Introduction
Insulinomas are rare neuroendocrine tumors with an
incidence of three to ten cases per million per year (1). Approximately 90% of insulinomas are
benign and the remaining 10% are malignant, with lymph node or
liver metastases often present at the time of diagnosis (2). Hypoglycemic symptoms are the usual
manifestations of these tumors, although patients can be
misdiagnosed as having cognitive, neurologic and psychiatric
disorders. Insulinomas are even rarer in patients with diabetes.
There are no formal epidemiologic studies of the incidence of the
condition in this population, and only a few cases have been
reported in the literature (3). The
diagnosis is challenging, as many of those patients never develop
hypoglycemic symptoms or do so only when the disease is advanced.
In addition, hypoglycemic episodes of non-endogenous etiology are
very common in diabetic patients.
In this report, we present a female patient with
type 2 diabetes mellitus (T2DM) who was finally diagnosed with a
large insulinoma after being investigated for an unexpected ‘cure’
of her diabetes.
Case report
A 62-year-old obese, diabetic female diagnosed with
T2DM 15 years earlier and treated with metformin and vildagliptin
since then suddenly developed episodes of hypoglycemia. She
reported confusion, lack of consciousness, blurred vision and
weakness. The symptoms were unrelated to meals. Although metformin
and vildagliptin do not typically cause hypoglycemia, they were
discontinued. Her blood glucose levels remained normal despite the
cessation of antidiabetic medication and milder hypoglycemic
episodes continued. The patient reported no weight loss [body mass
index (BMI) = 43], no change in her dietary habits, apart from
developing an increased appetite for sweeteners, and no alteration
in physical activity.
Due to the persistence of her symptoms, the patient
underwent a supervised 72 h fasting test. She developed symptoms of
hypoglycemia within 58 h with serum glucose concentration at 34
mg/dl, insulin plasma concentration at 10 µΙU/ml and C-peptide
concentration of 5.7 ng/ml. These values were over our
institution's threshold (>6 µΙU/ml for plasma insulin, >0.6
ng/ml for C-peptide and <45 mg/dl for glucose) and established
the biochemical diagnosis of endogenous hyperinsulinemia.
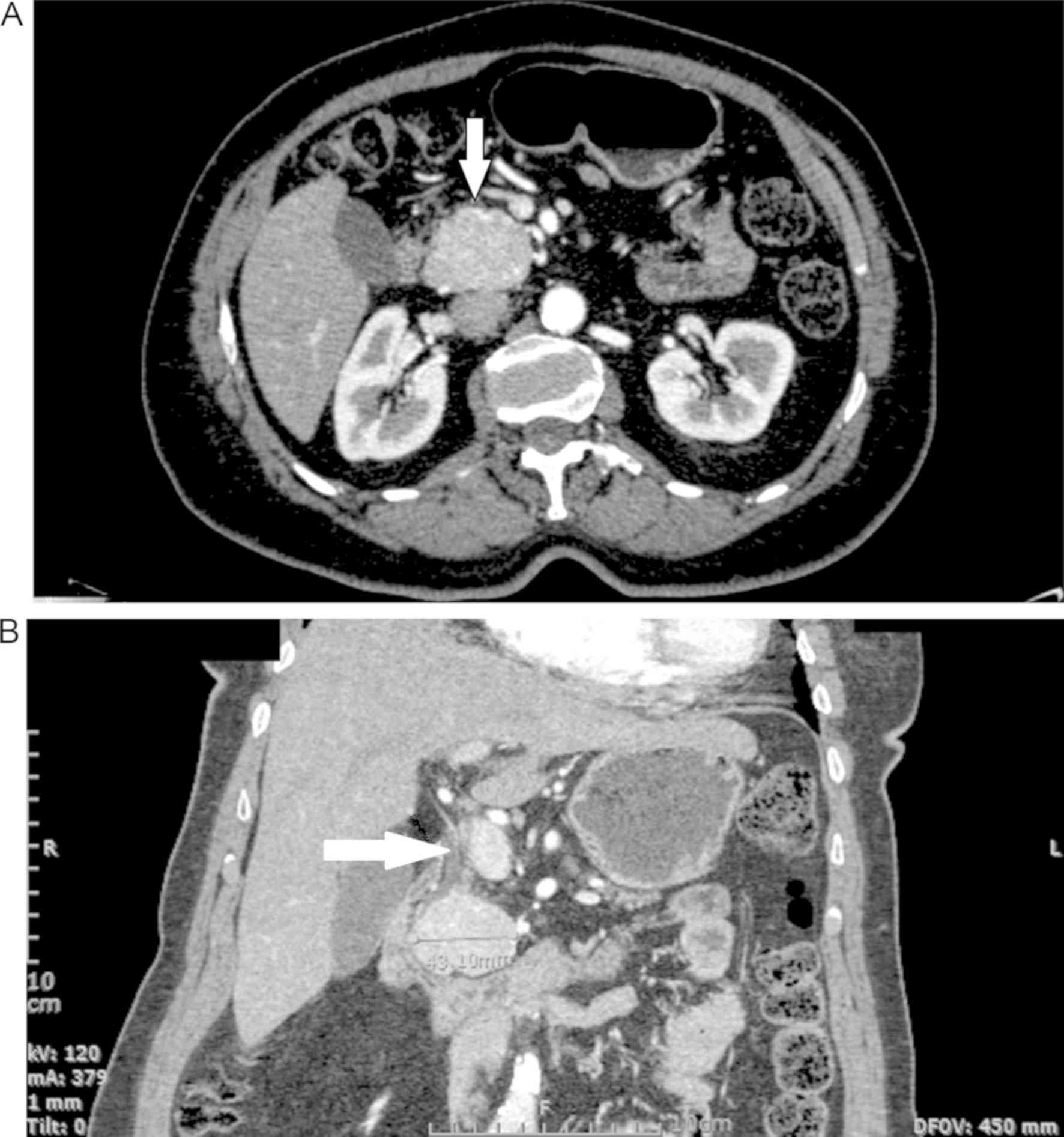
Moreover, a pancreatic computerized tomography (CT)
scan with significant contrast enhancement during the arterial
phase and without distention of the pancreatic duct revealed a
round, solid 4.8x3.6 cm lesion, with a few calcifications in the
head of the pancreas (Fig. 1A).
These findings were compatible with the diagnosis of a
neuroendocrine tumor of the pancreas. No other lesions were found
in the liver, thus excluding metastases. Indium-111 radiolabeled
octreotide scintigraphy was also performed and showed an area of
significant avidity in the pancreas. Brain CT was negative for
pituitary pathology, and calcium levels were normal. Serum CA 19-9
levels were within normal range, and serum insulin antibodies were
negative.
Exploratory laparotomy was negative for metastatic
disease, and multiple lesions were assessed with intraoperative
ultrasound. An enlarged hepatoduodenal lymph node was sent for
frozen section and was negative for malignancy. The tumor was in
contact with both the intra-pancreatic bile duct and the pancreatic
duct, so tumor enucleation was not possible (Fig. 1B). With the tumor larger than 2 cm in
diameter, malignancy could not be excluded, therefore the patient
underwent a pylorus-preserving pancreatoduodenectomy. As the
pancreatic duct was not distended and the pancreatic parenchyma was
very soft a jejunopancreatic anastomosis did not take place and the
remaining pancreas was ligated.
Pathology report confirmed the diagnosis of a grade
I insulinoma (4.5x4x3.5 cm) with low mitotic index and a Ki67
proliferation index of approximately 1%, positive for chromogranin
and synaptophysin, negative for CK19 and PgR and without
surrounding tissue or lymph node infiltration.
Postoperatively, serum glucose levels increased
significantly ranging from 162 to 210 mg/dl, and normoglycemia was
achieved with continuous intravenous insulin infusion. This was
discontinued on the seventh postoperative day when oral intake was
tolerated. The patient restarted her antidiabetic medication and
was prescribed oral pancreatin. She was discharged on the
fourteenth postoperative day. She continued with oral antidiabetics
to control blood glucose levels and did not report any symptoms
related to hypoglycemia at her one-year follow-up appointment. At
that follow-up, she presented with a significant weight loss and a
decrease in her BMI to 30.2. This was attributed mainly to the
pancreatoduodenectomy procedure and to the fact that the pancreatic
remnant was not anastomosed to the intestine. This led to exocrine
pancreatic insufficiency despite the pancreatic enzymes which were
prescribed to the patient. Serum glucose was 148 mg/dl in
accordance with here T2DM and c-peptide levels were 1.7 ng/ml.
Discussion
Insulinomas are rare pancreatic neuroendocrine
tumors; symptoms include hypoglycemia. Diagnosis can be challenging
as typical neuroglycopenic symptoms and hypoglycemia can be
mistaken for other pathology. Of additional interest in the case
described above, our patient experienced an unexpected resolution
of her diabetes.
There are very few reports in the literature of
insulinoma in diabetics. Insulinomas do not always produce
sufficient amounts of insulin to result in symptomatic hypoglycemia
(4). Moreover, patients with T2DM
have insulin resistance, which may explain the lack of typical
hypoglycemic symptoms. Obesity, conversely, increases insulin
resistance as part of the metabolic syndrome, and hyperinsulinism
causes weight gain which in turn increases insulin resistance.
Insulinomas can easily be missed in these patients as the reduced
blood glucose can be attributed to other factors such as excessive
exogenous insulin, antidiabetic medication, strenuous exercise,
fasting and inadequate diet. Insulinoma in type 1 diabetic patients
have been described even more rarely (5).
With respect to insulinomas tumorigenesis, the
molecular pathogenetic mechanisms include alterations in cell
physiology resulting from the accumulation of genetic or epigenetic
alterations. These alterations lead to uncontrolled cellular growth
and evasion of programmed cellular death (apoptosis), unlimited
replication potential, angiogenesis and possible tissue invasion
and metastases (6). The primary
mechanisms identified in insulinoma oncogenesis involve impaired
menin molecular interactions (including cyclin-dependent kinase
[CDK] inhibitor regulation such as p27 and p18), cell cycle
deregulation through growth signals (overexpression of cyclin-D1
and Akt1 gene), insensitivity to anti-growth signals (inactivation
of retinoblastoma protein gene-pRb and phosphatase with tensin
homology gene-PTEN), resistance to apoptosis (deregulated
expression of c-Myc, surviving and Bcl-2 gene), unrestricted
proliferation potential, angiogenesis and tissue invasion (6). More specifically, over-activation of
the phosphorylated mechanistic target of the rapamycin (p-mTOR)
signaling pathway has been found in insulinomas, resulting in tumor
formation commonly due to mutations of tumor suppressor PTEN gene
and mutations in band 11q13 (Menin gene) (7,8). K-ras
mutations have been linked with malignancy (9). CYR61 has also recently been found to be
an oncogenic factor (10).
This clinical case and all other similar case
reports in the literature pose clinical questions that cannot
easily be addressed. Can diabetes be a disorder that can
chronically stimulate β cells of the pancreas and force them to
proliferate into the formation of tumors? It was initially thought
that pancreatic endocrine tumors were of hyperplastic origin rather
than of primarily neoplastic origin (11). Based on this, it could be speculated
that insulin resistance or chronic treatment with sulfonylureas may
induce β cell hyperplasia and/or over-activation and result in
tumor formation (12). Functional β
cell hyperplasia has been shown in chronic hyperglycemia or insulin
resistance when glucose levels are normal (13). β cell hyperplasia has also been
reported after bariatric surgical procedures and is usually related
to dumping syndrome (14). Obesity
has been proven to cause functional β cell hyperplasia due to
insulin resistance. In the same context, high lipid diet and a diet
high in free fatty acids (FFA) may account for the compensatory
upregulation of β cell function in response to insulin resistance
(15). Conversely, long-term
exposure to FFA suppresses glucose-stimulated insulin secretion and
has been suggested to result in impaired glucose metabolism,
reduced insulin biosynthesis and β cell loss (16). However, the progression from β cell
hyperplasia to insulinoma formation has been proven only in
experimental models, not in human studies (17). More specifically, a multistep
developmental sequence of insulinoma has been postulated in a
Moloney murine sarcoma virus-simian virus-40 (MSV-SV40) large T
transgenic mouse model. The sequence has been described as a
transformation from nesidioblastosis to islet cell hyperplasia and
dysplasia and finally to tumor formation (18). Conversely, patients with a reduction
in the number of β cells (as is the case in autoimmune type 1
diabetes and non-obese type 2 diabetes) could be thought to have a
negative predisposition for developing insulinoma. A correlation,
either positive or negative, between insulinomas and
insulin-dependent diabetes has not been proven (19).
There are no reports of increased incidence of
insulinoma in patients with diabetes compared to non-diabetics, as
there are millions of diabetics worldwide and only a few cases of
insulinoma in diabetics reported to date. However, our review of
the literature interestingly revealed that reported cases
insulinoma in patients with diabetes are rather more advanced when
diagnosed. Therefore, it can be speculated that these tumors are
underdiagnosed in patients with diabetes. Current literature
suggests that insulinomas are malignant in 10% of cases (3). We found over 50 cases of insulinoma
presenting in those with diabetes. Of these, five presented in
patients with type 1 (5,20-22),
and the remaining cases were diagnosed in patients with T2DM. In
contrast to the malignancy rate in other patients, the incidence of
malignancy in these reported cases was at least 25% (3,5,20,22-32).
Additionally, although it is reported that 90% of insulinoma are
typically less than 2 cm in diameter at diagnosis in other patients
(3), among the cases describing
insulinoma in diabetics, tumor size was >2 cm in a significant
proportion of patients (3,12,20,21,23,33-36).
Although this could be based on publication bias, this may be
explained by the fact that insulinoma diagnosis is difficult in
these patients and only those with more overt disease present with
symptoms and receive a definitive diagnosis.
One could also argue that, in diabetic patients,
endogenous hyperinsulinemia could be beneficial, especially in the
absence of severe hypoglycemic symptoms. This would challenge the
mainstream surgical therapy of insulinoma in this patient group.
However, the surgical removal of insulinomas could be justified for
three reasons. First, it is not possible to preoperatively
determine whether an insulinoma is benign or malignant. Second,
insulinomas do not secrete insulin in a physiologic pattern defined
by blood glucose levels and therefore patients cannot achieve
predictable glucose homeostasis (37). Third, high insulin levels have been
associated with an increase in plasminogen activator inhibitor
type-1 (PAI-1). This has been implicated as a determinant of
atherogenesis and particularly of plaques prone to rupture,
precipitating acute coronary syndromes (38).
In conclusion, insulinomas are rarely reported in
patients with diabetes mellitus, possibly due to the masking of the
disease by insulin resistance and the rare occurrence of episodes
of hypoglycemia. Clinical suspicion is very important in order to
make the diagnosis in these patients. Although diabetes mellitus
appears to be resolved in these patients when they develop
insulinomas, surgical management is still the mainstream
therapeutic option.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CN, AV and ND conceived and designed the study. DG
and NKl performed the literature data collection and acquired the
patient data and follow-up information. MM, DD, NKl and NKa
analyzed the bibliographic data and built the database with the
patient's characteristics from all of the reported cases in the
literature. DG, NKa and ND wrote the article. CN, AV, NKa and ND
critically revised the article. All authors have read and approved
the final version of this manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient.
Patient consent for publication
The patient gave written informed consent for the
publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dadan J, Wojskowicz P and Wojskowicz A:
Neuroendocrine tumors of the pancreas. Wiad Lek. 61:43–47.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hirshberg B, Cochran C, Skarulis MC,
Libutti SK, Alexander HR, Wood BJ, Chang R, Kleiner DE and Gorden
P: Malignant insulinoma: Spectrum of unusual clinical features.
Cancer. 104:264–272. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kamocki ZK, Wodynska NA and Pryczynicz A:
Co-existence of insulinoma and diabetes: A case report. Oncol Lett.
8:1697–1700. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Doherty GM, Doppman JL, Shawker TH, Miller
DL, Eastman RC, Gorden P and Norton JA: Results of a prospective
strategy to diagnose, localize, and resect insulinomas. Surgery.
110:989–997. 1991.PubMed/NCBI
|
|
5
|
Lablanche S, Chobert-Bakouline M, Risse O,
Laverriere MH, Chabre O and Benhamou PY: Malignant insulinoma may
arise during the course of type 1 diabetes mellitus: A case report.
Diabetes Metab. 41:258–261. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jonkers YM, Ramaekers FC and Speel EJ:
Molecular alterations during insulinoma tumorigenesis. Biochim
Biophys Acta. 1775:313–332. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhan HX, Cong L, Zhao YP, Zhang TP, Chen
G, Zhou L and Guo JC: Activated mTOR/P70S6K signaling pathway is
involved in insulinoma tumorigenesis. J Surg Oncol. 106:972–980.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bhatti TR, Ganapathy K, Huppmann AR,
Conlin L, Boodhansingh KE, MacMullen C, Becker S, Ernst LM, Adzick
NS, Ruchelli ED, et al: Histologic and molecular profile of
pediatric insulinomas: Evidence of a paternal Parent-of-Origin
effect. J Clin Endocrinol Metab. 101:914–922. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pavelic K, Hrascan R, Kapitanovic S,
Vranes Z, Cabrijan T, Spaventi S, Korsic M, Krizanac S, Li YQ,
Stambrook P, et al: Molecular genetics of malignant insulinoma.
Anticancer Res. 16:1707–1717. 1996.PubMed/NCBI
|
|
10
|
Huang YT, Lan Q, Ponsonnet L, Blanquet M,
Christofori G, Zaric J and Rüegg C: The matricellular protein CYR61
interferes with normal pancreatic islets architecture and promotes
pancreatic neuroendocrine tumor progression. Oncotarget.
7:1663–1674. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Heitz PU, Kasper M, Polak JM and Klöppel
G: Pancreatic endocrine tumors. Hum Pathol. 13:263–271.
1982.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sakurai A, Aizawa T, Katakura M, Sato Y,
Kaneko G, Yoshizawa K and Hashizume K: Insulinoma in a patient with
non-insulin-dependent diabetes mellitus. Endocr J. 44:473–477.
1997.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Weir GC and Bonner-Weir S: Islet β cell
mass in diabetes and how it relates to function, birth, and death.
Ann N Y Acad Sci. 1281:92–105. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ouyang D, Dhall D and Yu R: Pathologic
pancreatic endocrine cell hyperplasia. World J Gastroenterol.
17:137–143. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stefanovski D, Richey JM, Woolcott O,
Lottati M, Zheng D, Harrison LN, Ionut V, Kim SP, Hsu I and Bergman
RN: Consistency of the disposition index in the face of diet
induced insulin resistance: Potential role of FFA. PLoS One.
6(e18134)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cerf ME: Beta cell dysfunction and insulin
resistance. Front Endocrinol (Lausanne). 4(37)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zumkeller W: Nesidioblastosis. Endocr
Relat Cancer. 6:421–428. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gotz W, Schucht C, Roth J, Theuring F and
Herken R: Endocrine pancreatic tumors in MSV-SV40 large T
transgenic mice. Am J Pathol. 142:1493–1503. 1993.PubMed/NCBI
|
|
19
|
Weir GC: Non-insulin-dependent diabetes
mellitus: Interplay between B-cell inadequacy and insulin
resistance. Am J Med. 73:461–464. 1982.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gjelberg HK, Hoem D, Verbeke CS, Eide J,
Cooper JG and Molven A: Hypoglycemia and decreased insulin
requirement caused by malignant insulinoma in a type 1 diabetic
patient: When the hoof beats are from a zebra, not a horse. Clin
Case Rep. 5:761–768. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Oikawa Y, Katsuki T, Kawasaki M,
Hashiguchi A, Mukai K, Handa K, Tomita M, Kabeya Y, Asai Y, Iwase
K, et al: Insulinoma may mask the existence of type 1 diabetes.
Diabet Med. 29:e138–e141. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Svartberg J, Stridsberg M, Wilander E,
Andersson DE and Eriksson B: Tumour-induced hypoglycaemia in a
patient with insulin-dependent diabetes mellitus. J Intern Med.
239:181–185. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abbasakoor NO, Healy ML, O'Shea D, Maguire
D, Muldoon C, Sheahan K and O'Toole D: Metastatic insulinoma in a
patient with type 2 diabetes mellitus: Case report and review of
the literature. Int J Endocrinol. 2011(124078)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ademoglu E, Unluturk U, Agbaht K, Karabork
A and Corapcioglu D: Type 2 diabetes mellitus in a patient with
malignant insulinoma manifesting following surgery. Diabet Med.
29:e133–e137. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Atkinson AB, Buchanan KD, Carson DJ,
Kennedy T, O'Hare MM, Sloan JM and Hadden DR: Insulinoma (apud cell
carcinoma) in a diabetic. Br Med J. 2:1397–1398. 1978.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Campos-Olive N, Ferrer-García JC and
Safont M: Malignant insulinoma in a patient with type 2 diabetes
mellitus. Acta Endo Buc. 6:103–109. 2010. View Article : Google Scholar
|
|
27
|
Ferrer-Garcia JC, Tolosa-Torrens M,
Hernando-Melia C, Arribas-Palomar L and Sanchez-Juan C: Everolimus
resolving hypoglycemia, producing hyperglycemia, and necessitating
insulin use in a patient with diabetes and nonresectable malignant
insulinoma. Endocr Pract. 17:e17–e20. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Grycewicz J, Scibor Z, Cwikla JB, Lewinski
A and Cypryk K: Recurrent hypoglycaemia in a type 2 diabetes
patient-diagnostic difficulties. Arch Med Sci. 6:126–129.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Holstein A, Morgenstern T, Dienst H and
Hiller W: Insulinoma as rare cause of severe post-partum
hypoglycemia. J Obstet Gynaecol Res. 41:1848–1850. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Podzolkov VI, Dragomiretskaia NA, Koroleva
TV, Kavtaradze NN, Iakovleva HH and Podzolkov AV: A rare case of
hypoglycemia in an elderly patient with type 2 diabetes mellitus:
Malignant metastasizing insulinoma. Klin Med (Mosk). 92:65–70.
2014.(In Russian). PubMed/NCBI
|
|
31
|
Schmitt J, Boullu-Sanchis S, Moreau F,
Drui S, Louis B, Chabrier G, Pinget M and Jeandidier N: Association
of malignant insulinoma and type 2 diabetes mellitus: A case
report. Ann Endocrinol (Paris). 69:69–72. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Siraj ES, Samuel G, Saber S, Samuel S,
Hamrahian AH and Reddy SS: Metastatic malignant insulinoma in a
patient with type 2 diabetes mellitus: Case presentation and
literature review. Endocr Pract. 12:411–416. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lei WY, Wang TE, Chen TL, Chang WH, Yang
TL and Wang CY: Insulinoma causing hypoglycemia in a patient with
type 2 diabetes. J Formos Med Assoc. 106:392–396. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Madathil A and Weaver J: Insulinoma
presenting as postprandial hypoglycaemia. BMJ Case Rep.
2011:2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shimizu M, Suzuki K, Tsuchida K, Kojima M,
Hiraishi H and Aso Y: Insulinoma in a patient with chronic renal
failure due to type 2 diabetes mellitus treated effectively with
diazoxide. Intern Med. 54:621–625. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wildbrett J, Nagel M, Theissig F, Gaertner
HJ, Gromeier S, Fischer S and Hanefeld M: An unusual picture of
insulinoma in type-2 diabetes mellitus and morbid obesity. Dtsch
Med Wochenschr. 124:248–252. 1999.(In German). PubMed/NCBI View Article : Google Scholar
|
|
37
|
Iglesias P and Diez JJ: Management of
endocrine disease: A clinical update on tumor-induced hypoglycemia.
Eur J Endocrinol. 170:R147–R157. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Levine RA and Sobel BE: Insulinoma, type 2
diabetes and plasminogen activator inhibitor type-I. Coron Artery
Dis. 12:333–336. 2001.PubMed/NCBI View Article : Google Scholar
|