumor size |
| 0.002 |
| 0.186 |
| 0.327 |
|
|
T1-T2 | 48 (66) |
| 38 (75) |
| 28 (90) |
| 322 (82) |
|
T3-T4 | 25 (34) |
| 13 (25) |
| 3 (10) |
| 70 (18) |
| Clinical
staging |
| 0.484 |
| 0.253 |
| 0.142 |
|
| I | 12 (16) |
| 16 (31) |
| 12 (39) |
| 87 (22) |
| II | 46 (63) |
| 23 (45) |
| 14 (45) |
| 221 (56) |
|
III | 15 (21) |
| 12 (24) |
| 5 (16) |
| 84 (21) |
| Histological
type |
| 0.018 |
| 0.033 |
| 0.252 |
|
| Ductal
invasive carcinoma | 56 (77) |
| 32 (63) |
| 27 (87) |
| 301 (77) |
| Lobular
invasive carcinoma | 1 (1) |
| 11 (22) |
| 3 (10) |
| 38 (10) |
|
Other | 16 (22) |
| 8 (16) |
| 1 (3) |
| 53 (14) |
 | Table III.Treatment strategy according to the
presence of mutation. |
Table III.
Treatment strategy according to the
presence of mutation.
| Treatment
strategy | BRCA1, n=76
(%) | CHEK2, n=51
(%) | NOD2,
n=31 | Control group,
n=392 (%) |
|---|
| Chemotherapy
regimen |
|
|
|
|
|
ACFAC | 48 (66) | 22 (44) | 12 (40) | 328 (84) |
| AC +
taxanes | 18 (25) | 8 (16) | 5 (17) | 41 (10) |
|
CMF | 5 (7) | 2 (4) | 1 (3) | 3 (1) |
|
Without | 0 (0) | 18 (35) | 12 (39) | 20 (5) |
| Trastuzumab
therapy |
|
|
|
|
|
Yes | 1 (1) | 8 (16) | 2 (6) | 179 (46) |
| No | 72 (99) | 43 (84) | 29 (94) | 213 (54) |
| Hormonotherapy |
|
|
|
|
|
Yes | 28 (38) | 44 (86) | 22 (71) | 255 (65) |
| No | 45 (62) | 7 (14) | 9 (29) | 137 (35) |
| Local
treatment |
|
|
|
|
|
Mastectomy | 51 (70) | 35 (69) | 17 (55) | 264 (67) |
| Breast
conservation surgery (BCT) | 14 (19) | 15 (29) | 14 (45) | 106 (27) |
| Without
surgery | 8 (11) | 1 (2) | 0 (0) | 22 (6) |
| Radiotherapy |
|
|
|
|
|
Yes | 51 (70) | 27 (53) | 24 (77) | 282 (72) |
| No | 22 (30) | 24 (47) | 7 (23) | 110 (28) |
Methods
The status of CHEK2*1100delC and I157T
mutations (GenBank NM_007194.3) was assessed by ASA-PCR and
RFLP-PCR techniques, respectively. The present study examined the
most common mutations in BRCA1 (c.68_69delAG, c.181T>G,
c.4034delA, c.5266dupC and c.3700_3704del5; GenBank NM_007294.3)
and BRCA2 (c.5946delT and c.9403delC; GenBank NM_000059.3)
present in the Silesian population. The presence of
thec.3016_3017insC mutation of NOD2 (GenBank NM_022162.1)
was also evaluated in the study group (Table IV). Each patient provided informed
consent prior to venous blood collection for a genetic test.
Genomic DNA was isolated from peripheral blood leucocytes.
 | Table IV.Mutation sites of all analyzed
molecular factors. |
Table IV.
Mutation sites of all analyzed
molecular factors.
| Mutation sites | n | % |
|---|
| CHEK2 | 51 |
|
|
c.470T>C | 48 | 94 |
|
c.1100delC | 3 | 6 |
| NOD2 | 31 |
|
|
c.3016_3017insC | 31 | 100 |
| BRCA1 | 73 |
|
|
c.5266dupC | 41 | 56 |
|
c.181T>G | 25 | 34 |
|
c.68_69delAG | 2 | 3 |
|
c.3700_3704delGTAAA | 2 | 3 |
|
c.1692_1693delTG | 1 | 1 |
|
c.213-12A>G | 1 | 1 |
|
c.5346G>A | 1 | 1 |
Statistical analysis
Statistical analysis was performed using STATISTICA
13 software (StatSoft, Inc., Tulsa, OK, USA). The frequency of side
effects was counted. The qualitative features are presented as the
percentage of their occurrence and were evaluated with Fisher's
test and χ2 test with the Yates correction. P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinical factors and mutations
BRCA1 mutation carriers were significantly
younger in comparison with patients without detected mutations
(P=0.0001). The median age of BRCA1 mutation carriers was 43
years (range, 25–74 years) and for the control group it was 53
years (range, 26–78 years). Patients with BRCA-associated
breast cancer were also significantly more often in the
premenopausal age range compared with the control group (71 vs.
49%; P=0.0005). The median age at menarche was 14 years old, which
was similar in the two groups (P=0.559). However, the median number
of births was significantly lower in patients with BRCA1
mutations (P=0.0001). The median age of CHEK2 carriers was
50 years (range, 26–71). In the present analysis, CHEK2
carriers were younger, although not significantly, compared with
the control group (P=0.081). No significant differences were
identified between CHEK2 mutation carriers and the control
group according to postmenopausal status (47 vs. 51%; P=0.656). The
median age at breast cancer diagnosis for the carriers of the
NOD2 mutation was 47 years (range, 27–68). All mutation
carriers were younger compared with patients in the control group.
The youngest patients were BRCA1 mutation carriers (median
age, 43 years), followed by those with the NOD2 mutation
(median age, 47 years) and those with the CHEK2 mutation
(median age, 50 years) (Table I).
There were no differences in age between BRCA1 and
NOD2 mutation carriers (P=0.338) or between patients with
CHEK2 and NOD2 mutations (P=0.268). BRCA1
mutation carriers were younger in comparison with patients with
CHEK2 mutation (P=0.015). BRCA1 mutation carriers
were more frequently in the premenopausal period compared with
patients with the CHEK2 (P=0.037) or NOD2 mutation
(P=0.054).
A history of cancer in the family was reported in
123 (31%) in control group and 80 (52%) patients with mutations
(P=0.0001). A family history of cancer was observed more frequently
in CHEK2 mutation carriers (61 vs. 31%; P=0.0001) and
NOD2 mutation carriers (58 vs. 31%; P=0.005) compared with
control group patients. There was a detected tendency towards a
family history of cancer in patients with BRCA1 mutations
(42 vs. 31%; P=0.065). A family history of breast cancer was
reported in patients with mutations in BRCA1 (25 vs. 10%;
P=0.0009), CHEK2 (27 vs. 10%; P=0.001) or NOD2 (35
vs. 10%; P=0.0003) in comparison with the control group. Colorectal
cancer (14 vs. 5%; P=0.017) and gastric cancer (10 vs. 3%; P=0.044)
within the family history were observed more frequently in patients
with CHEK2 mutation compared with the control group.
Similarly, a significant family history of CNS tumors was
identified in patients with CHEK2 mutations (8 vs. 1%;
P=0.013) (Table I). There was
reported no association between other types of cancer in family
history and BRCA1, CHEK2 or NOD2 mutations.
Histopathological factors and
mutations
Clinicopathological analysis was conducted.
BRCA1-associated cancer had significantly more frequent
negative steroid receptor status compared with the control group
(62 vs. 31%; P=0.0001). HER2 overexpression was significantly more
frequently detected in women without mutations compared with
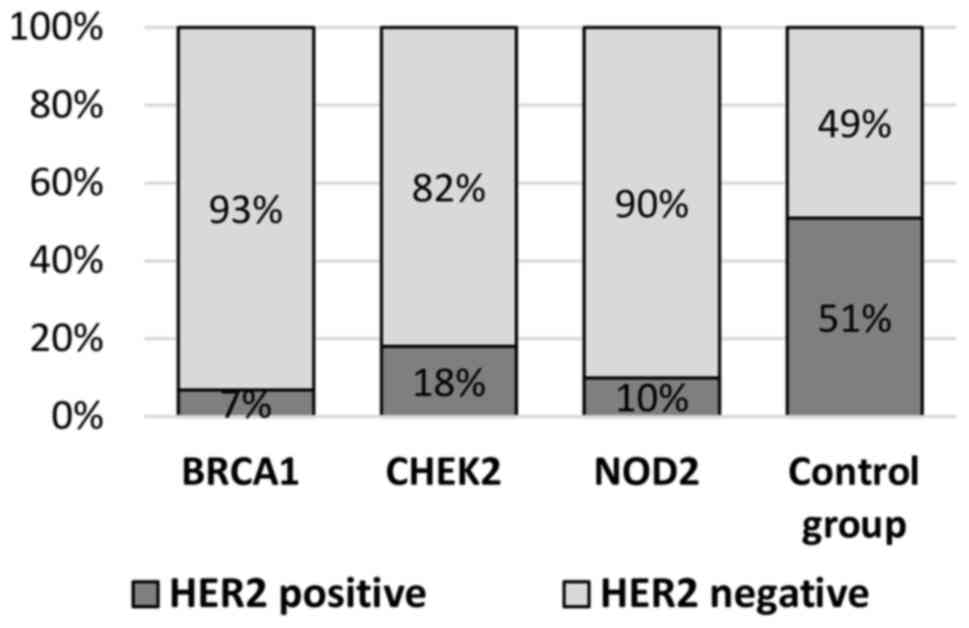
BRCA1 carriers (51 vs. 7%; P=0.0001; Table II, Fig.
1). Histological type G3 was detected more frequently in
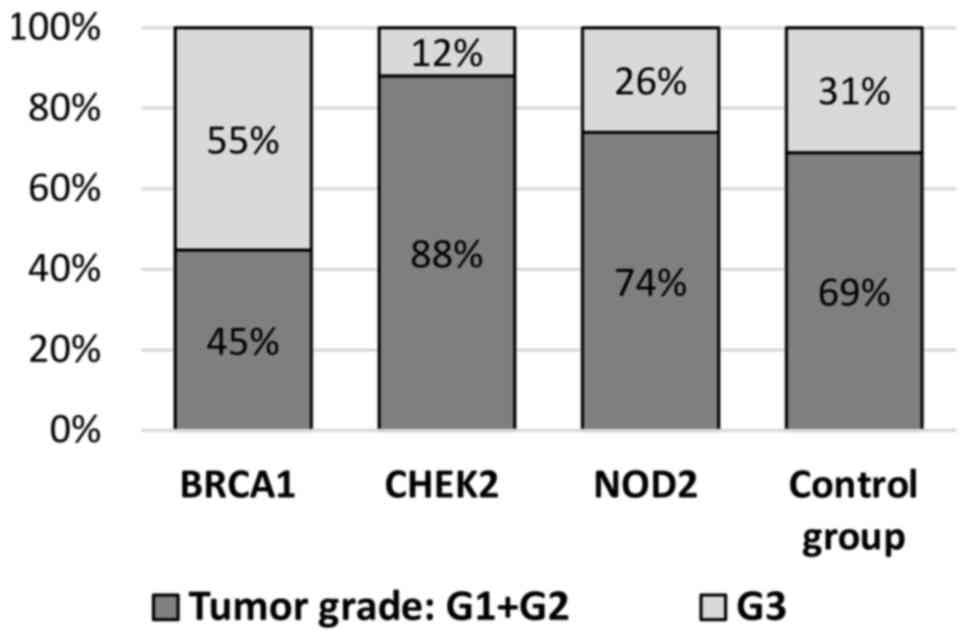
patients with BRCA1 mutations (55 vs. 31%; P=0.0001;
Fig. 2). There were observed
differences between two of the analyzed groups (CHEK2
carriers and the control group) with respect to ER-positive status
(82 vs. 66%; P=0.001), PR-positive status (78 vs. 59%; P=0.009) and
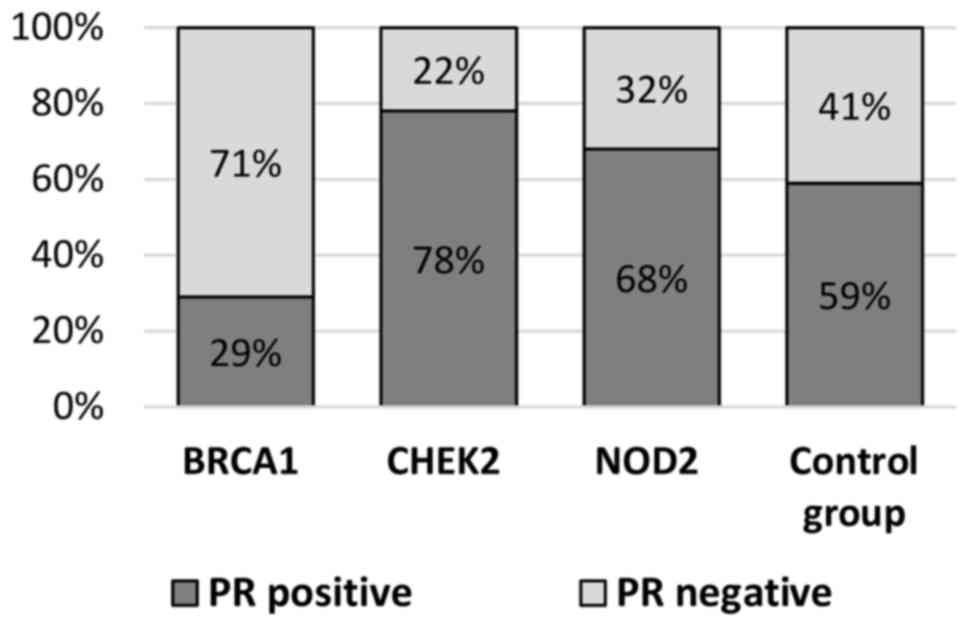
HER2 overexpression (18 vs. 51%; P=0.0001; Table II, Figs.
1, 3 and 4). The histological grade of breast cancer
differed between patients with CHEK2 mutations and the
control group (12% G3 vs. 31% G3; P=0.003; Table II, Fig.
2). The absence of HER2 overexpression was observed
significantly more frequently in NOD2 mutation carriers (90
vs. 49%; P=0.0001) compared with the control group (Table II, Fig.
1). By contrast, there were no differences between NOD2
mutation carriers and the control group with respect to ER (29 vs.
34%; P=0.253) and PR (32 vs. 41%; P=0.447) negative steroid
receptor status (Table II, Figs. 3 and 4). A lower histological grade, G1-G2, was
observed with similar frequency in NOD2 mutation carriers
and the control group (74 vs. 69%; P=0.687; Table II, Fig.
2). G3 tumors were detected in 26% of mutation carriers and 31%
of subjects in the control group.
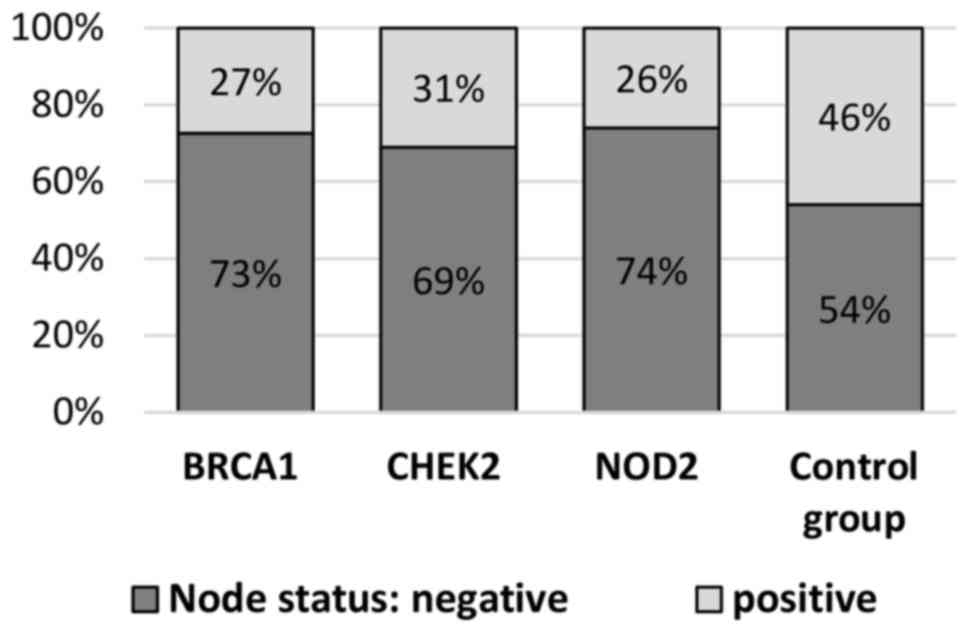
Lymph nodes metastases occurred more frequently in
the control group of patients compared with the group with
BRCA1 mutations (46 vs. 27%; P=0.004). There was an observed
tendency towards the presence of lymph node metastases in patients
of the control group compared with CHEK2 mutation carriers
(46 vs. 31%; P=0.071). Lymph nodes without metastases (N0) were
reported more frequently in patients with NOD2 mutations
compared with the control group (74% vs. 54%; P=0.038) (Table II, Fig.
5).
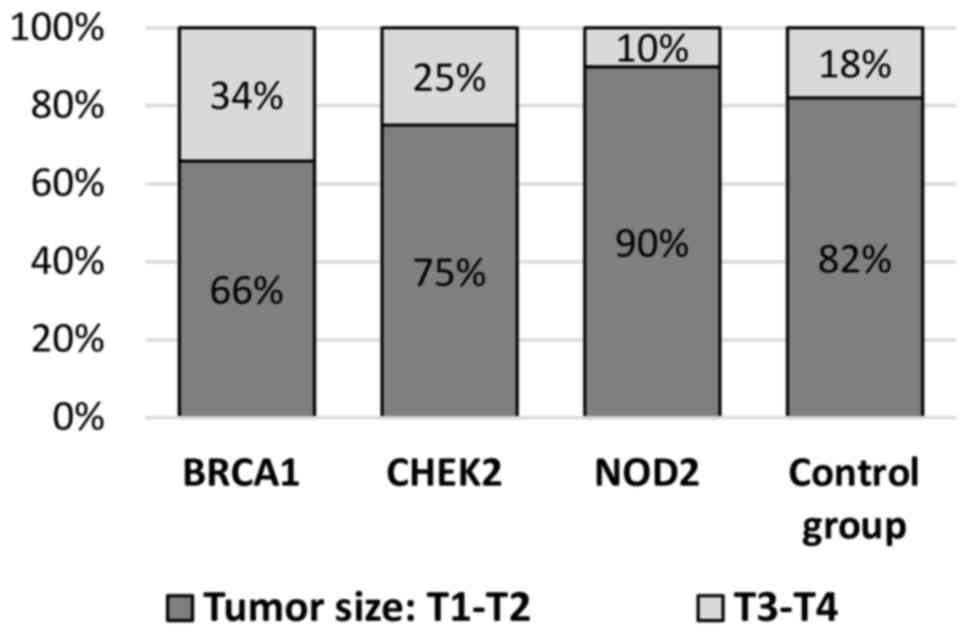
In the present study, patients with BRCA1
mutations had significantly more frequent large tumor sizes (T3-T4)
compared with the control group (34 vs. 18%; P=0.002). CHEK2
mutation carriers were slightly more likely to present with locally
advanced breast cancer (T3-T4) compared with the control group (25
vs. 18%; P=0.186) and patients with NOD2 mutations exhibited
no differences in T grade (Table
II, Fig. 6).
The majority of patients in studied groups had the
ductal invasive carcinoma subtype: 77% of BRCA1 mutation
carriers, 63% of CHEK2 mutation carriers and 87% of patients
with NOD2 mutations. The lobular type of breast cancer was
detected significantly more frequently in CHEK2 mutation
carriers compared with the control group (22 vs. 10%; P=0.033)
(Table II).
Compared with the control group, BRCA1
mutation carriers were younger, and more frequently had higher
tumor sizes (T3-T4), G3 tumors, negative steroid receptor status
(ER-) and tumors without HER2 overexpression. CHEK2 mutation
carriers more frequently had tumors without HER2 overexpression,
ER-positive receptor status and lower histological grade (G1-G2).
Patients with NOD2 mutation were younger and frequently had
tumors without HER2 overexpression, when compared with the control
group.
Molecular subtypes of breast cancer in
patients with mutations
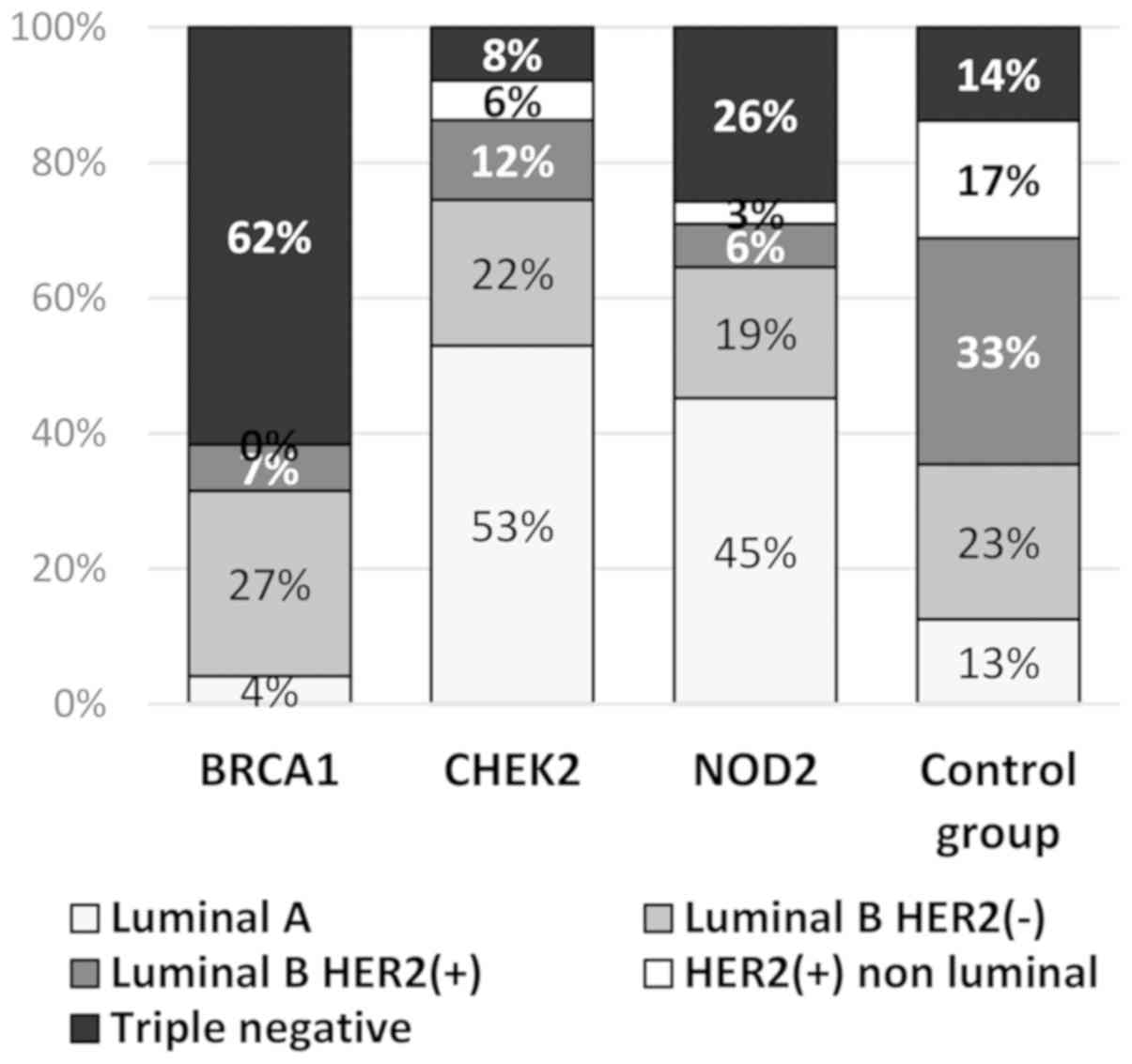
The distributions of the molecular types in breast
cancer patients with the BRCA1, CHEK2 and NOD2
mutations differed significantly from the distributions of the
subtypes in the control group (Table
V, Fig. 7). The
BRCA1-associated cancers were significantly more often
triple negative (TNBC) compared with the tumors in the sporadic
cancer cases (62 vs. 14%; P=0.0001). Luminal B subtypes,
particularly Luminal B HER2-positive subtypes, were reported more
frequently in the control group (56 and 33%, respectively) in
comparison with the mutation carriers: BRCA1 (34 and 7%,
respectively); CHEK2 (33 and 12%, respectively) and
NOD2 (26 and 6%, respectively). Luminal A type breast cancer
was diagnosed more frequently in CHEK2 mutation carriers
(53%) and NOD2 mutation carriers (45%) compared with the
control group (13%) (Table V).
 | Table V.Molecular subtype of breast cancer
according to St Gallen. |
Table V.
Molecular subtype of breast cancer
according to St Gallen.
| Molecular
subtype | BRCA1, n=76
(%) | P-value
BRCA1 vs. Control group | CHEK2, n=51
(%) | P-value,
CHEK2 vs. Control group | NOD2, n=31
(%) | P-value NOD2
vs. Control group | Control group,
n=392 (%) |
|---|
| Luminal A | 3 (4) |
| 27 (53) |
| 14 (45) |
| 49 (13) |
| Luminal B HER2
negative | 20 (27) |
| 11 (22) |
| 6 (19) |
| 90 (23) |
| Luminal B HER2
positive | 5 (7) | 0.0001 | 6 (12) | 0.0001 | 2 (6) | 0.0001 | 131 (33) |
| HER2 positive non
luminal | 0 (0) |
| 3 (6) |
| 1 (3) |
| 68 (17) |
| Triple
negative | 45 (62) |
| 4 (8) |
| 8 (26) |
| 54 (14) |
Discussion
In previous studies, patients with BRCA1
mutation were characterized by their younger age, negative steroid
receptor status (ER-), HER2 negative and triple negative tumors
(19). The basal type of cancer was
also significantly associated with BRCA1 expression
(20). The present results confirmed
those of previous studies. In the analyzed group, the median age of
patients with BRCA1 mutations was significantly lower
compared with that of patients in the control group (43 vs. 53
years; P<0.0001). These mutation carriers were also more
frequently in the premenopausal period (71 vs. 49%; P=0.0005), had
more locally advanced primary tumors (34 vs. 18%; P=0.002) and were
more often triple negative (62 vs. 14%; P=0.0001). The more
advanced disease stage and TNBC subtype in BRCA1 mutation
carriers may be associated with a more aggressive
clinicopathological tumor type. These observations are consistent
with those from previous studies (21–24).
A study conducted by de Bock et al (25) demonstrated that patients with a
CHEK2 mutation were significantly younger than patients
without this mutation (49.0 vs. 53.2 years; P=0.03). Patients with
a germline CHEK2*1100delC mutation more frequently had tumors with
positive steroid receptor status [ER (91 vs. 69%; P=0.03); PR (81
vs. 53%; P=0.04)] in comparison with non-carriers. By contrast, no
significant differences between these two groups were reported with
respect to tumor size, histological subtype, grade, or surgical
procedure, or in the choice of adjuvant systemic therapy or
radiotherapy (25). In patients with
early-onset breast cancer from Poland, ER-positive status was
observed more frequently in carriers of CHEK2 truncating
mutations compared with non-carriers (72 vs. 58%; P=0.01). Women
with a CHEK2 mutation had a fourfold increased risk of
ER-positive breast cancer in the Polish population (26). A correlation between
CHEK2*1100delC mutation status and tumor characteristics was
also reported in trial conducted by Kilpivaara et al
(27). In that study, no association
was observed between this mutation and hormone receptor status,
tumor histology or lymph node status. Another analyzed risk factor
was ionizing radiation treatment. In a study conducted by Broeks
et al (28), an association
was observed between BRCA1, BRCA2 and CHEK2 germline
mutation carriers and the risk of radiation-induced contralateral
breast cancer in comparison with non-carriers [odds ratio (OR),
2.51; 95% confidence interval, 1.03–6.10; P=0.049]. In the present
analysis, the presence of luminal A type breast cancer was reported
in CHEK2 mutation carriers more frequently than in the
control group (53% vs. 13%; P=0.0001). HER2 overexpression was
detected more frequently in the control group. In the present
study, there was observed tendency towards a family history of
cancer in patients of the control group compared with mutation
carriers (46 vs. 31%; P=0.071). The present study also observed a
tendency towards a family history of cancer, particularly gastric,
colorectal or CNS cancer, in the carrier groups. Breast cancer
within the family history was observed significantly more often in
CHEK2 mutation carriers in comparison with the control group
(27 vs. 10%; P=0.001). Lower histological grade was observed
significantly more often in patients with CHEK2 mutations
compared with the control group (88 vs. 69%; P=0.003). The carriers
also had locally advanced breast cancer (T3-T4) slightly more
frequently than the control group (25 vs. 18%; P=0.186). Domagala
et al (29) reported a
significant association between CHEK2 mutations and
molecular breast cancer subtype classification (P=0.004). Patients
with mutations in the CHEK2 gene primarily had luminal
subtypes of breast cancer (108/117=92.3%). The CHEK2-I157T variant
was associated with the luminal A subtype (P=0.01), whereas
CHEK2-truncating mutations were associated with the luminal
B subtype (P=0.005).
In certain studies, there was an observed
association between the NOD2 3020insC mutation and early
breast cancer (OR=1.9; P=0.01) (30). Similarly, ductal invasive carcinoma
breast cancer with an in-situ component was more frequently
reported in mutation carriers (OR=2.2; P=0.006) (30). In the present group, all patients had
early breast cancer. Ductal invasive carcinoma was also observed
more frequently in NOD2 mutation carriers (87 vs. 77%;
P=0.252) in comparison with the control group. Other
clinicopathological factors were also analyzed. Janiszewska et
al (31) did not report any
NOD2 mutations in patients diagnosed with breast cancer
after the age of 50 years. There was no reported association
between NOD2 mutations and a strong family history of breast
cancer. This mutation frequency (11.4%) was two times higher in
women from families with a single case of breast cancer. The
association of NOD2 mutations with other common types of
cancer, including digestive tract cancer, was described. The median
age at breast cancer diagnosis in the present group of patients was
47 years (range, 26–68) for the carriers of the NOD2
mutation and 53 years (range, 26–78) for the control group.
Differences were observed with respect to age between the two
groups. The other factors associated with NOD2 mutations in
the present study were: HER2 negative tumors (HER2-) and lymph
nodes without metastases (N-). The most common type of breast
cancer in this group was the Luminal type A and the TNBC
subtype.
In conclusion, the presence of mutations was
associated with a younger age of disease diagnosis, independent of
mutation type (BRCA1, CHEK2 and NOD2). BRCA1
mutation was associated with TNBC cancer and the Luminal B
HER2-negative breast cancer subtype, CHEK2 mutation with the
Luminal A and Luminal B HER2-negative subtypes, and NOD2
mutation with Luminal A breast cancer and the TNBC subtype.
CHEK2 and NOD2 mutation carriers had favorable
prognostic profiles, such as G1-G2, N (−) and HER2-negative
tumors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
JH is responsible for the study design, preparation
of the manuscript and final approval of the version to be
published. ZK conducted the statistical analysis, corrected the
manuscript, provided intellectual content and gave final approval
of the version to be published.
Ethics approval and consent to
participate
Not applicable. The present study was
retrospective.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yersal O and Barutca S: Biological
subtypes of breast cancer: Prognostic and therapeutic implications.
World J Clin Oncol. 10:412–424. 2014. View Article : Google Scholar
|
|
2
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th. Oxford;
UK: Wiley Blackwell: 2017
|
|
3
|
Senkus E, Kyriakides S, Ohno S,
Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S and Cardoso
F; ESMO Guidelines Committee, : Primary breast cancer: ESMO
clinical practice guidelines. Ann Oncol. 26 (Suppl 5):v8–v30. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nadji M, Gomez-Fernandez C, Ganjei-Azar P
and Morales AR: Immuno-histochemistry of estrogen and progesterone
receptors reconsidered: Experience with 5,993 breast cancers. Am J
Clin Pathol. 123:21–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ragab HM, Samy N, Afify M, Maksoud NA and
Shaaban HM: Assessment of Ki-67 as a potential biomarker in
patients with breast cancer. J Genet Engineering and Biotechnol.
16:479–484. 2018. View Article : Google Scholar
|
|
6
|
Gnant M, Thomssen C and Harbeck N: St.
Gallen/Vienna 2015: A Brief summary of the consensus discussion.
Breast Care (Basel). 10:124–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carey LA, Perou CM, Livasy CA, Dressler
LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S,
et al: Race, breast cancer subtypes, and survival in the Carolina
breast cancer study. JAMA. 295:2492–24502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Creighton CJ: The molecular profile of
luminal B breast cancer. Biologics. 6:289–297. 2012.PubMed/NCBI
|
|
9
|
Hubalek M, Czech T and Müller H:
Biological subtypes of triple-negative breast cancer. Breast Care
(Basel). 12:8–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Godet I and Gilkes DM: BRCA1 and BRCA2
mutations and treatment strategies for breast cancer. Integr Cancer
Sci Ther. 4:2017.PubMed/NCBI
|
|
11
|
Apostolou P and Papasotiriou I: Current
perspectives on CHEK2 mutations in breast cancer. Breast Cancer
(Dove Med Press). 9:331–335. 2017.PubMed/NCBI
|
|
12
|
Silwal-Pandit L, Vollan HK, Chin SF, Rueda
OM, McKinney S, Osako T, Quigley DA, Kristensen VN, Aparicio S,
Børresen-Dale AL, et al: TP53 mutation spectrum in breast cancer is
subtype specific and has distinct prognostic relevance. Clin Cancer
Res. 20:3570–3580. 2014. View Article : Google Scholar
|
|
13
|
Southey MC, Winship I and Nguyen-Dumont T:
PALB2: Research reaching to clinical outcomes for women with breast
cancer. Hered Cancer Clin Pract. 14:92016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huszno J, Nożyńska EZ, Lange D, Kołosza Z
and Nowara E: The association of tumor lymphocyte infiltration with
clinicopathological factors and survival in breast cancer. Pol J
Pathol. 68:26–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montagna E, Vingiani A, Maisonneuve P,
Cancello G, Contaldo F, Pruneri G and Colleoni M: Unfavorable
prognostic role of tumor-infiltrating lymphocytes in
hormone-receptor positive, HER2 negative metastatic breast cancer
treated with metronomic chemotherapy. Breast. 34:83–88. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huszno J, Kołosza Z and Grzybowska E:
BRCA1 mutation in breast cancer patients: Analysis of prognostic
factors and survival. Oncol Lett. 17:1986–1995. 2019.PubMed/NCBI
|
|
17
|
Huszno J, Budryk M, Kołosza Z, Tęcza K,
Pamuła Piłat J, Nowara E and Grzybowska E: A comparison between
CHEK2*1100delC/I157T mutation carrier and noncarrier breast cancer
patients: A clinicopathological analysis. Oncology. 90:193–198.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huszno J, Kołosza K, Tęcza T, Pamuła-Piłat
J, Mazur M and Grzybowska E: Comparison between NOD2 gene
mutation carriers (3020insC) and non-carriers in breast cancer
patients: A clinicopathological and survival analysis. AMS
Civilization Dis. 3:10e–15e. 2018. View Article : Google Scholar
|
|
19
|
Triantafyllidou O, Vlachos IS, Apostolou
P, Konstantopoulou I, Grivas A, Panopoulos C, Dimitrakakis C,
Kassanos D, Loghis C, Bramis I, et al: Epidemiological and
clinicopathological characteristics of BRCA-positive and
BRCA-negative breast cancer patients in Greece. J BUON.
20:978–984. 2015.PubMed/NCBI
|
|
20
|
Kutomi G, Ohmura T, Suzuki Y, Kameshima H,
Shima H, Takamaru T, Satomi F, Otokozawa S, Mori M and Hirata K:
Clinicopathological characteristics of basal type breast cancer in
triple-negative breast cancer. J Cancer Therapy. 3:836–840. 2012.
View Article : Google Scholar
|
|
21
|
Evans DG, Lalloo F, Howell S, Verhoef S,
Woodward ER and Howell A: Low prevalence of HER2 positivity amongst
BRCA1 and BRCA2 mutation carriers and in primary BRCA screens.
Breast Cancer Res Treat. 155:597–601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peshkin BN, Alabek ML and Isaacs C:
BRCA1/2 mutations and triple negative breast cancers. Breast Dis.
32:25–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huszno J, Budryk M, Kołosza Z and Nowara
E: The influence of BRCA1/BRCA2 mutations on toxicity related to
chemotherapy and radiotherapy in early breast cancer patients.
Oncology. 85:278–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kirova YM, Savignoni A, Sigal-Zafrani B,
de La Rochefordiere A, Salmon RJ, This P, Asselain B,
Stoppa-Lyonnet D and Fourquet A: Is the breast conserving treatment
with radiotherapy appropriate in BRCA1/2 mutation carries?
Long-term results and review of the literature. Breast Cancer Res
Treat. 120:119–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Bock GH, Mourits MJ, Schutte M,
Krol-Warmerdam EM, Seynaeve C, Blom J, Brekelmans CT,
Meijers-Heijboer H, van Asperen CJ, Cornelisse CJ, et al:
Association between the CHEK2*1100delC germ line mutation and
estrogen receptor status. Int J Gynecol Cancer. 16:552–555. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cybulski C, Huzarski T, Byrski T, Gronwald
J, Debniak T, Jakubowska A, Gorski B, Wokolorczyk D, Masojc B,
Narod SA and Lubiński J: Estrogen receptor status in CHEK2-positive
breast cancers: Implications for chemoprevention. Clin Genet.
75:72–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kilpivaara O, Bartkova J, Eerola H,
Syrjäkoski K, Vahteristo P, Lukas J, Blomqvist C, Holli K, Heikkilä
P, Sauter G, et al: Correlation of CHEK2 protein expression and
c.1100delC mutation status with tumor characteristics among
unselected breast cancer patients. Int J Cancer. 113:575–580. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Broeks A, Braaf LM, Huseinovic A, Nooijen
A, Urbanus J, Hogervorst FB, Schmidt MK, Klijn JG, Russell NS, Van
Leeuwen FE and Van't Veer LJ: Identification of women with an
increased risk of developing radiation-induced breast cancer: A
case only study. Breast Cancer Res. 9:R262007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Domagala D, Wokolorczyk D, Cybulski C,
Huzarski T, Lubinski J and Domagala W: Different CHEK2 germline
mutation are associated with distinct immunophenotyping molecular
subtypes of breast cancer. Breast Cancer Res Treat. 132:937–945.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huzarski T, Lener M, Domagala W, Gronwald
J, Byrski T, Kurzawski G, Suchy J, Chosia M, Woyton J, Ucinski M,
et al: The 3020insC allele of NOD2 predisposes to early-onset
breast cancer. Breast Cancer Res Treat. 89:91–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Janiszewska H, Haus O, Lauda-Swieciak A,
Bak A, Mierzwa T, Sir J and Laskowski R: The NOD2 3020insC mutation
in women with breast cancer from the Bydgoszcz region in Poland.
First results. Hered Cancer Clin Pract. 4:15–19. 2006. View Article : Google Scholar : PubMed/NCBI
|