Introduction
Breast ductal carcinoma in situ (DCIS) is
characterized by a proliferation of malignant epithelial cells
confined to the mammary ducts without light-microscopic evidence of
invasion through the basement membrane into the surrounding stroma
(1). According to the criteria of
the American Joint Committee on Cancer (AJCC), DCIS with a
microscopic focus of invasion ≤1 mm in the longest diameter is
defined as ductal carcinoma in situ with microinvasion
(DCISM) (2). The proportion of DCIS
and DCISM cases detected by screening has markedly increased
(3). Recent studies have revealed
that DCISM, with its potential for invasion and metastasis, might
represent a distinct entity differing from DCIS (4–6).
Patients with DCISM had worse cancer-specific survival, disease
free survival, and overall survival and a higher mortality rate
than patients with DCIS (3,7,8).
Distinguishing DCISM from DCIS via preoperative imaging would help
to predict the prognosis of patients. Yao et al (6), indicated that DCISM was more likely to
have calcifications in the mass and a high degree of
vascularization on sonography. However, the study by Yao et
al (6) included only DCIS cases
that appeared as masses on sonography, and DCIS cases that were
non-mass lesions or negative on sonography were excluded. No study
has compared the mammographic features of DCIS and DCISM.
Therefore, the purpose of our study was to compare the sonographic
and mammographic features between patients with DCISM and DCIS.
Patients and methods
Patients
The Sun Yat-sen Memorial Hospital Ethics Committee
approved this retrospective study. The pathologic database of our
hospital was searched to identify patients with a pathologic
diagnosis of DCIS and DCISM on surgical specimens diagnosed between
December 2012 and September 2015. We excluded patients who received
neoadjuvant chemotherapy before surgery or who underwent excisional
biopsy outside of our hospital. Therefore, 94 DCIS cases and 53
DCISM cases were included. The clinical features, sonographic and
mammographic images and pathology records were reviewed.
Clinical features
The clinical features, including clinical
presentation and history, were obtained from the medical records.
The clinical presentation included observation of a palpable mass,
nipple discharge or no symptoms. The clinical history included age,
menopausal status, family history of breast cancer and personal
history of breast cancer.
Sonography and mammography
Sonographic examinations were performed by one of
seven dedicated breast radiologists who specialized in breast
ultrasound using an ACUSON S2000 system (Siemens, Germany). The
sonographic findings were classified as masses, non-mass
abnormalities or no abnormality. A non-mass abnormality was defined
as: i) layered duct-like structures without a distinct mass with a
zebra pattern or with small nodules less than 3 mm in diameter and
ii) ductal dilation (9). When a mass
was present, the sonographic findings (shape, orientation, margin,
echo pattern and posterior features) were described according to
the American College of Radiology Breast Imaging Reporting and Data
System (BI-RADS) lexicon (10).
Whether calcifications and vascularity were present in a mass or
non-mass were also analyzed.
Bilateral digital mammograms with two standard
imaging planes (mediolateral oblique and craniocaudal) were
obtained using a digital mammographic unit (Planmed Nuance,
Planmed, Helsinki, Finland) in our institution. Because 23 patients
in the DCIS group and 14 patients in the DCISM group had mammograms
from other hospitals, they did not undergo mammography in our
hospital. Therefore, 71 DCIS patients and 39 DCISM patients were
included. Mammograms were reviewed for breast composition and
lesion characteristics according to the BI-RADS lexicon (10). Based on visual evaluation, the breast
composition was classified into the following four categories: i)
almost entirely fatty breasts; ii) t scattered areas of
fibroglandular density; iii) t heterogeneously dense breasts, and
iv) extremely dense breasts. All lesions were classified as either
calcifications only, a mass, asymmetry or architectural distortion.
The morphology of calcification is divided into three categories:
Amorphous, coarse heterogeneous and fine pleomorphic. The
distribution of calcification is also divided into three
categories: Grouped, regional and segmental.
Ultrasonograms and mammograms were reviewed
retrospectively by two breast imaging radiologists who were blinded
to the pathologic information but not blinded to the clinical
information. When the descriptor differed between the two
radiologists, a consensus was reached by discussion.
Histological analysis
All pathologic reports were reviewed. A diagnosis of
DCISM was rendered when a microscopic focus of invasion ≤1 mm in
the longest diameter within an area of DCIS was present (2). The histopathologic features included
the nuclear grade and the presence or absence of comedo-type
necrosis. Biological markers including estrogen receptor (ER),
progesterone receptor (PR), and human epidermal growth factor
receptor 2 (HER2) and the Ki-67 index were examined by
immunohistochemical analysis as a routine pathologic assessment in
our hospital. ER and PR positivity were defined as nuclear staining
in 1% or more of tumor cells. HER2 status was graded as 0, 1+, 2+,
and 3+ by immunohistochemistry. HER2 0 and 1+ were considered
negative, whereas HER2 3+ was considered positive. Ki-67 expression
was quantified using a visual grading system. An estimated
percentage of Ki-67 positive cells was determined, and the cutoff
for positivity was established at 20% (11).
Statistical analysis
A Mann-Whitney U test was used to compare the age
and maximum diameter of patients with DCISM to those of patients
with DCIS. The χ2 test and Fisher's exact test were used
to compare sonographic and mammographic characteristics,
clinicopathologic findings and biomarkers and between the two
groups of patients. Multivariate logistic regression analysis was
used to calculate the odds ratio (OR) and 95% confidence intervals
(CI) in the analysis of sonographic findings that were significant
in the univariate analysis. P<0.05 was considered to indicate a
statistically significant difference. Data analysis was performed
using SPSS v.19.0 statistical software (IBM Corp.).
Results
Clinical and pathologic features
The clinical and pathologic characteristics are
summarized in Table I. In addition
to the presence of a mass, nipple discharge was more common in
patients with DCISM. However, patient age, menopausal status, and
family and personal history of breast cancer were not significantly
different between the two disease entities (all P>0.05).
Patients with DCISM were more likely to have larger and higher
grade tumors with comedo-type necrosis, ER negativity, PR
negativity, HER2 positivity, and a higher Ki-67 index than patients
with DCIS (all P<0.05).
 | Table I.Clinical-pathologic parameters in
patients with DCIS (n=94) and DCISM (n=53). |
Table I.
Clinical-pathologic parameters in
patients with DCIS (n=94) and DCISM (n=53).
| Clinical-pathologic
parameters | DCIS, n=94 (%) | DCISM, n=53 (%) | P-value |
|---|
| Age (years) | 48±11a | 46±8a | 0.267 |
| Menopausal
status |
|
| 0.728 |
|
Premenopausal | 63 (67.0) | 37(69.8) |
|
|
Postmenopausal | 31 (33.0) | 16(30.2) |
|
| Clinical
presentation |
|
| 0.015 |
|
Mass | 58 (61.7) | 38 (71.7) |
|
| Nipple
discharge | 16 (17.0) | 13(24.5) |
|
|
Asymptomatic | 20 (21.3) | 2 (3.8) |
|
| Family history of
breast cancer |
|
| 0.509 |
|
Yes | 2 (2.1) | 3 (5.7) |
|
| No | 92 (97.9) | 50 (94.3) |
|
| Personal history of
breast cancer |
|
| 0.480 |
|
Yes | 3 (3.2) | 0 (0.0) |
|
| No | 91 (96.8) | 53 (100.0) |
|
| Maximum diameter
(cm)a |
2.1±1.5a |
3.4±1.5a | <0.001 |
| Nuclear grade |
|
| <0.001 |
| Low or
intermediate | 68 (72.3) | 11 (20.8) |
|
|
High | 26 (27.7) | 42 (79.2) |
|
| Comedo-type
necrosis |
|
| <0.001 |
|
Yes | 3 (3.2) | 17 (32.1) |
|
| No | 91 (96.8) | 36 (67.9) |
|
| Estrogen
receptor |
|
| <0.001 |
|
Positive | 86 (91.5) | 31 (58.5) |
|
|
Negative | 8 (8.5) | 22 (41.5) |
|
| Progesterone
receptor |
|
| <0.001 |
|
Positive | 76 (80.9) | 16 (30.2) |
|
|
Negative | 18 (19.1) | 37 (69.8) |
|
| HER2 status |
|
| 0.001 |
| 0,
1+ | 46 (48.9) | 11 (20.8) |
|
| 3+ | 16 (17.1) | 21 (39.6) |
|
| 2+ | 32 (34.0) | 21 (39.6) |
|
| Ki-67 index
(%) |
|
| 0.006 |
|
>20 | 28 (29.8) | 28 (52.8) |
|
|
≤20 | 66 (70.2) | 25 (47.2) |
|
Sonographic features
No abnormal signs were found in 11 patients from the
DCIS group. Thus, 83 mass or non-mass abnormalities were included
in the DCIS group. Univariate analysis of sonographic features
indicated that non circumscribed margins, the presence of
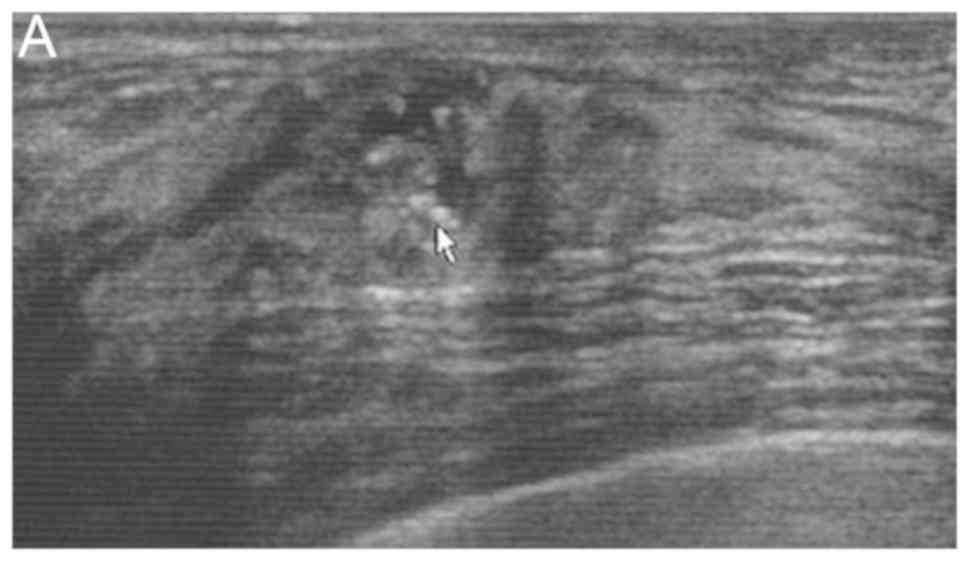
vascularity and calcification (Fig.
1) were significantly more common in DCISM cases (Table II). Multivariate analysis showed
that the presence of vascularity and calcification were independent
variables associated with DCISM (Table
III).
 | Table II.Univariate analysis of sonographic
features in DCIS and DCISM. |
Table II.
Univariate analysis of sonographic
features in DCIS and DCISM.
| Sonographic
features | DCIS, n=83 (%) | DCISM, n=53
(%) | P-value |
|---|
| Mass | 47 (56.6) | 25 (47.2) | 0.281 |
| Shape |
|
| 0.158 |
|
Oval | 9 (19.1) | 1 (4.0) |
|
|
Irregular | 38 (80.9) | 24 (96.0) |
|
| Orientation |
|
| 0.364 |
|
Parallel | 38 (80.9) | 23 (92.0) |
|
| Not
parallel | 9 (19.1) | 2 (8.0) |
|
| Margin |
|
| 0.015 |
|
Circumscribed | 12 (25.5) | 0 (0.0) |
|
| Not
circumscribed | 35 (74.5) | 25 (100.0) |
|
| Echo pattern |
|
| 0.502 |
|
Hypoechoic | 44 (93.6) | 25 (100.0) |
|
| Complex
cystic and solid | 3 (6.4) | 0 (0.0) |
|
| Posterior
features |
|
| 0.999 |
| No
posterior features or enhancement | 42 (89.4) | 23 (92.0) |
|
|
Shadowing or combined
pattern | 5 (10.6) | 2 (8.0) |
|
| Non-mass
abnormality | 36 (43.4) | 28 (52.8) | 0.381 |
| Layered
duct-like structures without a distinct mass with a zebra pattern
or with small nodules less than 3 mm in diameter | 28 (77.8) | 25 (89.3) |
|
| Ductal
dilation | 8 (22.2) | 3 (10.7) |
|
| Vascularity |
|
| 0.002 |
|
Absent | 37 (44.6) | 10 (18.9) |
|
|
Present | 46 (55.4) | 43 (81.1) |
|
| Calcification |
|
| 0.003 |
|
Absent | 56 (67.5) | 22 (41.5) |
|
|
Present | 27 (32.5) | 31 (58.5) |
|
 | Table III.Multivariate analysis of sonographic
features in ductal carcinoma in situ and ductal carcinoma
in situ with microinvasion. |
Table III.
Multivariate analysis of sonographic
features in ductal carcinoma in situ and ductal carcinoma
in situ with microinvasion.
| Sonographic
features | OR (95% CI) | P-value |
|---|
| Vascularity |
| 0.025 |
|
Absent | Reference |
|
|
Present | 2.660
(1.132–6.251) |
|
| Calcification |
| 0.038 |
|
Absent | Reference |
|
|
Present | 2.220
(1.044–4.723) |
|
Mammographic features
No abnormal signs on mammography were found in 21
patients in the DCIS group and 3 patients in the DCISM group. Among
24 cases, the classification of the breast composition was as
follows: 7 cases were extremely dense, 16 cases were
heterogeneously dense, and 1 case displayed scattered areas of
fibroglandular density. Therefore, 50 abnormalities in the DCIS
group and 36 abnormalities in the DCISM group were included. Larger
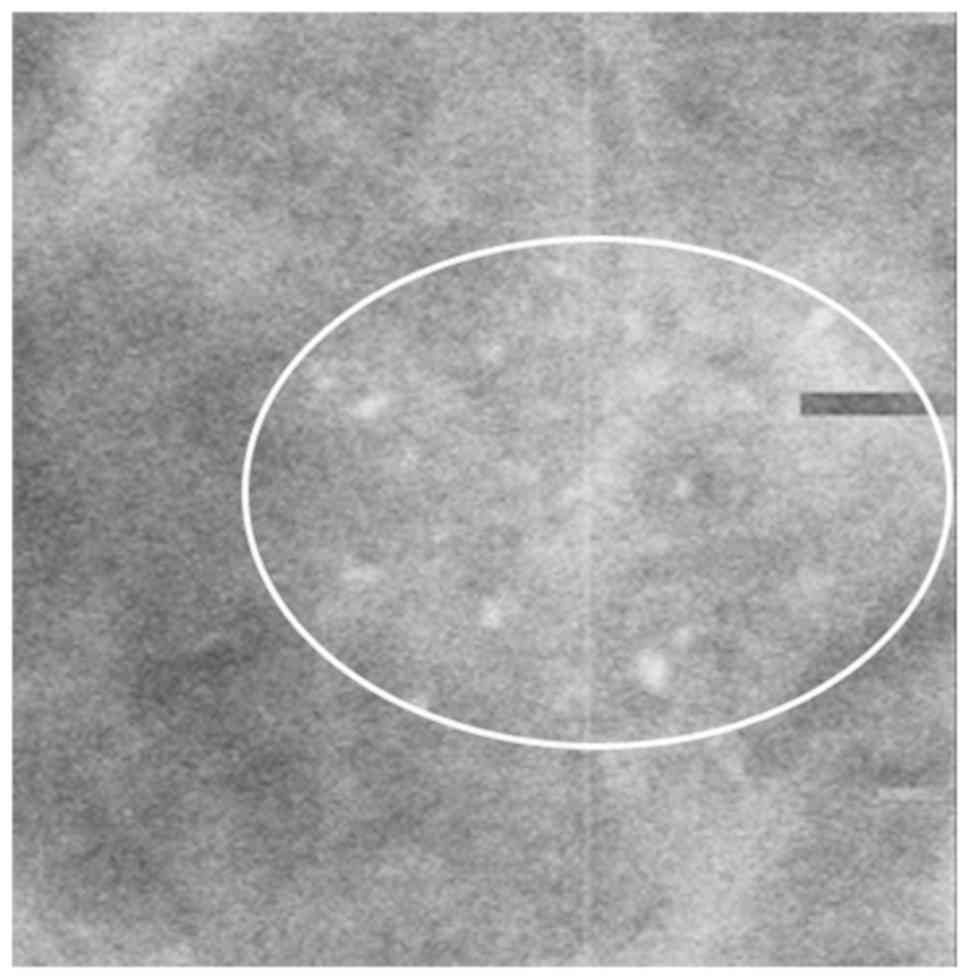
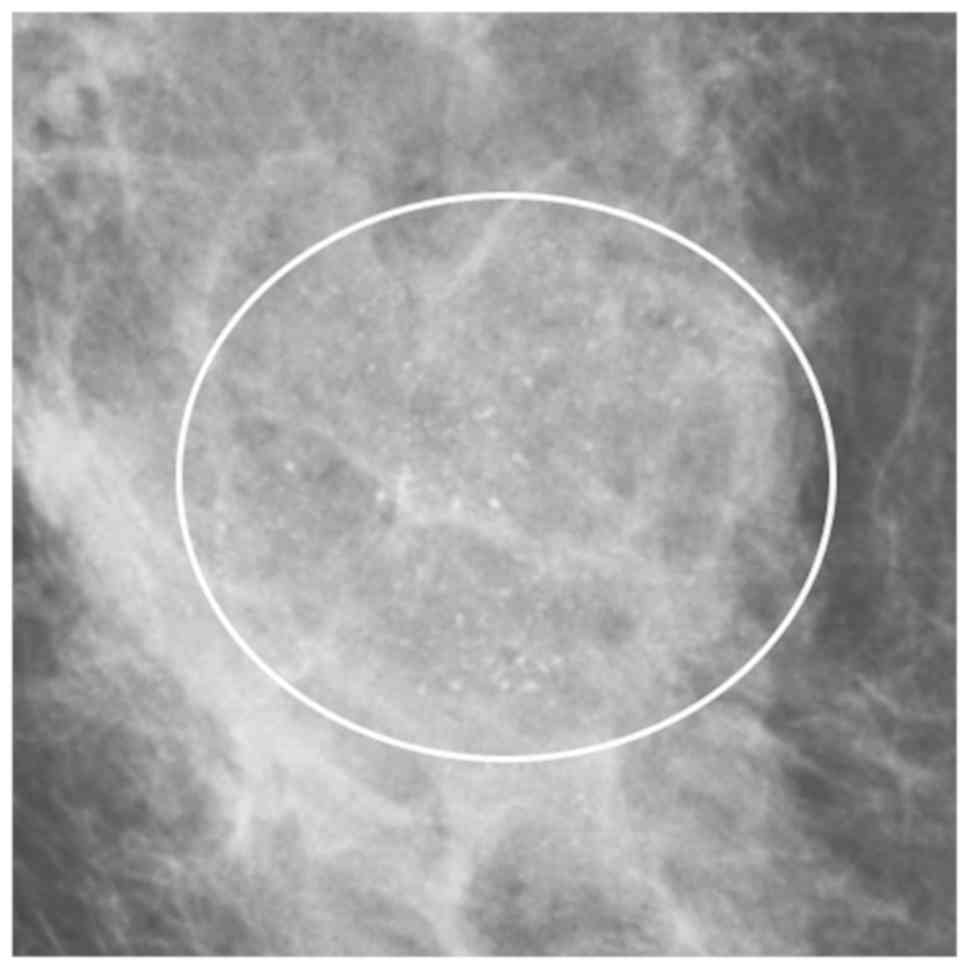
distribution of calcifications was associated with DCISM (Table IV; Figs.
2 and 3).
 | Table IV.Mammographic features in DCIS and
DCISM. |
Table IV.
Mammographic features in DCIS and
DCISM.
| Mammographic
feature | DCIS, n=50 (%) | DCISM, n=36
(%) | P-value |
|---|
| Calcifications | 37 (74.0) | 23 (63.9) | 0.195 |
| Morphology |
|
| 0.130 |
|
Amorphous | 19 (51.4) | 6 (26.1) |
|
| Coarse
heterogeneous | 2 (5.4) | 1 (4.3) |
|
| Fine
pleomorphic | 16 (43.2) | 16 (69.6) |
|
| Distribution |
|
| 0.002 |
|
Grouped | 25 (67.6) | 5 (21.8) |
|
|
Regional | 8 (21.6) | 11 (47.8) |
|
|
Segmental | 4 (10.8) | 7 (30.4) |
|
|
Massa | 4 (8.0) | 7 (19.4) |
|
| Focal
asymmetryb | 6 (12.0) | 6 (16.7) |
|
|
Architectural
distortionc | 3 (6.0) | 0 (0.0) |
|
Discussion
A palpable mass was the main symptom of DCIS and
DCISM lesions (5,12). In addition, nipple discharge was
commonly encountered in DCISM. Factors including age, menopausal
status, and family and personal history of breast cancer were not
found to be associated with the presence of microinvasion, which
was consistent with a study by Ozkan-Gurdal et al (13). Pathologically, DCISM tended to be
larger and have a higher nuclear grade, comedo-type necrosis, ER
negativity, PR negativity, HER2 positivity and a higher Ki-67 index
than DCIS, which was similar to the results of previous studies
(6,12–14).
Only one study performed a detailed comparison of
the sonographic features of DCIS and DCISM (6). However, Yao et al (6) investigated only lesions observed as
masses on sonography. Previous studies have shown that the
sonographic findings of DCIS could include a mass, a non-mass or no
abnormal findings and approximately 49 to 60% of DCIS cases appear
as non-mass abnormalities on sonography (15,16).
According to previous studies, we classified the sonographic
findings of DCIS and DCISM as masses, non-mass lesions or no
abnormality. The proportion of non-mass lesions was greater in the
DCISM group than in the DCIS group (52.8% vs. 43.4%), but the
difference was not significant. Yao et al (6), reported that DCISM was more likely to
have calcifications and a high degree of vascularization than DCIS.
In our study, univariate and multivariate analyses also showed that
the presence of calcifications and vascularity were variables
associated with DCISM. Calcifications are associated with a high
nuclear grade and necrosis (6,17,18). The
pathogenesis of DCISM may be related to the rapid growth and active
metabolism of tumors, in which oxygen and nutrition are
insufficient, leading to the development of local ischemic necrosis
and calcium salt deposition, which is detected as calcifications
(6). Although sonography is less
sensitive than mammography for the identification of calcifications
(18), the ability to visualize
calcifications on sonography has been described (16,19). In
a study using mammography as the gold standard, Yang (19) reported that ultrasound achieved a
sensitivity of 95%, a specificity of 87.8% and an accuracy of 91%
for the detection of calcification. Other studies have reported
that calcifications of DCIS were demonstrated on sonography in 54.1
to 74% of DCIS lesions (15,17). In our study, no sonographic
abnormalities were found in 11 patients in the DCIS group. However,
calcifications were found on mammography. Identifying isolated
calcifications within normal breast tissue, that consists of
increased hyperechoic and heterogeneous fibrous tissue, is thought
to be more difficult using sonography. This is primarily due to the
lack of contrast between the normal parenchyma with hyperechoic
heterogeneous fibrous structures and calcifications (20). Angiogenesis is the formation of new
capillaries from the existing vascular network and is essential for
tumor growth and dissemination (21). Cao et al (22), established that the comedo-type and
high nuclear grade DCIS, which has a high potential for invasive
transformation, are significantly associated with high microvessel
counts. In our study, a significant correlation was observed
between high grade tumors, comedo-type necrosis and DCISM. Thus,
the presence of vascularity on sonography is associated with
DCISM.
Mammography is widely accepted as the most important
imaging method for the detection of DCIS. DCIS manifests as
calcifications in 62–98% of cases (23–25). In
our study, calcifications were found more commonly in DCIS than in
DCISM (74% vs. 63.9%), but the difference was not significant. The
distribution of calcifications was mainly regional and segmental in
DCISM cases and mainly grouped and regional in DCIS cases,
resulting in a statistically significant difference between the two
groups (P<0.05). Similar results regarding the association
between a higher cluster area of the calcifications and an
increased likelihood of invasion were reported by Lagios et
al (25). Regarding the 24
lesions that were not visible on mammography, 10 lesions manifested
as masses and 14 manifested as non-mass abnormalities on
sonography. Dense breasts composed 95.8% (23/24) of the cases.
Mammographic sensitivity is significantly inversely related to
breast density and decreases from 100% in fatty breasts to 45–48%
in extremely dense breasts (26,27). Our
findings suggested that sonography may be more effective than
mammography for the detection of DCIS in patients with dense
breasts or lesions without calcification.
There are a few limitations in the present study.
First, it was a retrospective study. Additionally, several patients
who had mammograms performed at other hospitals did not undergo
mammography in our hospital. Therefore, we did not match the
mammography and sonography results for lesions. Second, we did not
consider the distribution and degree of vascularization, and three
color Doppler indices (peak systolic velocity, pulsatility index,
and resistive index), elastography on sonography and circulating
tumor cell were not used for comparisons (6). The reasons were as follows (1), Because of the small sample size, it was
difficult to classify the distribution and degree of
vascularization (2). Color Doppler
indices, elastography on sonography and circulating tumor cell were
not routinely used during the study period. Third, other methods of
diagnostics, for example a thermography device, are not available
at our institute.
In summary, imaging features including the presence
of calcifications and vascularity on sonography and a larger area
of calcifications on mammography were associated with DCISM. Even
so, pathological analysis is still the gold standard. Additional
larger prospective studies are needed for further research.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from The
National Natural Science Foundation of China (grant no.
81372817).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HLW made substantial contributions to the study
design, and the acquisition, analysis and interpretation of data,
and drafted the manuscript. JJL, JGL and CT participated in the
design of the study and the analysis of the data, and helped to
draft the manuscript. YPY helped conduct the statistical analysis.
RG, XFJ, FTL and YH participated in data acquisition and manuscript
revision. FXS contributed to the study design and was involved in
revising the manuscript critically for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This retrospective study was approved by the Ethics
Committee of Sun Yat-sen Memorial Hospital. Written informed
consent was not required for the review of these images and
data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DCISM
|
ductal carcinoma in situ with
microinvasion
|
|
DCIS
|
ductal carcinoma in situ
|
|
AJCC
|
American Joint Committee on Cancer
|
|
BI-RADS
|
breast imaging reporting and data
system
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Schnitt SJ, Silen W, Sadowsky NL, Connolly
JL and Harris JR: Ductal carcinoma in situ (intraductal carcinoma)
of the breast. N Engl J Med. 318:898–903. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singletary SE, Allred C, Ashley P, Bassett
LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, et
al: Revision of the American Joint Committee on Cancer staging
system for breast cancer. J Clin Oncol. 20:3628–3636. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Zhu W, Du F, Luo Y and Xu B: The
demographic features, clinicopathological characteristics and
cancer-specific outcomes for patients with microinvasive breast
cancer: A SEER database analysis. Sci Rep. 7:420452017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okumura Y, Yamamoto Y, Zhang Z, Toyama T,
Kawasoe T, Ibusuki M, Honda Y, Iyama K, Yamashita H and Iwase H:
Identification of biomarkers in ductal carcinoma in situ of the
breast with microinvasion. BMC Cancer. 8:2872008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vieira CC, Mercado CL, Cangiarella JF, Moy
L, Toth HK and Guth AA: Microinvasive ductal carcinoma in situ:
Clinical presentation, imaging features, pathologic findings, and
outcome. Eur J Radiol. 73:102–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao JJ, Zhan WW, Chen M, Zhang XX, Zhu Y,
Fei XC and Chen XS: Sonographic features of ductal carcinoma in
situ of the breast with microinvasion: Correlation with
clinicopathologic findings and biomarkers. J Ultrasound Med.
34:1761–1768. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sopik V, Sun P and Narod SA: Impact of
microinvasion on breast cancer mortality in women with ductal
carcinoma in situ. Breast Cancer Res Treat. 167:787–795. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang Y, Wu J, Wang W, Fei X, Zong Y, Chen
X, Huang O, He J, Chen W, Li Y, et al: Biologic behavior and
long-term outcomes of breast ductal carcinoma in situ with
microinvasion. Oncotarget. 7:64182–64190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gwak YJ, Kim HJ, Kwak JY, Lee SK, Shin KM,
Lee HJ, Kim GC, Jang YJ, Han MH, Park JY and Jung JH:
Ultrasonographic detection and characterization of asymptomatic
ductal carcinoma in situ with histopathologic correlation. Acta
Radiol. 52:364–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Breast Imaging Reporting and Data System
(BI-RADS). 5th. American College of Radiology; Reston, VA: 2013
|
|
11
|
Penault-Llorca F, André F, Sagan C,
Lacroix-Triki M, Denoux Y, Verriele V, Jacquemier J, Baranzelli MC,
Bibeau F, Antoine M, et al: Ki67 expression and docetaxel efficacy
in patients with estrogen receptor-positive breast cancer. J Clin
Oncol. 27:2809–2815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Gao EL, Zhou YL, Zhai Q, Zou ZY,
Guo GL, Chen GR, Zheng HM, Huang GL and Zhang XH: Different
distribution of breast ductal carcinoma in situ, ductal carcinoma
in situ with microinvasion, and invasion breast cancer. World J
Surg Oncol. 10:2622012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozkan-Gurdal S, Cabioglu N, Ozcinar B,
Muslumanoglu M, Ozmen V, Kecer M, Yavuz E and Igci A: Factors
predicting microinvasion in Ductal Carcinoma in situ. Asian Pac J
Cancer Prev. 15:55–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sue GR, Lannin DR, Killelea B and Chagpar
AB: Predictors of microinvasion and its prognostic role in ductal
carcinoma in situ. Am J Surg. 206:478–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee MH, Ko EY, Han BK, Shin JH, Ko ES and
Hahn SY: Sonographic findings of pure ductal carcinoma in situ. J
Clin Ultrasound. 41:465–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Watanabe T, Yamaguchi T, Tsunoda H, Kaoku
S, Tohno E, Yasuda H, Ban K, Hirokaga K, Tanaka K, Umemoto T, et
al: Ultrasound image classification of ductal carcinoma in situ
(DCIS) of the breast: Analysis of 705 DCIS lesions. Ultrasound Med
Biol. 43:918–925. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagashima T, Hashimoto H, Oshida K, Nakano
S, Tanabe N, Nikaido T, Koda K and Miyazaki M: Ultrasound
demonstration of mammographically detected microcalcifications in
patients with ductal carcinoma in situ of the breast. Breast
Cancer. 12:216–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rauch GM, Kuerer HM, Scoggins ME, Fox PS,
Benveniste AP, Park YM, Lari SA, Hobbs BP, Adrada BE, Krishnamurthy
S and Yang WT: Clinicopathologic, mammographic, and sonographic
features in 1187 patients with pure ductal carcinoma in situ of the
breast by estrogen receptor status. Breast Cancer Res Treat.
139:639–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang WT, Suen M, Ahuja A and Metreweli C:
In vivo demonstration of microcalcification in breast cancer using
high resolution ultrasound. Br J Radiol. 70:685–690. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gufler H, Buitrago-Téllez CH, Madjar H,
Allmann KH, Uhl M and Rohr-Reyes A: Ultrasound demonstration of
mammographically detected microcalcifications. Acta Radiol.
41:217–221. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao Y, Paner GP, Kahn LB and Rajan PB:
Noninvasive carcinoma of the breast: Angiogenesis and cell
proliferation. Arch Pathol Lab Med. 128:893–896. 2004.PubMed/NCBI
|
|
23
|
Dershaw DD, Abramson A and Kinne DW:
Ductal carcinoma in situ: Mammographic findings and clinical
implications. Radiology. 170:411–415. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stomper PC, Connolly JL, Meyer JE and
Harris JR: Clinically occult ductal carcinoma in situ detected with
mammography: Analysis of 100 cases with radiologic-pathologic
correlation. Radiology. 172:235–241. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lagios MD, Westdahl PR, Margolin FR and
Rose MR: Duct carcinoma in situ. Relationship of extent of
noninvasive disease to the frequency of occult invasion,
multicentricity, lymph node metastases, and short-term treatment
failures. Cancer. 50:1309–1314. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berg WA, Gutierrez L, NessAiver MS, Carter
WB, Bhargavan M, Lewis RS and Ioffe OB: Diagnostic accuracy of
mammography, clinical examination, US, and MR imaging in
preoperative assessment of breast cancer. Radiology. 233:830–849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kolb TM, Lichy J and Newhouse JH:
Comparison of the performance of screening mammography, physical
examination, and breast US and evaluation of factors that influence
them: An analysis of 27,825 patient evaluations. Radiology.
225:165–175. 2002. View Article : Google Scholar : PubMed/NCBI
|