Introduction
Pancreatic cancer (PC) is one of the most lethal
malignant neoplasms and a major cause of cancer-related death in
developed countries (1–3). Surgical resection has contributed to
favorable prognosis, and progress in surgical techniques and
perioperative care have reduced the rates of mortality and severe
complications (4–6). However, the long-term survival rate has
plateaued over the last three decades (7). Most patients present with advanced
stage at initial diagnosis (8), and
effective drugs are still under development because of the
complexity of PC at the genomic, epigenetic, and metabolic levels
(9–11). Particularly, patients with advanced
stage pancreatic head cancer (PHC) can often only undergo
non-curative operation, as the tumor cells tend to invade adjacent
main vessels. However, chemotherapy and/or radiation therapy prior
to operation, known as neoadjuvant chemoradiotherapy (NAC-RT), has
been recently introduced as a treatment strategy, and has achieved
several successful outcomes (7,9,12–14).
Borderline resectable PHC (BR-PHC) is defined as a
tumor of low resectability because it is accompanied by vascular
invasion, especially portal venous and arterial involvement
(15–17). The possibility of complete resection
of BR-PHC depends on the efficacy of preoperative NAC-RT, i.e.,
stable disease or complete or partial response (9). Jang et al (14). reported that the 2-year prognosis of
patients who received neoadjuvant treatment was significantly
better than the prognosis of those receiving upfront surgery. There
are several reasons for this, including early systemic treatment
for undetected micrometastases, no residual tumor (R0) rate
increment, and optimal selection of patients for surgery (14). Above all, R0 resection is closely
linked to the regression of local vascular invasion, more
specifically, invasion to the portal and superior mesenteric
veins.
Japanese pathologists routinely make meticulous
diagnoses using the Japanese Classification of Pancreatic Cancer
(18), which requires individual
evaluation of invasion of the bile duct, duodenum, anterior or
posterior pancreatic tissue, portal venous system, arterial system,
extrapancreatic nerve plexus and surgical margin. In contrast,
these parameters are combined in the World Health Organization
(WHO) classification, thus the diagnosis of T3 tumors remains only
a rough estimate. Since PHC deserves careful attention, our
approach of separate evaluation seems to be indispensable to
specify important factors for the prognosis of PC.
This study compared the clinicopathological features
of BR-PHC with NAC-RT and resectable PHC and tried to clarify the
critical factors influencing the prognosis of PHC.
Materials and methods
Patient selection
This study was a retrospective cohort study using
clinicopathological data from the Kurume University Hospital
between 2009 and 2016. It was approved by the ethical committee of
Kurume University (approved # 17226). Twenty-nine patients with
BR-PHC who received NAC-RT were reevaluated. Patients with
unresectable PC (UR-PC) were excluded from the study. All patients
received a combination of chemotherapy with gemcitabine (600
mg/m2/week) S-1 (50 mg/m2/day) and
radiotherapy (50.4 Gy). Approximately one month after conclusion of
this neoadjuvant therapy, pancreaticoduodenectomy (PD) was
performed under the conditions of no disease progression,
metastasis, or contraindications to major abdominal surgery.
Pre-treatment cytology under EUS-FNA or ERCP (19) and imaging findings were reviewed in
all PHC patients with NAC-RT. Clinical follow-up data were
available for overall survival (OS). A control group consisted of
resectable 55 PHC patients who underwent PD during the same
period.
Resected specimens (pancreas and surrounding tissue)
were fixed with 10% buffered formalin; they were then totally
sectioned (18 to 42 slides) and embedded in paraffin for
microscopic examination. All slides were consecutively cut to 4-mm
thickness, stained with hematoxylin and eosin, and evaluated by two
pathologists (Y.N., M.T, M.N.). Histological diagnosis was
performed based on the 2010 WHO classification, and, according to
the Japanese Classification of Pancreatic Cancer (18); invasion to the bile duct, duodenum,
serosal side of the anterior pancreatic tissue, retropancreatic
tissue, portal venous system, arterial system, extrapancreatic
nerve plexus and surgical margin were assessed separately. The
extent of residual carcinoma in specimens of BR-PHC after NAC-RT
was also evaluated; histological response was classified based on
the residual rate of viable cancer cells in post-treatment surgical
specimens (Table I) (18). In this study, arterial system
invasion was not observed, but cases with portal venous system
invasion were defined as local invasion. After HE staining, regions
in which viable tumor cells remained were selected and measured as
the tumor diameter. For R assessment, cases without exposed tumor
cells on the surgically dissected surface were categorized as R0,
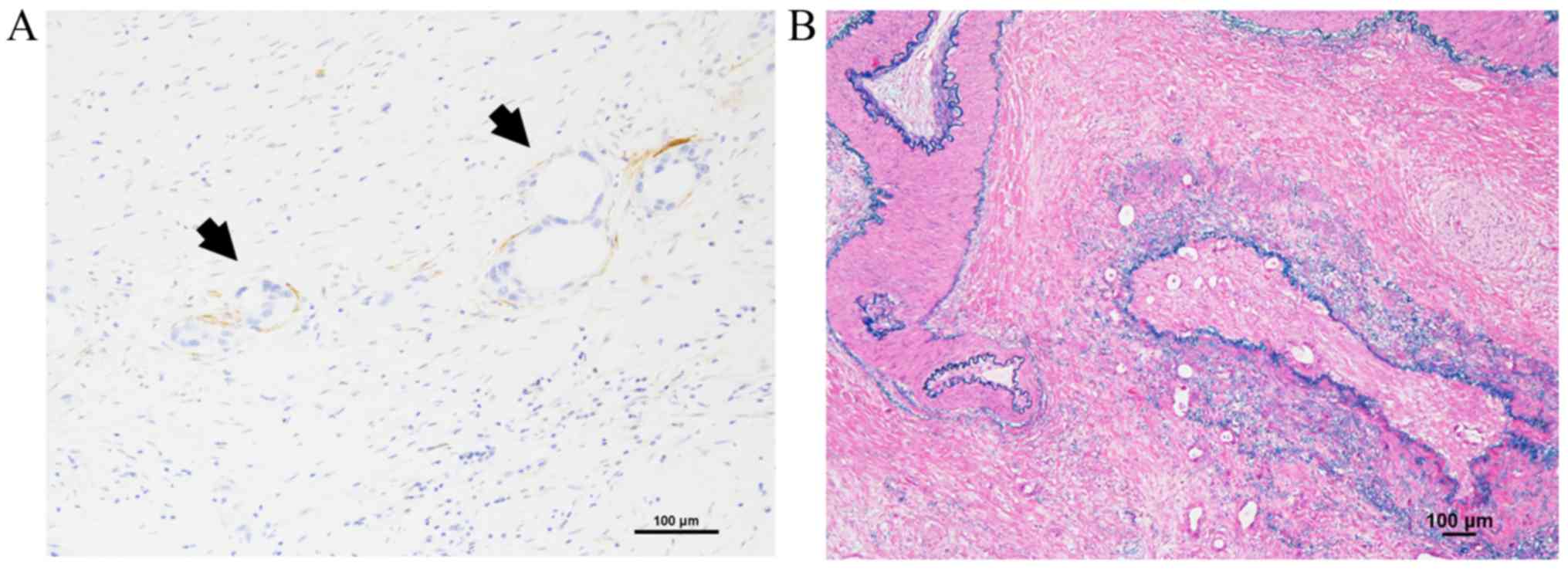
and cases with exposed tumor cells were categorized as R1. D2-40
immunohistochemical staining (clone D2-40, Nichirei, Japan) for
lymphovascular invasion (LVI) and Elastica van Gieson or Victoria
blue H&E staining for microvessel invasion (MVI) were prepared
for accurate evaluation. Tumor cell invasion into lymph ducts
comprised of D2-40 positive endothelial cells was categorized as
LVI. Tumor cell invasion findings in veins with elastic fiber
measuring more than half the diameter on EVG or Victoria blue
H&E staining were categorized as MVI.
 | Table I.Histological assessment of
preoperative therapeutic effects. |
Table I.
Histological assessment of
preoperative therapeutic effects.
| A, Japanese
classification of pancreatic cancer |
|---|
| Grade 1 | Poor or no
response |
|
|
| Grade 1a | Estimated residual
rate ≥90% |
|
| Grade 1b |
|
| Grade 2 | Moderate
response | 10%≤ estimated
residual rate <50% |
| Grade 3 | Marked
response |
|
|
| Estimated residual
rate <10% |
|
| Grade 4 | Complete
response |
|
|
| B, Evans grading
system |
|
| Grade I | <10% or no tumor
cell destruction is evident |
|
| Grade II | IIa | Destruction of
10–50% of tumor cells |
|
| IIb | Destruction of
51–90% of tumor cells |
| Grade III | <10%
viable-appearing tumor cells are present |
|
| Grade IV | No viable tumor
cells are present |
|
|
| C, CAP grading
system |
|
| Grade 0 | Complete
response |
|
| Grade 1 | Marked
response |
|
| Grade 2 | Moderate
response |
|
| Grade 3 | Poor or no
response |
|
Statistical analysis
Continuous variables were expressed as medians and
ranges and categorical variables as numbers and percentages.
Clinicopathological variables were compared using Wilcoxon rank
sum, Chi-square, or Fisher exact tests. The survival function for
OS was estimated using the Kaplan-Meier method. The log-rank test
was used to compare differences in survival rates according to
clinicopathological variables. All statistical analyses were
performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and
R version 3.4.4. All statistical tests were two-tailed, and
P-values <0.05 was considered statistically
significant.
Results
Histological assessment of the
preoperative therapeutic effect of NAC-RT on BR-PHC
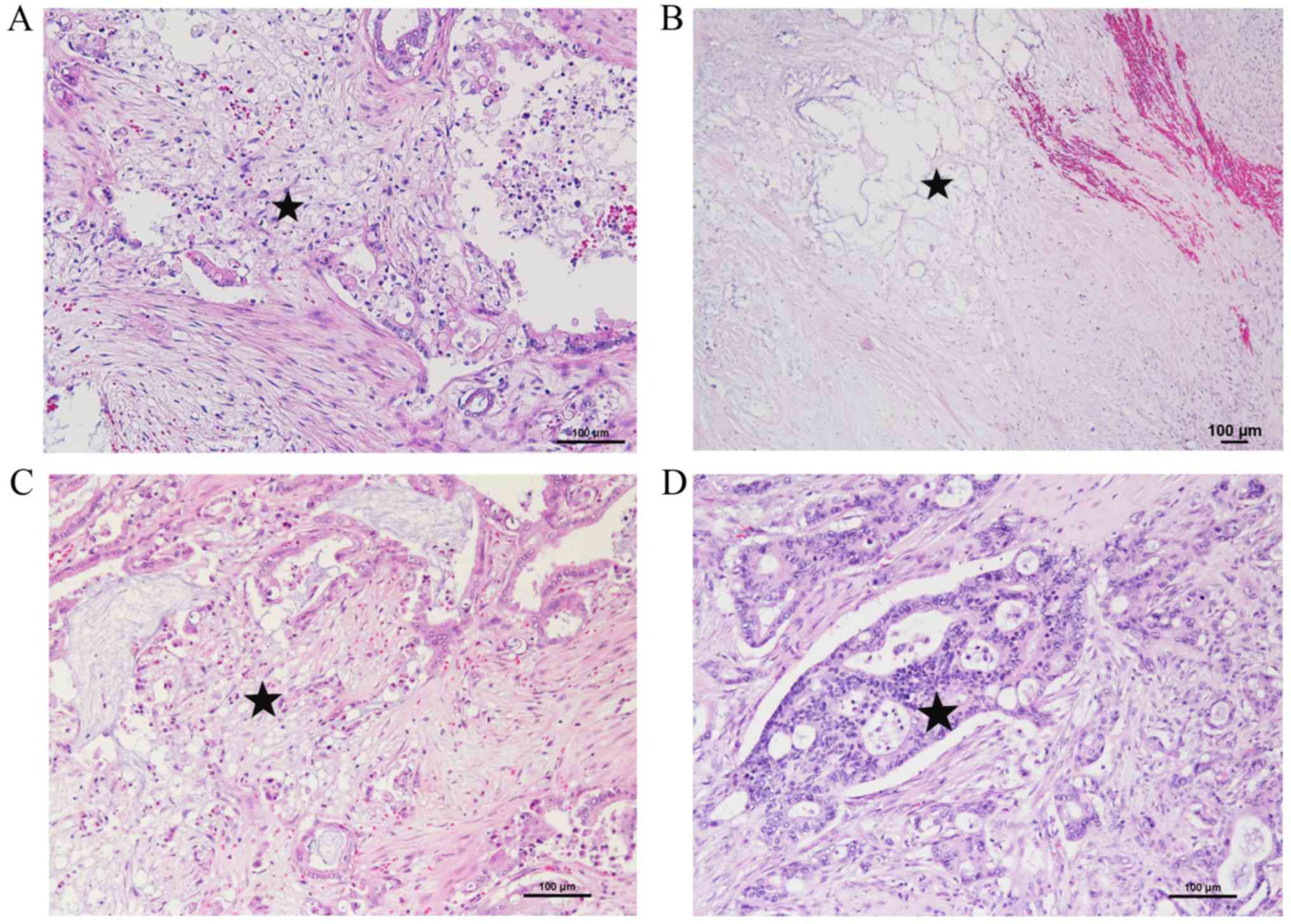
Representative morphopathological features of BR-PHC
after NAC-RT are shown in Fig. 1.
The post-therapeutic response of tumor cells was represented by
clear cytoplasm, pyknosis, loss of nuclei, and indistinct cell
borders. In some cases, mucin pools or xanthogranuloma-like
features with coarse fibrosis were observed as the host tissue
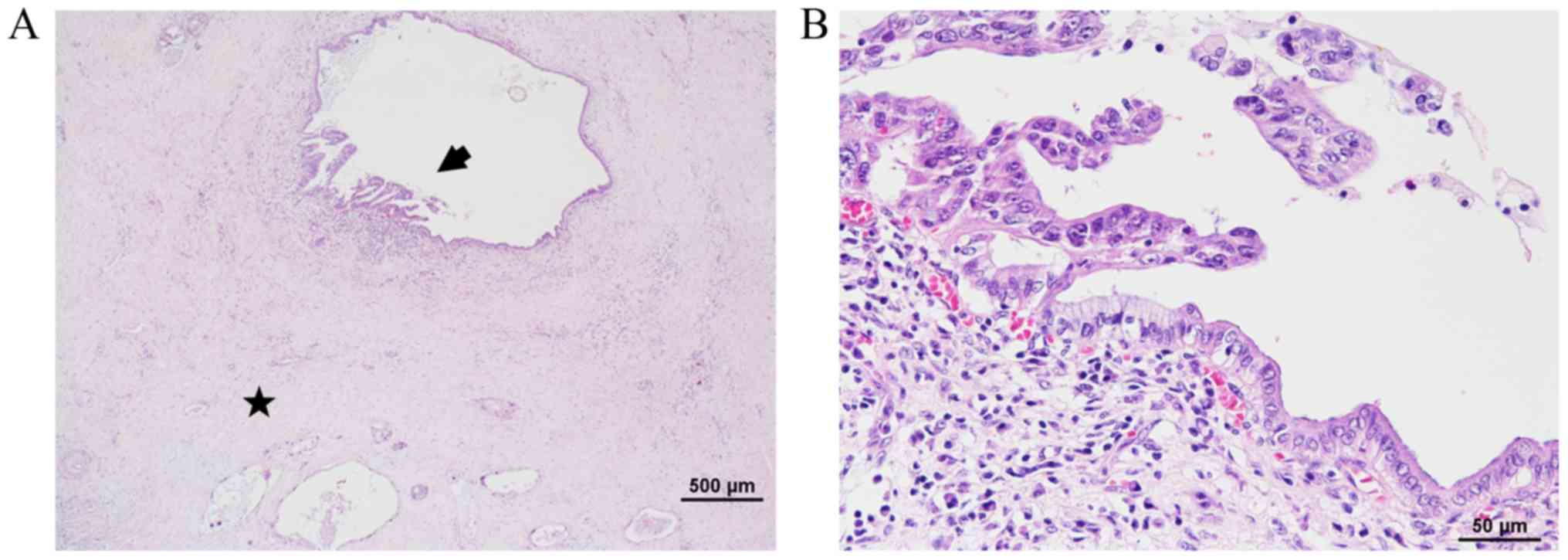
response. Interestingly, intraductal components corresponding to
high grade pancreatic intraepithelial neoplasia (PanIN) remained in
the pancreatic ducts (Fig. 2).
Clinicopathological data of the BR-PHC with NAC-RT and control
groups are shown in Table II. The
NAC-RT group consisted of 16 men and 13 females and the median age
was 66.0 (range 50–78) years. The median tumor size was 20 (range
0–43) mm. Upon comparison of the post therapy tumor stages
(ypT0/T1/T2 vs. ypT3), ypT3 was predominant (9 and 23,
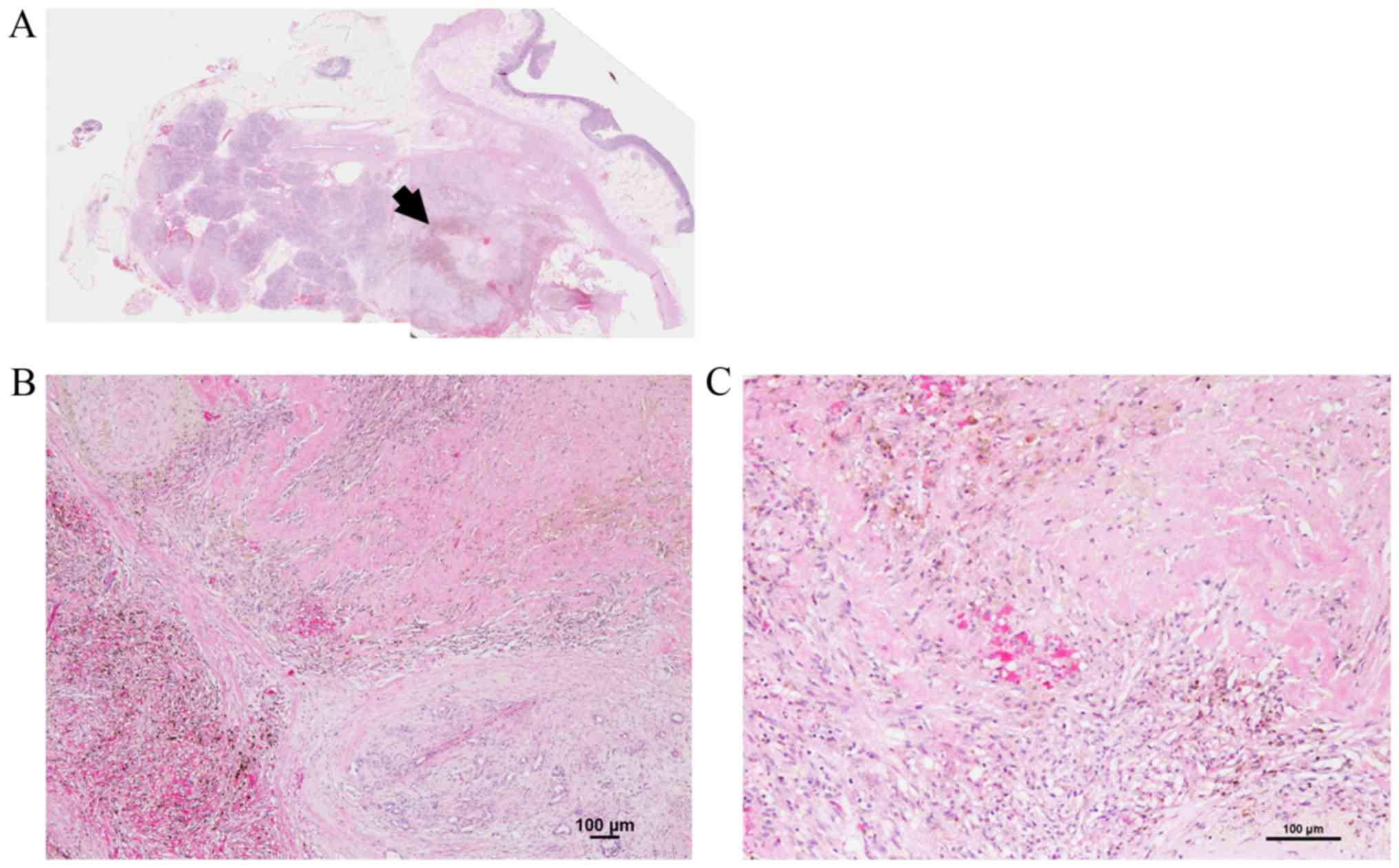
respectively). For the one patient with pCR (ypT0) (Fig. 3), (20) the pretreatment computerized
tomography showed a mass lesion in the pancreas head, and the
pretreatment cytology diagnosis was adenocarcinoma. Six patients
(21.0%) with lymph node metastasis were classified with ypStage IIB
disease. According to the WHO classification standards, 27 of the
29 (93.1%) cases with viable tumors were well to moderately
differentiated and 1 (3.4%) was poorly differentiated. In one case,
there was no viable cancer cells in any of the specimens (pCR)
(20). R0 resection was achieved in
25 (86%) patients. Pathological evaluation of local invasion among
the BR-PHC with NAC-RT and the control groups is shown in Table III. Based on the Japanese
Classification of Pancreatic Cancer protocol, the histological
response to NAC-RT was classified as Grade 1a, Grade 1b, Grade 2,
Grade 3, or Grade 4. Grade 1b (55.0%) was the most common, and only
one case (4.0%) was Grade 4 (this case achieved pCR) (Table IV). The Grade 4 case had no
recurrence for 4 years.
 | Table II.Clinicopathological data of BR-PHC
with NAC-RT and control groups. |
Table II.
Clinicopathological data of BR-PHC
with NAC-RT and control groups.
|
Characteristics | Categories | BR-PHC with
NAC-RT | (%) | Control PHC | (%) | P-value |
|---|
| No. of
patients |
| 29 |
| 55 |
|
|
| Age, y median
(min-max) |
| 66 (50–78) |
| 68 (37–81) |
| 0.281 |
| Sex | Male | 16 | (55.2) | 36 | (65.5) | 0.356 |
|
| Female | 13 | (44.8) | 19 | (34.5) |
|
| Operation | PD | 19 | (65.5) | 46 | (83.6) | 0.098 |
|
| PD with PV | 10 | (34.5) | 9 | (16.4) |
|
| Tumor size (mm)
median (min-max) |
| 20 (0–43) |
| 28 (0.8-85) |
| 0.006 |
| Tumor stage
(T) | T0/1/pT2 | 9 | (31.0) | 8 | (14.5) | 0.074 |
|
| T3 | 20 | (69.0) | 47 | (85.5) |
|
| Regional LN | N0 | 23 | (79.3) | 20 | (36.4) | <0.001 |
|
| N1 | 6 | (20.7) | 35 | (63.6) |
|
| Stage | 0/IA/IB | 8 | (27.6) | 5 | (9.1) | <0.001 |
|
| IIA | 15 | (51.7) | 15 | (27.3) |
|
|
| IIB | 6 | (20.7) | 35 | (63.6) |
|
|
Histologya | G1/2 | 27 | (93.1) | 46 | (83.6) | 0.153 |
|
| G3/4 | 1 | (3.4) | 9 | (16.4) |
|
| LVI | Present | 15 | (51.7) | 46 | (83.6) | 0.002 |
|
| Absent | 14 | (48.3) | 9 | (16.4) |
|
| MVI | Present | 18 | (62.1) | 47 | (85.5) | 0.015 |
|
| Absent | 11 | (37.9) | 8 | (14.5) |
|
| NI | Present | 22 | (75.9) | 50 | (90.9) | 0.098 |
|
| Absent | 7 | (24.1) | 5 | (9.1) |
|
| Surgical margin
(R) | Present | 4 | (13.8) | 6 | (10.9) | 0.731 |
|
| Absent | 25 | (86.2) | 49 | (89.1) |
|
 | Table III.Pathological evaluation of local
invasion among BR-PHC with NAC-RT and control groups. |
Table III.
Pathological evaluation of local
invasion among BR-PHC with NAC-RT and control groups.
|
| BR-PHC with NAC-RT
(n=29) | % | Control PHC
(n=55) | % | P-value |
|---|
| CH |
|
Present | 4 | (13.8) | 32 | (58.2) | <0.001 |
|
Absent | 25 | (86.2) | 23 | (41.8) |
|
| DU |
|
Present | 13 | (44.8) | 30 | (54.5) | 0.397 |
|
Absent | 16 | (55.2) | 25 | (45.5) |
|
| S |
|
Present | 5 | (17.2) | 15 | (27.3) | 0.305 |
|
Absent | 24 | (82.8) | 40 | (72.7) |
|
| RP |
|
Present | 10 | (34.5) | 31 | (56.4) | 0.057 |
|
Absent | 19 | (65.5) | 24 | (43.6) |
|
| PV |
|
Present | 5 | (17.2) | 4 | (7.3) | 0.264 |
|
Absent | 24 | (82.8) | 51 | (92.7) |
|
| A |
|
Present | 0 | (0.0) | 0 | (0.0) | – |
|
Absent | 29 | (100.0) | 55 | (100.0) |
|
| PL |
|
Present | 1 | (3.4) | 5 | (9.1) | 0.659 |
|
Absent | 28 | (96.6) | 50 | (90.9) |
|
| OO |
|
Present | 0 | (0.0) | 0 | (0.0) | – |
|
Absent | 29 | (100.0) | 55 | (100.0) |
|
 | Table IV.Grading of histological response to
neoadjuvant chemoradiotherapy in BR-PHC. |
Table IV.
Grading of histological response to
neoadjuvant chemoradiotherapy in BR-PHC.
| CAP system | Evans
classification | JPS
classification | Patients (%) |
|---|
| Grade 3 | Grade I | Grade 1a | 5
(17.0) |
|
| Grade IIa | Grade 1b | 16 (55.0) |
| Grade 2 | Grade IIb | Grade 2 | 7
(24.0) |
| Grade 1 | Grade III | Grade 3 | 0 (0.0) |
| Grade 0 | Grade IV | Grade 4 |
(4.0) |
Correlation of survival with
histologic parameters of local invasion of the residual tumor
Comparison of PHC between the NAC-RT and control
groups is shown in Table II. The
median tumor size was significantly smaller in the NAC-RT group
(P=0.006). Lymph node metastases were significantly
less common in the NAC-RT group than in the control group
(P<0.001). Moreover, the NAC-RT group had
significantly lower rates of LVI (51.7%) and MVI (62.1%) than the
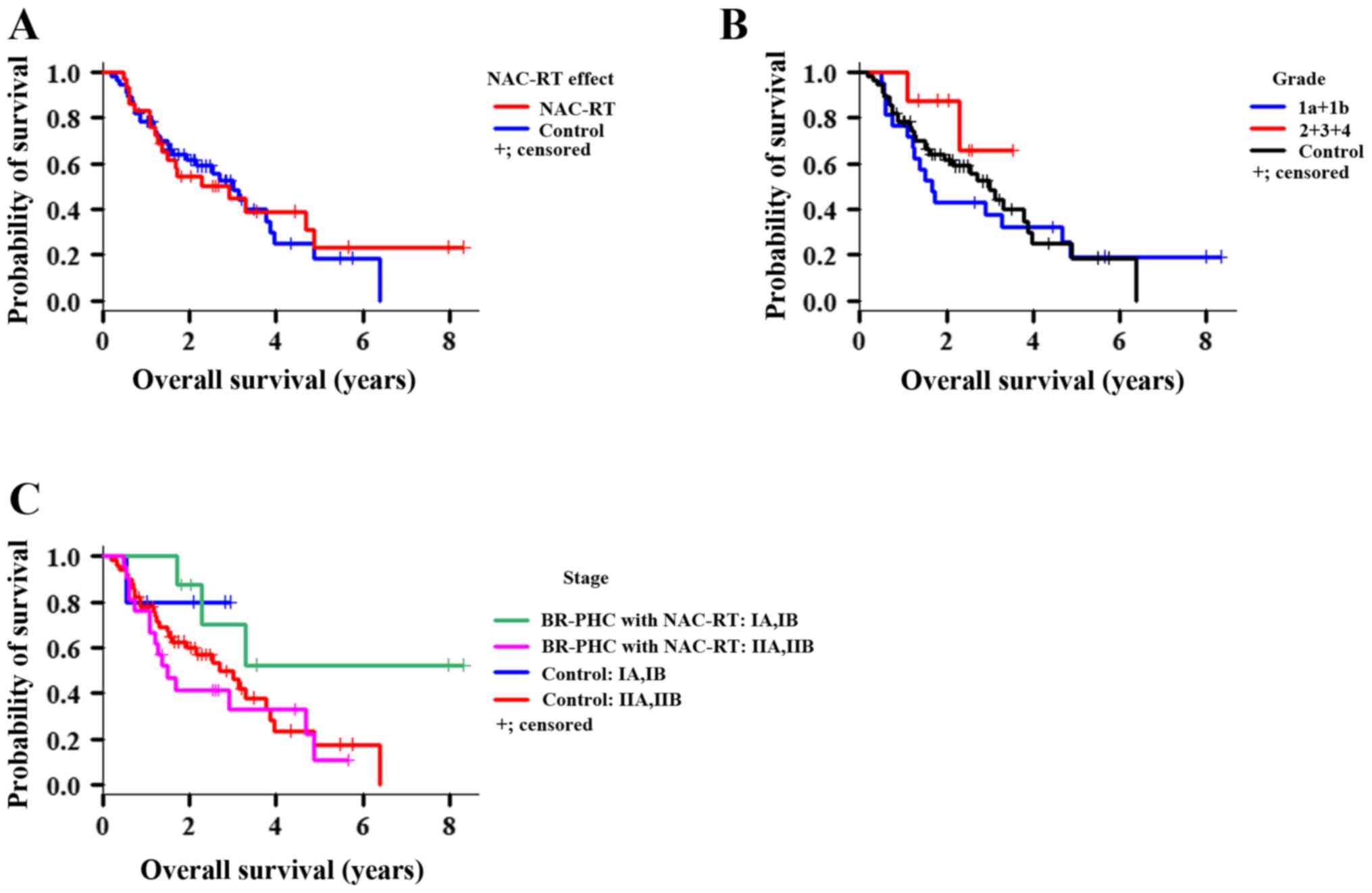
control group (MVI: P=0.015, LVI: P=0.02; Fig. 4). OS was similar between the BR-PHC
with NAC-RT and control groups (P=0.831; Fig. 5A). Additionally, there were no
significant differences in OS between the two groups based on grade
(Fig. 5B, P=0.470) or
stage (Fig. 5C,
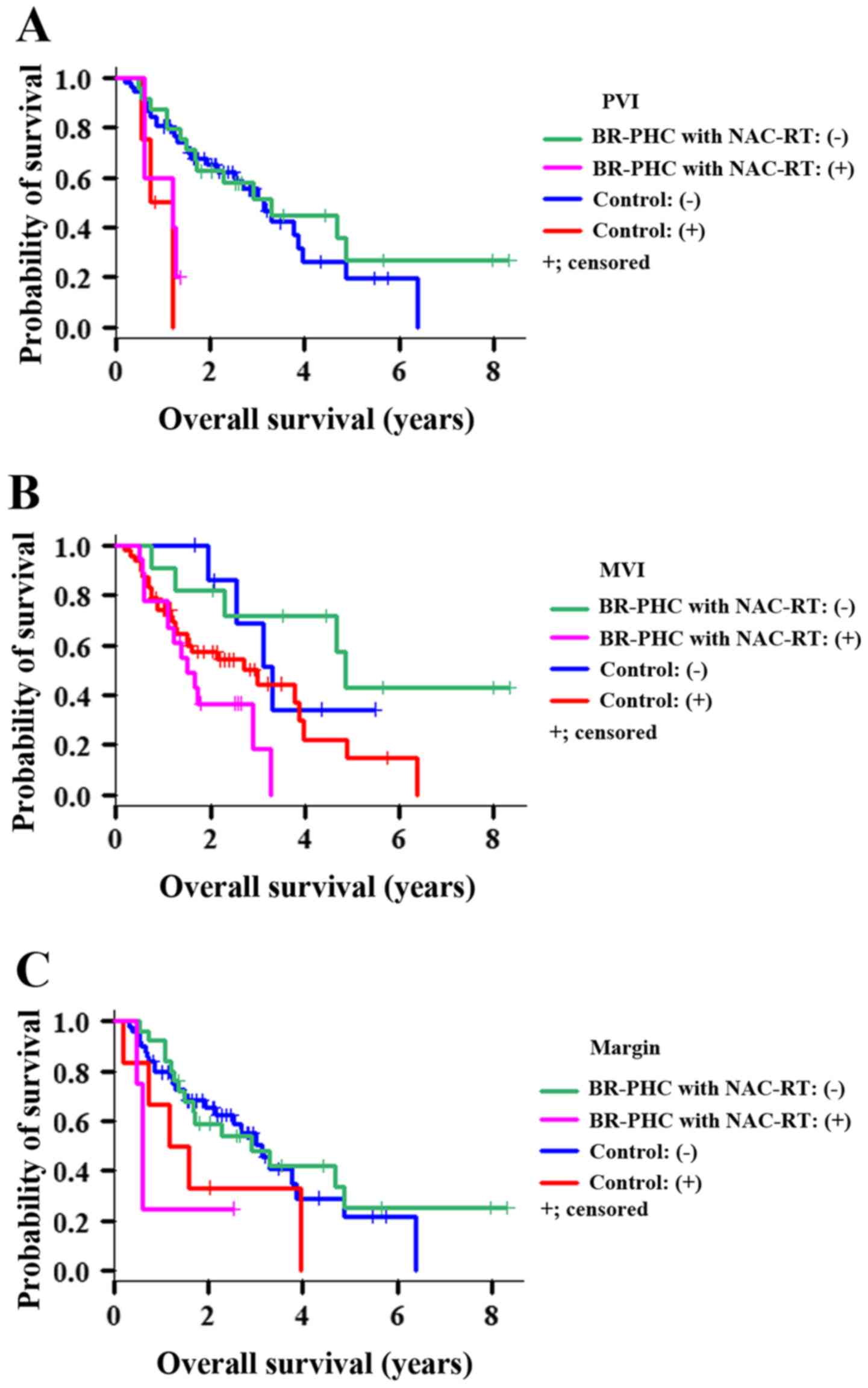
P=0.167). However, patients in the NAC-RT group with
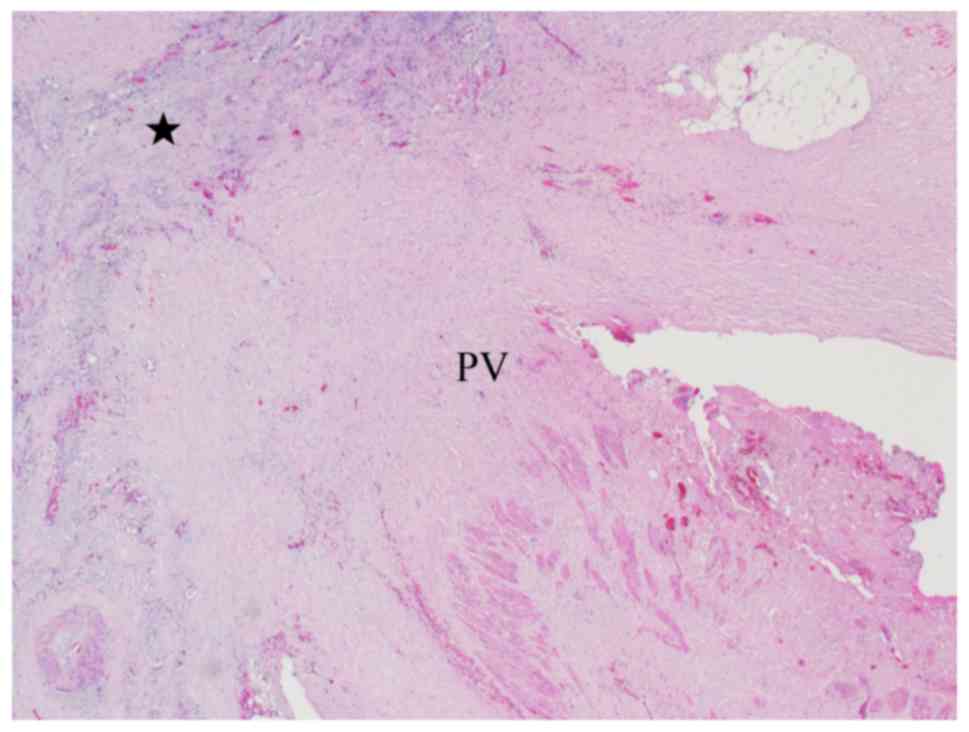
portal vein invasion (PVI) (Fig. 6),
MVI, and surgical margin factors had significantly shorter OS than
the corresponding patients in the control group (P=0.002,
P=0.011, P=0.043, respectively; Fig. 7). Conversely, the BR-PHC with NAC-RT
patients without PVI had a significantly better prognosis than
patients in the control group with PVI (P=0.002).
Discussion
We expected that BR-PHC patients who underwent
NAC-RT would have shorter OS than resectable PHC patients, but we
found no differences in survival between the two groups. There are
several potential reasons for this, including early systemic
treatment for undetected micrometastases via LVI and MVI, increases
in the R0 resection rate as a result of downsizing the primary
tumor and inhibiting local invasion, and optimal selection of
patients for surgery. In particular, patients in the NAC-RT group
without PVI had significantly better prognosis than patients in the
control group with PVI in this study. In this comparison, patients
with resectable pancreatic cancer with PVI, in other words
histologic BR-PHC, had better prognosis than PHC patients with
resected PVI with NAC-RT. Despite the small case-number limitation,
PVI resection with NAC-RT was considered to have important
implications. Although there were a limited number of pCR cases, we
observed that the majority of cases had some response to NAC-RT,
which inhibited local invasion and good OS.
In general, PHC patients are diagnosed with locally
advanced cancer or metastasis, and many cases are diagnosed as
UR-PC at admission. Anatomical relations between the pancreatic
head and surrounding tissue, such as the bile duct, major vessels,
and duodenum, contribute to the high frequency of extrapancreatic
invasion. Among such advanced PHC cases, our data showed that PVI
and surgical margins had an impact on survival outcomes. Previous
reports have demonstrated that adopting neoadjuvant treatment
potentially increases R0 resection rates (21,22).
There are competing ideas as to the effect of PVI on survival. One
stresses the importance of portal vein resection for PHC with PVI.
The other concludes that portal vein resection has no impact on
survival duration, and survival in patients who under portal vein
resection does not differ from those who undergo standard PD
(23).
The prognoses of BR-PHC patients who underwent
NAC-RT were unfavorable by preoperative assessment, but improved to
be comparable to that of resectable PHC patients, provided that
PVIs were dissolved. Most PHCs show a high incidence of peritoneal
dissemination or widespread metastasis, such as to the liver and
lung (24). These conditions are
strongly linked to hematogenous and lymphatic micrometastasis,
which are regarded as prognostic factors in PC (25,26). In
this study, the NAC-RT group had significantly lower rates of lymph
node metastasis, LVI, and MVI than the control group. Moreover, PVI
turned out to be a crucial factor affecting OS. We predict that
NAC-RT behaves as a protective measure in advanced PC to stem
further progression of the disease. However, the fact that the OS
of the NAC-RT group did not exceed that of the control group
indicates that other factors, not only control or inhibition of LVI
and MVI, should be taken into consideration to improve
prognosis.
In the present study, NAC-RT was expected to provide
good local control and to decrease MVI and LVI in the BR-PHC
microenvironment. However, remnants of high grade PanIN within the
pancreatic ducts were frequently observed. This may indicate that,
compared to invasive carcinoma, PanINs may be resistant to NAC-RT,
or NAC-RT has only a limited effect. High grade PanINs often
coexist with PC and cause high intraductal spread and shortens the
survival of PC patients (27,28).
Careful follow-up will be needed in order to detect local
recurrence or metastasis to other organs associated with high grade
PanINs at an early point.
Histological features of residual carcinoma in
post-therapy resection specimens has been shown to correlate with
the prognosis of patients with PC and several gastrointestinal
cancers (29–32). In this study, the post-therapeutic
response of tumor cells was represented by clear cytoplasm,
pyknosis, loss of nuclei, and indistinct cell borders.
Additionally, mucin pools or xanthogranuloma-like features with
coarse fibrosis were observed as the host tissue response. However,
the histology of the preoperative therapeutic effect of BR-PHC was
non-significantly correlated with OS in patients who received
NAC-RT in this study. Moreover, as seen in Fig. 5B, survival curves (Grades 1a and 1b
vs. Grades 2, 3, and 4) did not diverge until 8 years after
surgery, regardless of control or inhibition of PVI, LVI, and MVI.
Several studies have reported that chemotherapy such as FOLFIRINOX
and gemcitabine combined with protein-bound paclitaxel
(nab-paclitaxel) regimens are widely used due to the relatively
high response rate (33–36). In our study, all patients received a
combination of chemotherapy with gemcitabine or S-1. Therefore,
future studies may need to identify more effective systemic
treatments that control local disease and reduce systemic
metastasis after treatment.
In summary, NAC-RT could improve the prognosis of
BR-PHC patients, such that they have a prognosis similar to that of
patients with resectable PHC, if it successfully controls or
reduces local progression such as lymph node metastasis, LVI, and
MVI. Furthermore, the dissolution of PVI and, although it was rare,
complete response by NAC-RT led BR-PHC patients to have a better
prognosis than resectable PHC patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by the grant of the
2015 Clinical Research Promotion Foundation (No. 9).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YN, HI, ES, KS, HY and JA designed the study and
drafted the manuscript. YN and ES performed the statistical
analysis. YN, HI, YO, KT, RK, TH, MF, TU, YI, EO and KO. Collected
the clinical data. YT and HA performed immunohistochemistry. YN,
MT, YM, MN, RK and HK performed pathological analysis.
Ethics approval and consent to
participate
This retrospective study was approved by the ethical
committee of Kurume University (approval no. 17226), and the
requirement for informed consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Egawa S, Toma H, Ohigashi H, Okusaka T,
Nakao A, Hatori T, Maguchi H, Yanagisawa A and Tanaka M: Japan
pancreatic cancer registry; 30th year anniversary: Japan pancreas
society. Pancreas. 41:985–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuno S, Egawa S, Fukuyama S, Motoi F,
Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K, et al:
Pancreatic cancer registry in Japan: 20 years of experience.
Pancreas. 28:219–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winter JM, Cameron JL, Campbell KA, Arnold
MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS,
et al: 1423 pancreaticoduodenectomies for pancreatic cancer: A
single-institution experience. J Gastrointest Surg. 10:1199–1210;
discussion 1210–1191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grobmyer SR, Pieracci FM, Allen PJ,
Brennan MF and Jaques DP: Defining morbidity after
pancreaticoduodenectomy: Use of a prospective complication grading
system. J Am Coll Surg. 204:356–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimura H, Ohtsuka T, Matsunaga T, Watanabe
Y, Tamura K, Ideno N, Aso T, Miyazaki T, Osoegawa T, Aishima S, et
al: Predictors and diagnostic strategies for early-stage pancreatic
ductal adenocarcinoma: A retrospective study. Pancreas.
44:1148–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Serrano PE, Cleary SP, Dhani N, Kim PT,
Greig PD, Leung K, Moulton CA, Gallinger S and Wei AC: Improved
long-term outcomes after resection of pancreatic adenocarcinoma: A
comparison between two time periods. Ann Surg Oncol. 22:1160–1167.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garcea G, Dennison AR, Pattenden CJ, Neal
CP, Sutton CD and Berry DP: Survival following curative resection
for pancreatic ductal adenocarcinoma. A systematic review of the
literature. JOP. 9:99–132. 2008.PubMed/NCBI
|
|
9
|
Neoptolemos JP, Kleeff J, Michl P,
Costello E, Greenhalf W and Palmer DH: Therapeutic developments in
pancreatic cancer: Current and future perspectives. Nat Rev
Gastroenterol Hepatol. 15:333–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH
and Neoptolemos JP: Pancreatic cancer. Nat Rev Dis Primers.
2:160222016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Guo X, Fan Y, Wang D, Wu W, Wu L,
Liu T, Xu B, Feng Y, Wang Y, et al: Efficacy and safety comparison
of nabpaclitaxel plus S-1 and gemcitabine plus S-1 as first-line
chemotherapy for metastatic pancreatic cancer. Jpn J Clin Oncol.
48:535–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sherman WH, Hecht E, Leung D and Chu K:
Predictors of response and survival in locally advanced
adenocarcinoma of the pancreas following neoadjuvant GTX with or
without radiation therapy. Oncologist. 23:4–e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee
KH, Oh DY, Chie EK, Lee JM, Heo JS, et al: Oncological benefits of
neoadjuvant chemoradiation with gemcitabine versus upfront surgery
in patients with borderline resectable pancreatic cancer: A
prospective, randomized, open-label, multicenter phase 2/3 trial.
Ann Surg. 268:215–222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada S, Fujii T, Sugimoto H, Nomoto S,
Takeda S, Kodera Y and Nakao A: Aggressive surgery for borderline
resectable pancreatic cancer: Evaluation of national comprehensive
cancer network guidelines. Pancreas. 42:1004–1010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Isaji S, Mizuno S, Windsor JA, Bassi C,
Fernandez-Del Castillo C, Hackert T, Hayasaki A, Katz MHG, Kim SW,
Kishiwada M, et al: International consensus on definition and
criteria of borderline resectable pancreatic ductal adenocarcinoma
2017. Pancreatology. 18:2–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishikawa O, Ohigashi H, Imaoka S, Furukawa
H, Sasaki Y, Fujita M, Kuroda C and Iwanaga T: Preoperative
indications for extended pancreatectomy for locally advanced
pancreas cancer involving the portal vein. Ann Surg. 215:231–236.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uchida K and Masugi Y: Histological
assessment of therapeutic response. Classification of Pancreatic
Carcinoma: Japan Pancreas Society. Isaji S: Kanehara & Co.,
Ltd.; Tokyo: pp. 117–122. 2017
|
|
19
|
Ushijima T, Okabe Y, Ishida Y, Sugiyama G,
Sasaki Y, Kuraoka K, Yasumoto M, Taira T, Naito Y, Nakayama M, et
al: Evaluation of endoscopic cytological diagnosis of unresectable
pancreatic cancer prior to and after the introduction of endoscopic
ultrasound-guided fine-needle aspiration. Mol Clin Oncol.
2:599–603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakama Y, Kawahara R, Nomura Y, Muroya D,
Arai S, Ishikawa H, Yasunaga M, Horiuchi H, Akagi Y, Tanaka H, et
al: A case of pancreatic head cancer showing pathological complete
response to neoadjuvant chemoradiation therapy. Gan To Kagaku
Ryoho. 42:2376–2378. 2015.(In Japanese). PubMed/NCBI
|
|
21
|
Ravikumar R, Sabin C, Abu Hilal M,
Bramhall S, White S, Wigmore S, Imber CJ and Fusai G; UK Vascular
Resection in Pancreatic Cancer Study Group, : Portal vein resection
in borderline resectable pancreatic cancer: A United Kingdom
multicenter study. J Am Coll Surg. 218:401–411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yekebas EF, Bogoevski D, Cataldegirmen G,
Kunze C, Marx A, Vashist YK, Schurr PG, Liebl L, Thieltges S, Gawad
KA, et al: En bloc vascular resection for locally advanced
pancreatic malignancies infiltrating major blood vessels:
Perioperative outcome and long-term survival in 136 patients. Ann
Surg. 247:300–309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tseng JF, Raut CP, Lee JE, Pisters PW,
Vauthey JN, Abdalla EK, Gomez HF, Sun CC, Crane CH, Wolff RA and
Evans DB: Pancreaticoduodenectomy with vascular resection: Margin
status and survival duration. J Gastrointest Surg. 8:935–949;
discussion 949–950. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iacobuzio-Donahue CA, Fu B, Yachida S, Luo
M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P,
et al: DPC4 gene status of the primary carcinoma correlates with
patterns of failure in patients with pancreatic cancer. J Clin
Oncol. 27:1806–1813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamada Y and Nakayama Y: Aggressive venous
invasion in the area of carcinoma correlates with liver metastasis
as an index of metastasis for invasive ductal carcinoma of the
pancreas. Pancreatology. 17:951–955. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagakawa Y, Aoki T, Kasuya K, Tsuchida A
and Koyanagi Y: Histologic features of venous invasion, expression
of vascular endothelial growth factor and matrix
metalloproteinase-2 and matrix metalloproteinase-9, and the
relation with liver metastasis in pancreatic cancer. Pancreas.
24:169–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naito Y, Suda K, Nobukawa B, Kinoshita H
and Kojiro M: Histopathological study of invasive ductal carcinoma
(IDC) of the pancreas without associated cancerous occlusion of the
main pancreatic duct. J Hepatobiliary Pancreat Surg. 13:556–561.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamasaki S, Suda K, Nobukawa B and Sonoue
H: Intraductal spread of pancreatic cancer. Clinicopathologic study
of 54 pancreatectomized patients. Pancreatology. 2:407–412. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chatterjee D, Katz MH, Rashid A,
Varadhachary GR, Wolff RA, Wang H, Lee JE, Pisters PW, Vauthey JN,
Crane C, et al: Histologic grading of the extent of residual
carcinoma following neoadjuvant chemoradiation in pancreatic ductal
adenocarcinoma: A predictor for patient outcome. Cancer.
118:3182–3190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ajani JA, Mansfield PF, Crane CH, Wu TT,
Lunagomez S, Lynch PM, Janjan N, Feig B, Faust J, Yao JC, et al:
Paclitaxel-based chemoradiotherapy in localized gastric carcinoma:
Degree of pathologic response and not clinical parameters dictated
patient outcome. J Clin Oncol. 23:1237–1244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu TT, Chirieac LR, Abraham SC, Krasinskas
AM, Wang H, Rashid A, Correa AM, Hofstetter WL, Ajani JA and
Swisher SG: Excellent interobserver agreement on grading the extent
of residual carcinoma after preoperative chemoradiation in
esophageal and esophagogastric junction carcinoma: A reliable
predictor for patient outcome. Am J Surg Pathol. 31:58–64. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ryan R, Gibbons D, Hyland JM, Treanor D,
White A, Mulcahy HE, O'Donoghue DP, Moriarty M, Fennelly D and
Sheahan K: Pathological response following long-course neoadjuvant
chemoradiotherapy for locally advanced rectal cancer.
Histopathology. 47:141–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh
Rde W, Collisson E, Schwartz L, Frankel W, Martin R, Conway W, et
al: Preoperative modified FOLFIRINOX treatment followed by
capecitabine-based chemoradiation for borderline resectable
pancreatic cancer: Alliance for clinical trials in oncology trial
A021101. JAMA Surg. 151:e1611372016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okada KI, Hirono S, Kawai M, Miyazawa M,
Shimizu A, Kitahata Y, Ueno M, Hayami S and Yamaue H: Phase I study
of nab-paclitaxel plus gemcitabine as neoadjuvant therapy for
borderline resectable pancreatic cancer. Anticancer Res.
37:853–858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Okada KI, Shimokawa T, Hirono S, Kawai M,
Sho M, Satoi S, Matsumoto I, Eguchi H, Murakami Y, Yamada S, et al:
Effect of neoadjuvant nab-paclitaxel plus gemcitabine therapy on
overall survival in patients with borderline resectable pancreatic
cancer: A prospective multicenter phase II trial (NAC-GA Trial).
Oncology. 93:343–346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Von Hoff DD, Ramanathan RK, Borad MJ,
Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias
JL, et al: Gemcitabine plus nab-paclitaxel is an active regimen in
patients with advanced pancreatic cancer: A phase I/II trial. J
Clin Oncol. 29:4548–4554. 2011. View Article : Google Scholar
|