Introduction
Fucoidan is a complex sulfated polysaccharide that
is mostly found in brown marine algae. Fucoidan exhibits a broad
spectrum of biological activities, including anti-inflammatory,
immunomodulatory, antiviral, antioxidant, and anti-tumor activities
(1–4). Previous studies reported a potential
role for fucoidan as an anticancer agent based on its proven
anticancer and anti-metastatic effects in vivo and in
vitro (4–7). The mechanisms underlying the anticancer
effects of fucoidan have not yet been elucidated in detail;
however, immune modulation is one of the most promising areas for
the anticancer efficacy of fucoidan (8,9).
A cancer survivor is defined as anyone who has been
diagnosed with cancer, from the time of diagnosis and throughout
the remainder of their lifespan (10). Many cancer survivors are highly
motivated to seek information about food choices, physical
activity, dietary supplement use, and complementary nutritional
therapies in order to improve their response to treatment, speed of
recovery, and quality of life and also to reduce their risk of
recurrence (11).
A clinical trial was performed on fucoidan in
patients with advanced colon cancer. Ikeguchi et al reported
that the ingestion of 4.05 g per day of fucoidan derived from
Cladosiphon okamuranus reduced the clinical toxicity
indicator ‘fatigue’ in patients receiving conventional chemotherapy
(12). Furthermore, patients
administered fucoidan tolerated more cycles of chemotherapy. A
recent clinical trial on fucoidan in patients with different types
of advanced cancers identified the responsiveness of interleukin
(IL)-1β as a significant independent prognostic factor. However,
natural killer (NK) cell activity was not affected by a four-week
treatment with fucoidan derived from C. novae-caledoniae
Kylin (13).
NK cell cytotoxic activity represents an innate
immune component that plays an important role in defenses against
tumor cells (14). NK cells
recognize and induce the lysis of tumor cells without prior
sensitization. An epidemiologic survey of 11-year follow up shows a
link between low NK cell activity in peripheral blood and increased
cancer risk in adults (15). We
herein examined the effects of fucoidan extracted from C.
Okamuranus on NK cell activity in cancer survivors.
Patients and methods
Subjects
Cancer survivors willing to participate in the
present study were recruited from 4 medical clinics (Ono Clinic,
Kobe City; Hoshi Clinic, Maebashi City; Takeichi Clinic, Odawara
City and Daido Chuo Clinic, Naha City) in Japan between June 2016
and May 2017. Eleven patients who met the following inclusion
criteria were enrolled: i) cancer survivors following surgical
resection, radiation therapy, chemotherapy, and radiofrequency
ablation (RFA) without recurrence and metastasis; ii) cancer
survivors receiving chemotherapy without metastasis and iii) cancer
survivors before therapy without metastasis. Exclusion criteria are
as follows: i) cancer survivors with a performance status of 2, 3
and 4; ii) cancer survivors with obvious recurrence and metastasis
at enrollment and iii) cancer survivors complicated with liver
cirrhosis, renal insufficiency, advanced heart failure and
immunodeficiency.
The diagnosis of cancer was confirmed by
characteristic pathologies (Table
I). Demographic characteristics are shown in Table II. All subjects were ambulatory as
an outpatient with a normal food intake during the trial periods.
Although the primary cancer lesions markedly varied, recurrence and
metastasis were confirmed to be absent by an examination using
computed tomography (CT) and/or magnetic resonance imaging (NRI)
before enrollment. Primary tumor lesions of seven patients (Case 1,
2.3.4.5.8 and 9) were completely eradicated by surgical operation
or radiation therapy. The period from cancer therapy to fucoidan
trial was between 6 months and 8 years (3.0 years on the
average).
 | Table I.Characteristics and diagnosis of
subjects. |
Table I.
Characteristics and diagnosis of
subjects.
| Case | Sex | Age (years) | Diagnosis | Treatment |
|---|
| 1 | Male | 67 | Pharyngeal
cancer | Post-surgical
resection |
| 2 | Male | 81 | Hepatocellular
carcinoma | Post-radiofrequency
ablation |
| 3 | Male | 78 | Hepatocellular
carcinoma | Post-radiofrequency
ablation |
| 4 | Male | 70 | Renal cell
cancer | Post-surgical
resection |
| 5 | Male | 71 | Colon cancer | Post-surgical
resection |
| 6 | Male | 74 | Lung cancer | Undergoing
chemotherapy (Erlotinib) |
| 7 | Male | 76 | Prostate
cancer | Pre-surgical
operation |
| 8 | Female | 58 | Cervical
cancer | Post-surgical
resection |
| 9 | Female | 52 | Breast cancer | Post-surgical
resection |
| 10 | Female | 56 | Colon cancer | Post-surgical
resection |
| 11 | Female | 70 | Adult T-cell
leukemia | Undergoing
chemotherapy (Irinotecan) |
 | Table II.Demographic characteristics of
subjects. |
Table II.
Demographic characteristics of
subjects.
| Case | Sex | Age (years) | Height (cm) | Body weight
(kg) | Performance
status |
|---|
| 1 | Male | 67 | 171 | 68 | 0 |
| 2 | Male | 81 | 161 | 71 | 0 |
| 3 | Male | 78 | 167 | 62 | 0 |
| 4 | Male | 70 | 172 | 63 | 0 |
| 5 | Male | 71 | 168 | 64 | 0 |
| 6 | Male | 74 | 162 | 65 | 0 |
| 7 | Male | 76 | 163 | 71 | 0 |
| 8 | Female | 58 | 158 | 54 | 1 |
| 9 | Female | 52 | 167 | 50 | 0 |
| 10 | Female | 56 | 152 | 45 | 0 |
| 11 | Female | 70 | 153 | 54 | 1 |
Two cases (Cases 6 and 11) were undergoing standard
chemotherapy before the administration of fucoidan. Case 6 took
erlotinib for two years and Case 11 took irinotecan for five years.
Case 7 was awaiting surgical resection without chemotherapy. Case
10 was treated with a surgical operation for colon cancer two years
before the clinical trial.
The present study was performed in accordance with
the Declaration of Helsinki. The study protocol was approved by the
Ethics Committee of South Product Co., Ltd.
Fucoidan treatment
Fucoidan extracted from Okinawa mozuku (C.
okamuranus) was used in the present study. The main molecular
weight of fucoidan was 28.8 kDa, containing 70 mg/ml of L-fucose
and 10 mg/ml of sulfate (16).
Subjects were orally administered 50 ml of a drink that contained
1.5 g of fucoidan twice daily for 6 months. The drink was prepared
by South Product Co. Subjects took a meal without dietary
restriction during the trial period. Subjects receiving medicine(s)
continued with the same treatment as before, and anti-cancer drugs
were unchanged during the trial period. Complementary and
alternative medicine (CAM) was prohibited during the trial period,
because it may affect NK cell activity and/or absorption of
fucoidan. We checked use of CAM when subjects attended a
clinic.
Clinical assessment
The clinical status of subjects was recorded every
month during the ingestion of fucoidan. Subjects were asked about
any new symptoms or changes since the introduction of fucoidan.
Fucoidan was withheld if subjects developed adverse events and
laboratory toxicity.
Endpoints
NK cells are the prototype of innate lymphoid cells
with potent cytolytic function that provide immune surveillance
against cancer; whereas, the effect of fucoidan on NK cell activity
remains to be fully elucidated in cancer survivors. The primary
endpoint was changes in NK cell activity and immunoglobulin titers
because we focused on the immunomodulatory effects of fucoidan. We
have already performed a randomized double-blind trial of fucoidan
in healthy Japanese volunteers, in which oral administration of
fucoidan for 12 weeks significantly increased NK cell activity in
male, but not in female volunteers (unpublished data). In order to
certify the finding, we herein examined NK cell activity based on
sex.
Although there are no specific tumor markers used in
cancer screening, some markers can be used to assist in making a
diagnosis and determining a prognosis. They can be used to follow
in cases where the diagnosis is cancer through monitoring of the
disease recurrence and/or evaluating the response to therapy. In
order to elucidate the effect of fucoidan on anti-tumor activity,
the secondary endpoint was changes in tumor markers during the
trial period.
Metabolism of fucoidan for long period of oral
intake was not determined yet. The third endpoint was fucoidan
absorption following its oral administration. These endpoints were
monitored at the outpatient clinic using blood sampling before and
every six months during the ingestion of fucoidan. A urinalysis was
also simultaneously performed.
Blood tests
Blood samples were drawn to assess hematological
markers (white blood cells, neutrophils, lymphocytes, monocytes,
eosinophils, red blood cells, hemoglobin, and platelets); liver
function (albumin, bilirubin, alkaline phosphatase, alanine
aminotransferase, aspartate aminotransferase, γ-glutamyl
transferase); lipids (triglycerides, HDL cholesterol, LDL
cholesterol); renal function tests (blood urea nitrogen,
creatinine); uric acid; electrolytes (Na, K, Cl, Ca); NK cell
activity; immunoglobulin titers; tumor markers; and fucoidan
concentrations. Clinical biochemical and hematological parameters
were assayed using commercially available kits. Albumin was
measured by the modified PCP method (KAINOS Laboratories, Inc.).
Total bilirubin was measured by vanadic acid oxidation method (Wako
Laboratory Chemical Co., Ltd.). Aspartate aminotransferase (AST),
alanine aminotransferase (ALT), alkaline phosphatase (ALP) and
γ-glutamyl transferase (γ-GTP) were measured by the JSCC
transferable method (Kanto Chemical Co., Inc.). HDLchoresterol and
LDL choresterol were measured by selective solubilization method
(Kyowa Medex Co., Ltd.). Triglycerides (TG) was measured by
GK:GP-elimination of free glycerol method (Sekisui MedicalCo.,
Ltd.). Blood urea nitrogen (BUN) was measured by urease-LED method
(Kanto Chemical Co., Inc.). Creatinine (Cr) was measured by
enzymatic method (Sekisui Medicals Co., Ltd.). Uric acid (UA) was
measured by uricase-peroxidase method (KAINOS Laboratories, Inc.).
Sodium (Na) and Potassium (K) were measured by ion selective
electrode method (JOEL Ltd.). Calcium (Ca) was measured by Arsenazo
III method (KAINOS Laboratories, Inc.). Complete blood count was
measured by the automated method (Sysmex Corp.).
NK cell activity: NK cell activity was evaluated in
the k-562 cell line (Dainippon Pharmaceutical Co.) marked with
51Cr using a cytotoxicity test for 3.5 h (17). Blood samples taken from the cubital
vein were collected into heparinized tubes. After the
centrifugation of blood samples with a lymphocyte separation
medium, interface mononuclear cells were collected and suspended at
a cell density of 1×106/ml in RPMI-1640 medium (IBL) and
supplemented with 10% FBS. Peripheral blood monocytes
(2×105 cells) were added to round-bottomed 96-well
microplates containing 51Cr-labeled target cells
(1×104 cells) in 0.2 ml of RPMI-1640 medium supplemented
with 10% FBS. The effector cell/target cell ratio was 20. After
centrifugation at 800 r/min for 5 min using an exclusive centrifuge
for microplates, cells were incubated at 37°C for 3.5 h under 50
ml/l CO2 in air. After the incubation, the culture
supernatant was harvested using PETΣ-96 (Sohken; Tokyo, Japan), and
radioactivity was evaluated using a gamma counter (1272 clinigamma;
Wallac). The percentage of cytotoxicity was calculated as follows:
% cytotoxicity=(experimental 51Cr release-spontaneous
51Cr release)/(maximal 51Cr
release-spontaneous 51Cr release) ×100.
IgG and IgA titers were measured using a
turbidimetric immunoassay, and the IgE titer was assessed using the
FEIA method.
Tumor markers were assayed as follows. Cytokeratin
fragment (Cyfra) 21-1 (18),
α-fetoprotein (AFP) (19),
carcinoembryonic antigen (CEA) (20–22),
cancer antigen (CA) 125 (23), CA
15-3 (22), and soluble IL-II
receptor (sIL2R) (24) were assayed
using the CLEIA method. A protein induced by the absence of vitamin
K (PIVKA)-II (25) and squamous cell
carcinoma-related antigen (SCC-Ag) (26) were assayed using the ECLIA method.
C-reactive protein (CRP) (27) was
assayed using the Latex turbidimetric immunoassay method. Sialyl
Lewis X-i (SLX) (28) and
prostate-specific antigen (PSA) (29) were assayed using RIA and CLIA,
respectively.
Measurements of immunoglobulin titers, tumor
markers, and NK cell activity were entrusted to SRL of a reliable
outsourcing company in Japan.
Serum fucoidan levels were assayed using a sandwich
ELISA method developed by our laboratory (16). Reproducibility of the fucoidan ELISA
method was as follow.
The intra- and inter assay CVs for serum, plasma and
urine, using high and low concentration of fucoidan, were in the
range of 1.5–13.4%.
Statistical analysis
Statistical analyses were performed using SAS
version 9.4 (Statistical Analysis Software 9.4, SAS Institute
Inc.). Mean quantitative values were compared between the basal
value and the value at each time point during the trial period
using the Friedman test. Post hoc comparison was performed
using the Bonferroni method. Statistical correlation was analyzed
using Spearman's rank correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical assessment
No severe adverse effects were observed during the
trial period, and all subjects tolerated the ingestion of
fucoidan.
Primary outcome
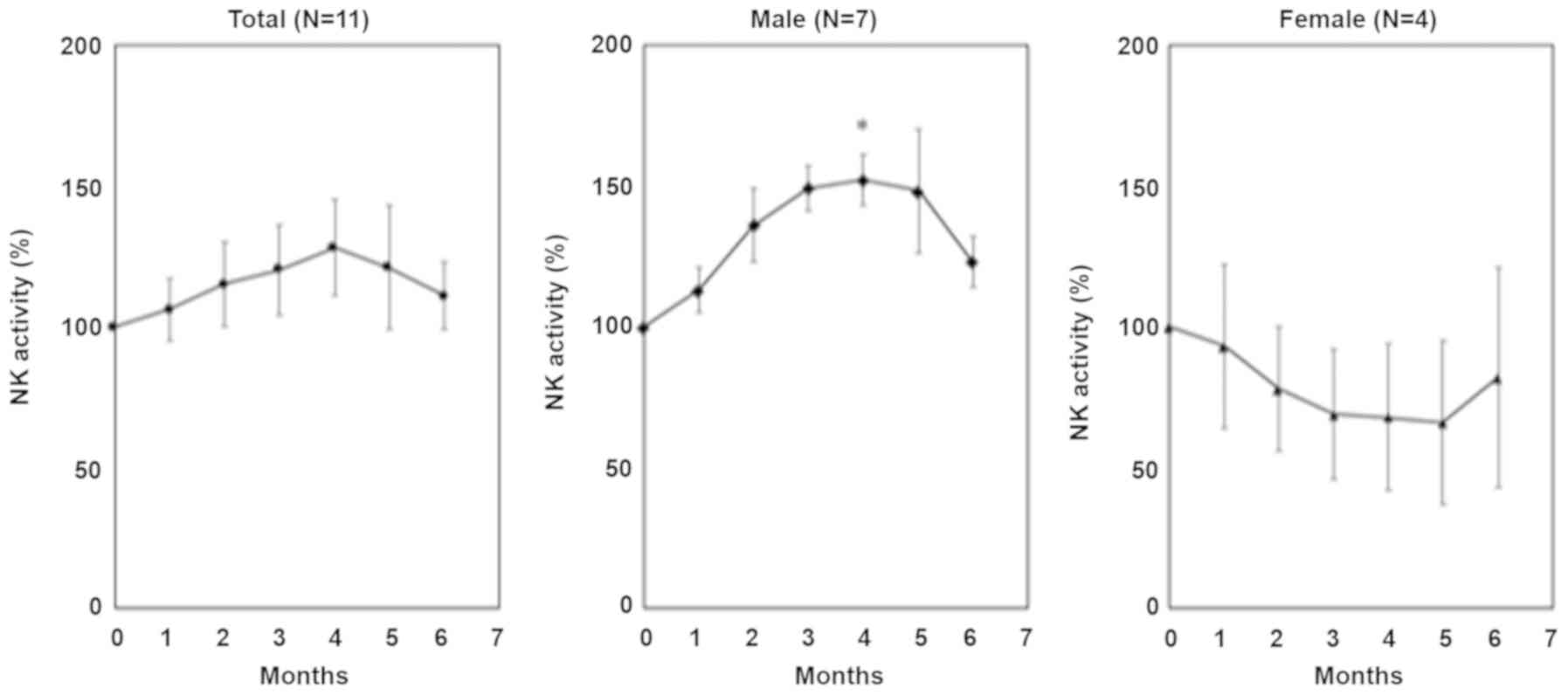
No significant changes were observed in the mean
activities of NK cells in total subject between before and after
the ingestion of fucoidan. An analysis of each sex revealed that NK
cell activity was elevated in seven male subjects, and was
significantly different 4 months after than before the ingestion of
fucoidan; however, changes in NK cell activity were not significant
in four female subjects (Fig.
1).
IgG, IgA, and IgE titers remained unchanged during
the trial period (Table SI).
According to the result of multiple regression
analysis, demographic characteristics of subjects, such as age,
height, and body weight were not a significant factor contributing
to NK cell activity and immunoglobulins titers (data not
shown).
Tumor markers. In Cases 1, 2, 3, 4, 5, 8, and 9, in
whom primary tumors were eradicated by surgical resection or RFA,
tumor markers remained within the reference range before and after
the ingestion of fucoidan. Basal values for tumor markers were
elevated in three cases. In Cases 6 and 11, who were receiving
chemotherapy, tumor markers slightly decreased in the former, but
increased in the latter after the ingestion of fucoidan. Case 7 was
awaiting surgery for prostate cancer. PSA levels gradually
increased during the trial period (Table III).
 | Table III.Changes of tumor markers after the
ingestion of fucoidan. |
Table III.
Changes of tumor markers after the
ingestion of fucoidan.
| Tumor marker
(reference range) | 0 | 1M | 2M | 3M | 4M | 5M | 6M |
|---|
| Male, 67 y.o,
pharyngeal cancer, post-surgical resection |
|
CYFRA21-1 (<5.0 ng/ml) | 1.0< | 1.0< | 1.0< | 1.0< | 1.0< | 1.0< | 1.0< |
| Male, 81 y.o,
hepatocellular carcinoma, post-radiofrequency ablation |
| AFP
(<10 ng/ml) | 2.8 | 3.6 | 3.4 | 3.0 | 3.5 | 2.9 | 2.9 |
|
PIVKA-II (<40 mAU/ml) | 19 | 21 | 16 | 18 | 22 | 23 | 18 |
| Male, 78 y.o,
hepatocellular carcinoma, post-radiofrequency ablation |
| AFP
(<10 ng/ml) | 2.6 | 3.0 | 3.2 | 2.7 | 2.9 | 2.8 | 2.5 |
|
PIVKA-II (<40 mAU/ml) | 25 | 27 | 29 | 28 | 32 | 22 | 27 |
| Male, 70 y.o, renal
cell cancer, post-surgical resection |
| CRP
(<0.3 mg/dl) | 0.03 | 0.03 | 0.02 | 0.06 | 0.05 | 0.05 | 0.20 |
| Male, 71 y.o, colon
cancer, post-surgical resection |
| CEA
(<5.0 ng/ml) | 1.8 | 1.9 | 1.7 | 1.5 | 1.5 | 1.6 | 1.8 |
| Male, 74 y.o, lung
cancer, undergoing chemotherapy |
| CEA
(<5.0 ng/ml) | 38.2 | 39.7 | 42.2 | 36.4 | 32.3 | 25.7 | 28.5 |
| SLX
(<38 U/ml) | 51 | 57 | 69 | 57 | 66 | 55 | 55 |
| Male, 76 y.o,
prostate cancer, pre-surgical operation |
| PSA
(<4.0 ng/ml) | 2.79 | 3.48 | 3.37 | 4.30 | 4.35 | 4.55 | 4.99 |
| Female, 58 y.o,
cervical cancer, post-surgical resection |
| CA125
(<35.0 U/ml) | 9.1 | 8.8 | 9.1 | 8.8 | 8.5 | 9.4 | ne |
| SCC
(<2.5 ng/ml) | 0.9 | 0.7 | 1.1 | 0.8 | 1.0 | 2.2 | ne |
| Female, 52 y.o,
breast cancer, post-surgical resection |
| CEA
(<5.0 ng/ml) | 2.8 | 2.7 | 2.4 | 2.4 | 2.3 | 2.4 | 2.5 |
| CA15-3
(<25.0 U/ml) | 12.5 | 12.5 | 11.6 | 10.7 | 14.2 | 13.1 | 15.8 |
| Female, 56 y.o,
colon cancer, post-surgical resection |
| CEA
(<5.0 ng/ml) | 8.1 | 12.1 | 19.0 | 23.4 | 23.0 | ne | ne |
| Female, 70 y.o,
adult T-cell leukemia, undergoing chemotherapy |
| sIL-2R
(154–510 U/ml) | 4,690 | 10,200 | 13,700 | 9,710 | 10,900 | 12,300 | 11,400 |
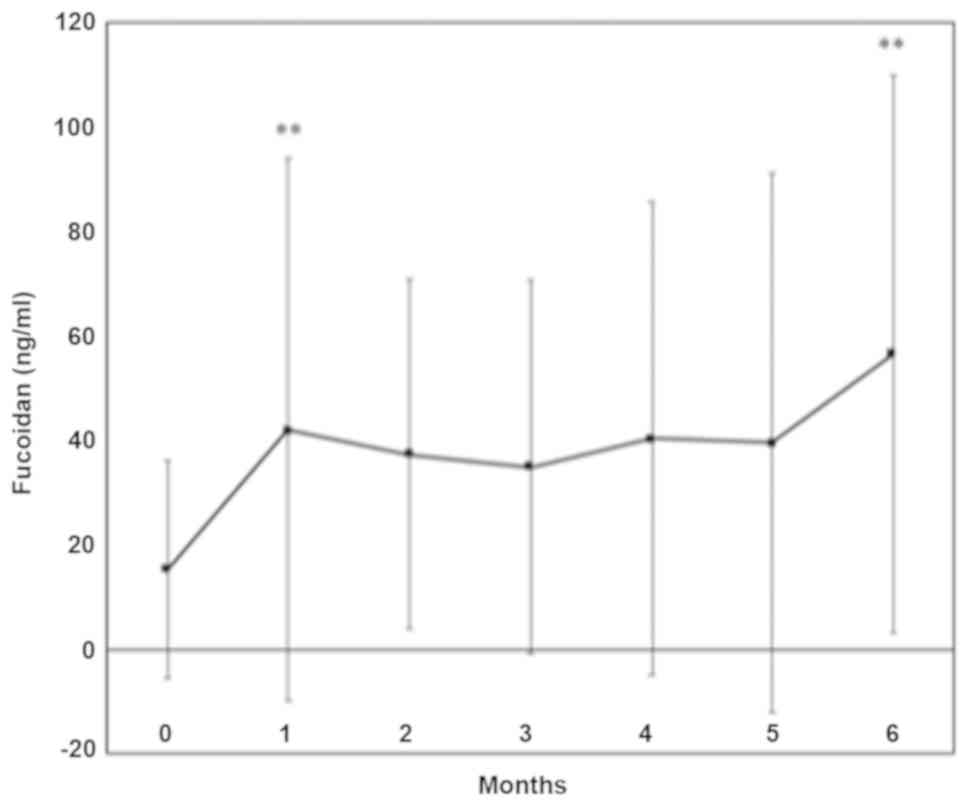
Fucoidan absorption. Mean serum fucoidan levels were
significantly increased one month and six months following the
ingestion of fucoidan; peak levels ranged between 30 and 198 ng/ml.
Fucoidan levels did not show a sex difference (Fig. 2).
Clinical laboratory test. Statistical analysis of
all subjects indicated that no significant changes were observed in
clinical parameters, such as hematology, liver function, renal
function, lipids, electrolytes, and the urinalysis, over the trial
period (Tables SII and SIII).
Abnormal laboratory parameters before treatment were
observed in a few cases. Two patients showed mild elevation of Cr
levels, and abnormal γ-GTP and AST levels were observed in two
patients with alcoholic liver injury. AST and ALT levels were
elevated temporally in a patient with drug induced liver injury;
elevation of AST and ALT levels was within 2 times the upper limit
of normal. Renal injury and liver damage were not so severe as to
affect the drug metabolism during the trial period.
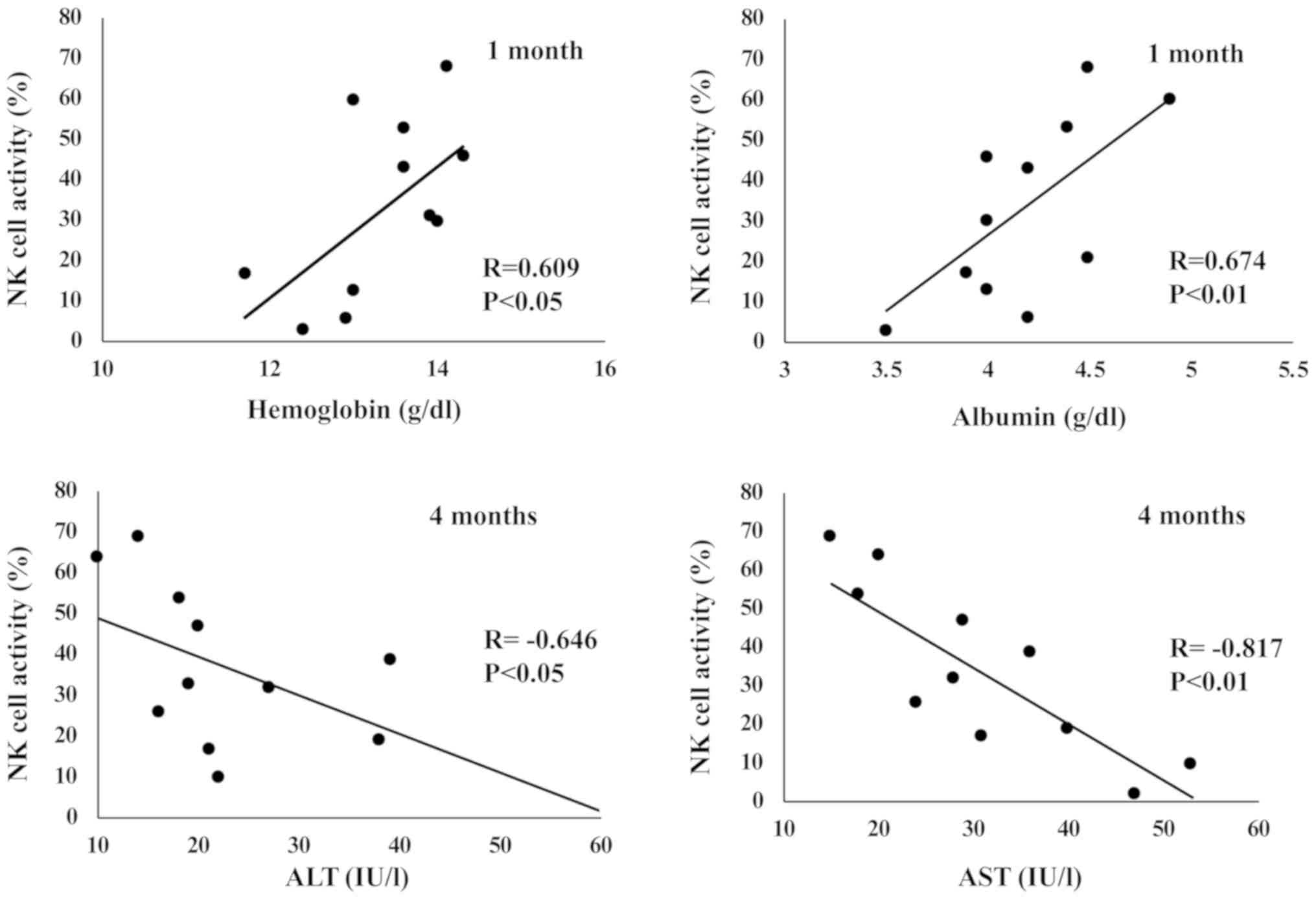
Correlation among NK cell activity, immunoglobulin
titers, and laboratory parameters. NK cell activity positively
correlated with albumin and hemoglobin levels; a significant
correlation was observed 1 month into the trial. NK cell activity
had a significant negative correlation with AST levels 1,3,4, and 5
months after the ingestion of fucoidan. NK cell activity had a
significant negative correlation with ALT levels 4 months into the
trial (Fig. 3).
IgG titers did not correlate with laboratory
parameters. IgA titers negatively correlated with lymphocyte counts
and positively correlated with γ-GTP levels. IgE titers positively
correlated with Cr and γ-GTP levels, and negatively correlated with
lymphocyte counts (data not shown).
Discussion
Numerous in vivo and in vitro studies
on the effects of fucoidan on NK cell activity have supported its
application as an anticancer drug (8,9,30,31).
Regarding in vivo studies on fucoidan, the dose and route of
administration were both shown to affect outcomes. Oral intake is
more convenient than an intravenous or subcutaneous injection for
the administration of fucoidan in clinical settings. Negishi et
al reported that the oral administration of 300 mg of Mekabu
fucoidan for 24 weeks to elderly subjects receiving influenza
vaccines attenuated the aging-related suppression of NK cell
activity (32). Based on these
findings, we examined the effects of fucoidan on immunomodulation
in cancer survivors with a performance status of 0 or 1. The
results obtained demonstrated that fucoidan extracted from C.
okamuranus did not increase NK cell activity in total patients,
but significantly increased in male cancer survivors. The finding
confirmed our unpublished data, in which fucoidan significantly
increased NK cell activity in male but not in female healthy
volunteers. The mechanism of different function of fucoidan
according to sex should be clarified in a future research.
The mechanisms underlying NK cell activation by
fucoidan have not yet been elucidated. Cytokines, such as
interferon (lFN)-α/β, lL-2, lL-12, and lL-15, activate NK cells and
produce lFN-γ (33). Since these
cytokines were not examined in the present study, further studies
are warranted to investigate the role of fucoidan on the induction
of NK cell activity by cytokines.
Interestingly, the present study showed that NK cell
activity negatively correlated with AST and ALT titers. The
percentage of NK cells among liver cells is five times as high as
the percentages among spleen or peripheral blood, suggesting that
NK cells may play an important role in the immune function of the
liver. Accumulating evidences were found that the NK cells were
modulated by liver diseases and were further involved in the
pathogenesis of liver injury and inflammation (34). Fucoidan was shown to benefit alcohol
and non-alcoholic liver disease (3).
Taken together with the present and previous studies, fucoidan may
activate NK cells via improvement of liver damage through
unexplained mechanism.
We evaluated changes in tumor markers caused by the
ingestion of fucoidan. In Cases 1, 2, 3, 4, 5, 8, and 9, in whom
primary tumors were eradicated by surgery or RFA, tumor markers
remained within the reference range before and after the ingestion
of fucoidan. These patients increased fucoidan levels and NK cell
activity after the ingestion of fucoidan. It is difficult to
clarify whether this effect is related to medical intervention or
fucoidan administration. Noteworthy, there was no recurrence of the
primary tumors in these cases during the trial period. Long-term
surveillance in large-scale and well-organized studies is needed to
clarify whether fucoidan alone is able to reduce their risk of
recurrence via NK cell activation in male cancer survivors after
conventional chemotherapy, radiofrequency ablation or
radiotherapy.
Regarding the two cases undergoing conventional
chemotherapy, tumor markers were continuously elevated in Case 11
but decreased in Case 6 following the administration of fucoidan.
Case 6 taking erlotinib revealed elevation of fucoidan levels and
NK cell activity following the ingestion of fucoidan. Case 11
taking irinotecan showed elevation of fucoidan levels and decrease
of NK cell activity. It is important to assess the effect of
chemotherapeutic regimens on circulating levels of fucoidan and NK
cells activity. In addition, the ability of fucoidan as an adjunct
therapy during conventional chemotherapy has been reported by a few
investigators (12,13); therefore, the advantage of fucoidan
as a supplemental therapy in the management of cancer patients is
needed to be certified in the future.
Given that subjects were heterogeneous, and heavily
treated, it is necessary to assess how the disease itself is
affecting circulating levels of fucoidan and therefore levels of NK
cells and immunoglobulins. Since fucoidan levels were similar in
the recovered cancer patients and the non-recovered cancer
patients, the disease itself is unlikely affecting the fucoidan
absorption in cancer survivors with performance status 0 or 1.
Whereas, fucoidan enhanced NK activity in the recovered cancer
patients, but it did not work in the non-recovered cancer patient.
It is a further study whether the disease itself affect the
fucoidan metabolism in cancer survivors.
Mean serum fucoidan levels were significantly higher
one month and six months after than before the ingestion of
fucoidan, even though the intestinal absorption of fucoidan was
very low, peaking at 198 ng/ml, in serum. The serum profiles after
fucoidan administration varied among patients. This result was
consistent with our previous findings (16,35).
Since subjects continued with the same medicine(s) as before, the
potential pharmacokinetic interactions between fucoidan and
medicines should be clarified in the future. Mild liver damage was
observed in three subjects and mild renal injury was complicated
with two subjects. Since these patients showed similar fucoidan
levels compared with those without liver and renal injury, the
fucoidan metabolism is unlikely to be influenced by mild liver and
renal injury.
The oral route is essential for the administration
of fucoidan in clinical studies. The maximum dose used heretofore
was 6 g of fucoidan for patients with human t-lymphotropic virus
type-1-associated neurological diseases. A previous study reported
that four out of 17 subjects developed diarrhea within 1 month of
trial initiation (36). Other
patients with advanced cancer received 4 g of fucoidan without
adverse effects for six months (12). The present results indicated that
since the intestinal absorption of fucoidan was so low, it may be
difficult to reach the serum levels needed to directly inhibit
cancer cell growth or metastasis in clinical trials with safety
dose of fucoidan for cancer survivors. Therefore, further studies
are needed to establish the effective serum concentration of
fucoidan needed for NK cell activation, tumor inhibition, and
anti-angiogenesis activity.
Our preliminary study had some limitations. The
number of subjects was small in the present study, with only four
females being enrolled. This may have affected the statistical
analysis; therefore, further studies are needed to confirm the
potential effects of fucoidan on NK cell activity using a large
number of subjects. The proinflammatory cytokines, including
interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α)
were reported to be reduced after fucoidan ingestion (13). Since cancer and inflammatory
responses are closely associated, we need to assess the
relationship between inflammatory markers and levels of fucoidan
and NK cells/immunoglobulins. Lack of measurement of inflammation
markers was the limitations of the present study. Subjects took a
meal without dietary restriction during the trial period. Since
this parameter may affect circulating levels of fucoidan, it may be
a limitation of the present study.
The oral administration of 3 g fucoidan was safe for
and tolerated well by cancer survivors. The present results showed
that fucoidan extracted from C. Okamuranus may enhance the
activation of NK cells in male cancer survivors. No recurrence of
primary tumors was shown in cancer survivors, in whom primary tumor
was eradicated by treatment.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Assistant Professor
Kenji Nakamura (Takasaki University of Health and Welfare) for his
helpful advice on the statistical analysis. In addition, the
authors thank Dr. Kazuhiro Ono (Medical doctor of Ono Clinic), Dr.
Sanae Takeichi (Medical doctor of Takeichi clinic) and Dr. Hiroto
Hoshi (Medical Doctor of Hoshi clinic) for their contributions to
clinical management of the participants.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TN designed the study, contributed to the analysis
and interpretation of data, and wrote the initial draft of the
manuscript. KK, MT, and MI contributed to the collection and
assembly of data. KN contributed to the analysis and interpretation
of data and assisted in the preparation of the manuscript. All
authors critically reviewed the manuscript and approved the final
version.
Ethics approval and consent to
participate
The Ethics Committee of South Product Co., Ltd.
approved the present study (approval no. 16-01). Following an
explanation of the study and its aim, written informed consent was
obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cumashi A, Ushakova NA, Preobrazhenskaya
ME, D'Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE,
Berman AE, Bilan MI, et al: A comparative study of the
anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive
activities of nine different fucoidans from brown seaweeds.
Glycobiology. 17:541–552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li B, Lu F, Wei X and Zhao R: Fucoidan:
Structure and bioactivity. Molecules. 13:1671–1695. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fitton JH, Stringer DN and Karpiniec SS:
Therapies from fucoidan: An update. Mar Drugs. 13:5920–5946. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Senthilkumar K, Manivasagan P, Venkatesan
J and Kim SK: Brown seaweed fucoidan: Biological activity and
apoptosis, growth signaling mechanism in cancer. Int J Biol
Macromol. 60:366–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwak JY: Fucoidan as a marine anticancer
agent in preclinical development. Mar Drugs. 12:851–870. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang P, Liu Z, Liu X, Teng H, Zhang C, Hou
L and Zou X: Anti-metastasis effect of fucoidan from Undaria
pinnatifida sporophylls in mouse hepatocarcinoma Hca-F cells. PLoS
One. 9:e1060712014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu L, Sun J, Su X, Yu Q, Yu Q and Zhang P:
A review about the development of fucoidan in antitumor activity:
Progress and challenges. Carbohydr Polym. 154:96–111. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atashrazm F, Lowenthal RM, Woods GM,
Holloway AF, Karpiniec SS and Dickinson JL: Fucoidan suppresses the
growth of human acute promyelocytic leukemia cells in vitro and in
vivo. J Cell Physiol. 231:688–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Oda T, Yu Q and Jin JO: Fucoidan
from Macrocystis pyrifera has powerful immune-modulatory effects
compared to three other fucoidans. Mar Drugs. 13:1084–1104. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Centers for Disease Control and Prevention
(CDC), . Cancer survivors-United States, 2007. MMWR Morb Mortal
Wkly Rep. 60:269–272. 2011.PubMed/NCBI
|
|
11
|
Rock CR, Doyle C, Demark-Wahnefried W,
Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK,
Grant B, McCullough M, et al: Nutrition and physical activity
guidelines for cancer survivors. CA Cancer J Clin. 62:243–274.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikeguchi M, Yamamoto M, Arai Y, Maeta Y,
Ashida K, Katano K, Miki Y and Kimura T: Fucoidan reduces the
toxicities of chemotherapy for patients with unresectable advanced
or recurrent colorectal cancer. Oncol Lett. 2:319–322. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi H, Kawaguchi M, Kitamura K,
Narumiya S, Kawamura M, Tengan I, Nishimoto S, Hanamure Y, Majima
Y, Tsubura S, et al: An exploratory study on the anti-inflammatory
effects of fucoidan in relation to quality of life in advanced
cancer patients. Integr Cancer Ther. 17:282–291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Whiteside TL and Herberman RB: The role of
natural killer cells in human disease. Clin Immunol Immunopathol.
53:1–23. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imai K, Matsuyama S, Miyake S, Suga K and
Nakachi K: Natural cytotoxic activity of peripheral-blood
lymphocytes and cancer incidence: An 11-year follow-up study of a
general population. Lancet. 356:1795–1799. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tokita Y, Nakajima K, Mochida H, Iha M and
Nagamine T: Development of a fucoidan-specific antibody and
measurement of fucoidan in serum and urine by sandwich ELISA.
Biosci Biotechnol Biochem. 74:350–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oshimi K, Oshimi Y, Satake M and Mizoguchi
H: Natural killer-mediated lysis of normal and malignant target
cells, and its regulation by monocytes. J Exp Med. 162:472–486.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alkotyfan K, Wiegand S, Müller HH,
Windfuhr JP, Werner JA and Sesterhenn AM: Cyfra 21-1 as a tumor
marker for follow-up of patients with squamous cell carcinoma of
the oropharynx. Anticancer Res. 30:2291–2296. 2010.PubMed/NCBI
|
|
19
|
Aoyagi Y: Carbohydrate-based measurements
on alpha-fetoprotein in the early diagnosis of hepatocellular
carcinoma. Glycoconj J. 12:194–199. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Das V, Kalita J and Pal M: Predictive and
prognostic biomarkers in colorectal cancer: A systematic review of
recent advances and challenges. Biomed Pharmacother. 87:8–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Triphuridet N, Vidhyarkorn S,
Worakitsitisatorn A, Sricharunrat T, Teerayathanakul N, Auewarakul
C, Chungklay N, Krongthong W, Luengingkasoot S, Sornsamdang G, et
al: Screening values of carcinoembryonic antigen and cytokeratin 19
fragment for lung cancer in combination with low-dose computed
tomography in high-risk populations: Initial and 2-year screening
outcomes. Lung Cancer. 122:243–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Dai D, Chen B, Tang H, Xie X and Wei
W: Clinicopathological and prognostic significance of cancer
antigen 15-3 and carcinoembryonic antigen in breast cancer: A
meta-analysis including 12,993 patients. Dis Markers.
2018:98630922018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laengsri V, Kerdpin U, Plabplueng C,
Treeratanapiboon L and Nuchnoi P: Cervical cancer markers:
Epigenetics and microRNAs. Lab Med. 49:97–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Umekita K, Hashiba Y, Kariya Y, Kubo K,
Miyauchi S, Aizawa A, Umeki K, Nomura H, Kawaguchi T, Matsuda M, et
al: The time-sequential changes of risk factors for adult T-cell
leukemia development in human T-cell leukemia virus-positive
patients with rheumatoid arthritis: A retrospective cohort study.
Mod Rheumatol. 29:795–801. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia J, Song P, Sun Z, Sawakami T, Jia M
and Wang Z: Advances of diagnostic and mechanistic studies of
γ-glutamyl transpeptidase in hepatocellular carcinoma. Drug Discov
Ther. 10:181–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Charakorn C, Thadanipon K, Chaijindaratana
S, Rattanasiri S, Numthavaj P and Thakkinstian A: The association
between serum squamous cell carcinoma antigen and recurrence and
survival of patients with cervical squamous cell carcinoma: A
systematic review and meta-analysis. Gynecol Oncol. 150:190–200.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teishima J, Ohara S, Shinmei S, Inoue S,
Hayashi T, Mochizuki H, Mita K, Shigeta M and Matsubara A:
Normalization of C-reactive protein levels following cytoreductive
nephrectomy in patients with metastatic renal cell carcinoma
treated with tyrosine kinase inhibitors is associated with improved
overall survival. Urol Oncol. 36:339.e9–339.e15. 2018. View Article : Google Scholar
|
|
28
|
Mizuguchi S, Nishiyama N, Iwata T, Nishida
T, Izumi N, Tsukioka T, Inoue K, Uenishi T, Wakasa K and Suehiro S:
Serum sialyl Lewis × and cytokeratin 19 fragment as predictive
factors for recurrence in patients with stage I non-small cell lung
cancer. Lung Cancer. 58:369–375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stephan C, Jung K, Lein M, Sinha P,
Schnorr D and Loening SA: Molecular forms of prostate-specific
antigen and human kallikrein 2 as promising tools for early
diagnosis of prostate cancer. Cancer Epidemiol Biomarkers Prev.
9:1133–1147. 2000.PubMed/NCBI
|
|
30
|
Ale MT, Maruyama H, Tamauchi H, Mikkelsen
JD and Meyer AS: Fucoidan from Sargassum sp. and fucus vesiculosus
reduces cell viability of lung carcinoma and melanoma cells in
vitro and activates natural killer cells in mice in vivo. Int J
Biol Macromol. 49:331–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azuma K, Ishihara T, Nakamoto H, Amaha T,
Osaki T, Tsuka T, Imagawa T, Minami S, Takashima O, Ifuku S, et al:
Effects of oral administration of fucoidan extracted from
Cladosiphon okamuranus on tumor growth and survival time in
a tumor-bearing mouse model. Mar Drugs. 10:2337–2348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Negishi H, Mori M, Mori H and Yamori Y:
Supplementation of elderly Japanese men and women with fucoidan
from seaweed increases immune responses to seasonal influenza
vaccination. J Nutr. 143:1794–1798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fehniger TA, Shah MH, Turner MJ, VanDeusen
JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F and
Caligiuri MA: Differential cytokine and chemokine gene expression
by human NK cells following activation with IL-18 or IL-15 in
combination with IL-12: Implications for the innate immune
response. J Immunol. 162:4511–4520. 1999.PubMed/NCBI
|
|
34
|
Liu P, Chen L and Zhang H: Natural killer
cells in liver disease and hepatocellular carcinoma and the NK
cell-based immunotherapy. J Immunol Res. 2018:12067372018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagamine T, Hayakawa K, Nakazato K and Iha
M: Determination of the active transport of fucoidan derived from
Okinawa Mozuku across the human intestinal Caco-2 cells as assessed
by size-exclusion chromatography. J Chromatogr B Analyt Technol
Biomed Life Sci. 997:187–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Araya N, Takahashi K, Sato T, Nakamura T,
Sawa C, Hasegawa D, Ando H, Aratani S, Yagishita N, Fujii R, et al:
Fucoidan therapy decreases the proviral load in patients with human
T-lymphotropic virus type-1-associated neurological disease.
Antivir Ther. 16:89–98. 2011. View
Article : Google Scholar : PubMed/NCBI
|