Introduction
Primary tumors of the chest wall, arising from soft
tissue, bone or cartilage are uncommon. At most, the reported
incidence is only 500 new cases per year in the United States
(1). Primary bone tumors of the
thoracic cage are relatively rare, representing up to 4-8% of all
non-secondary, non-metastatic bone neoplasias (2,3). Most
chest wall bony tumors demonstrate malignant behavior and the most
common primary malignancy is chondrosarcoma (1-4).
Waller and Newman (2), using data
from the Leeds bone tumor registry, reported that chondrosarcoma
constituted around 24% of all bone tumors of chest wall, while
other estimates place its incidence at 15-48.1% of the thoracic
wall malignancies (4-6).
Chondrosarcomas may arise as de novo lesions
or in the context of preexisting benign tumors of cartilage origin,
such as enchondromas, and are usually located in bones that undergo
endochondral ossification (1,7,8). Early recognition, preoperative
evaluation and adequate surgical intervention with wide margins are
crucial to prevent invasive local disease and metastasis (3,4,8). Awareness of the characteristics and
behavior of these tumors are necessary in order to avoid
misdiagnosis and inadequate or unnecessary therapeutic
interventions (4).
De novo malignancies have become one of the
leading causes of late mortality after renal transplantation, with
their incidence being 2-15-fold higher than in general population
(9). We report a case of large
thoracic chondrosarcoma in a kidney transplant recipient with
intra-abdominal invasion combined with vague
gastrointestinal-related symptoms.
Case report
A 79-year-old woman was referred to our clinic with
3 month history of mild epigastric pain and intermittent nausea,
without vomiting episodes. The patient underwent kidney
transplantation from deceased donor for end stage renal disease due
to diabetic nephropathy 20 years ago, complicated by chronic
rejection and return to dialysis the last 5 years. Her
immunosuppression regimen included steroids, cyclosporine, and
mycophenolate mofetil. Given the clinical presentation and
examination findings, a chest and abdomen computerized tomography
(CT) scan was performed at an outside facility in order to identify
the origin of the palpable mass. Per report, CT revealed a large
space-occupying, non-homogeneous solid mass accompanied by
intralesional calcifications and measuring approximately 17x15x12
cm (data not shown). In an effort to better characterize the mass
and delineate its margins, the patient underwent a magnetic
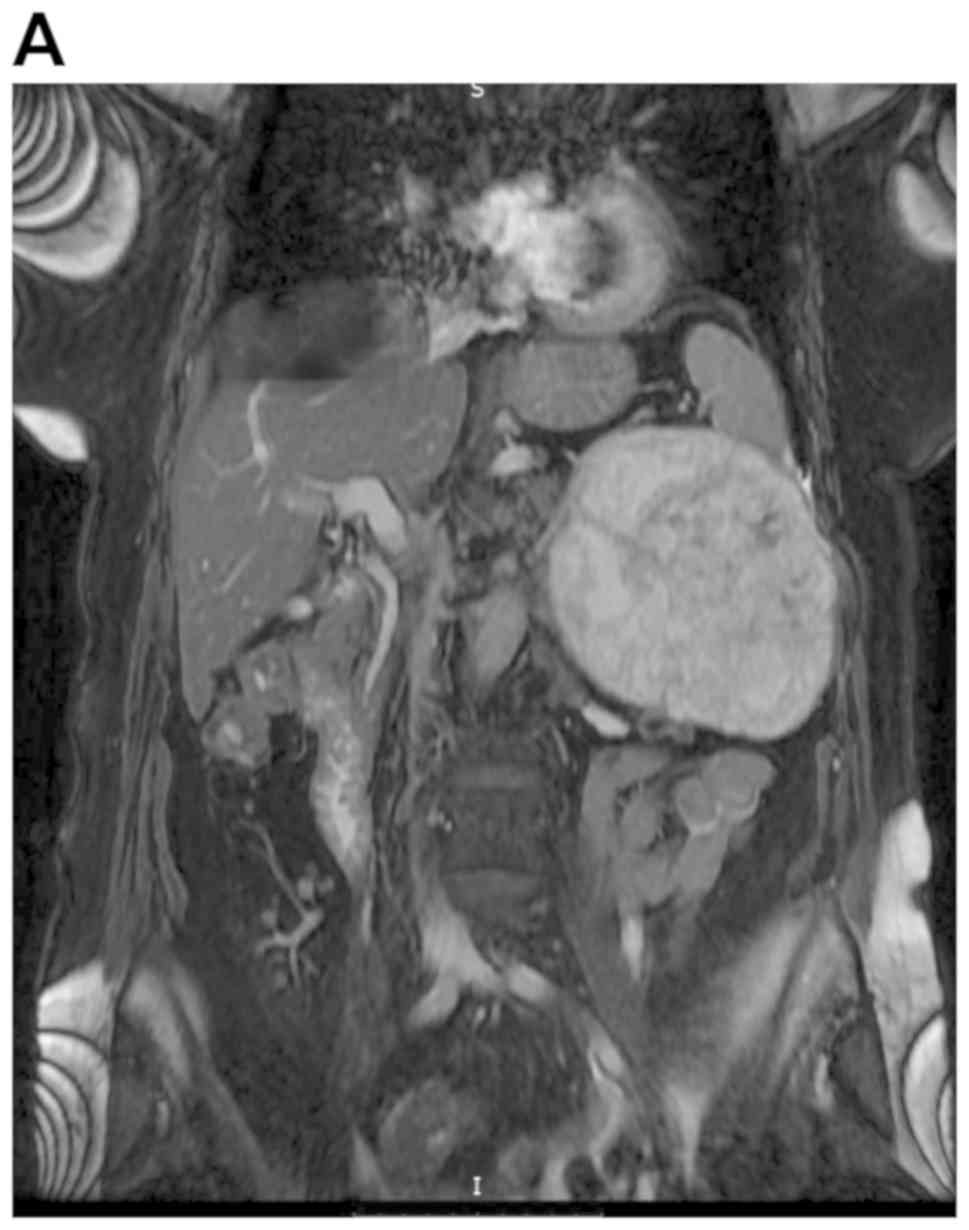
resonance imaging (MRI) scan one week later. It demonstrated a mass
arising from the 9th left rib with infiltration of the adjacent
subcutaneous fat tissue. Additionally, the mass exhibited
intra-abdominal subdiaphragmatic extension with compressive effects
on spleen, greater curvature of the stomach, body and tail of
pancreas, left colic flexure, left kidney and renal vein. The
lesion was well-circumscribed and demonstrated imaging
characteristics of neovascularized osteochondral tissue.
Preoperative MRI of chest and abdomen did not reveal any suspicious
intraparenchymal organ lesion or pathologically enlarged
intra-abdominal, retroperitoneal or pelvic lymph nodes (Fig. 1).
Considering the clinical presentation and imaging
findings, the decision was made to proceed with surgical
exploration and resection of the mass with curative intent.
Exploratory laparotomy was initiated with a left Kocher incision
extended to the xiphoid process and right subcostal area.
Intraoperatively, a botryoid, grayish lesion of cartilaginous
consistency was identified. Its smooth, membranous external surface
was carefully detached from the surrounding tissues with blunt
dissection. Despite being in close proximity to the mass capsule,
the adjacent organs, such as the spleen, greater curvature of the
stomach, pancreas, colon and left renal vein were not invaded. A
portion of the diaphragm measuring 12x12 cm was invaded and removed
via the abdominal incision. It corresponded to the left
costophrenic recess as well as 9 and 10th lateral rib shaft
parts.
The diaphragmatic defect was repaired with mesh. The
visceral antiadhesive surface was placed adjacent to the left lower
lung lobe and the parietal surface put in contact with the spleen
in order to avoid adhesion to the small bowel serosa. A left sided
chest tube and left subdiaphragmatic drain were also placed.
Subsequently, the abdominal wall incision was closed in anatomic
layers with simultaneous repair of 2 supraumbilical hernias caused
by previous laparotomy. The patient, was transferred to the
intensive care unit intubated and hemodynamically stable for
monitoring and further postoperative management.
The patient was gradually weaned off the ventilator
during the 3rd postoperative day and was subsequently transferred
to the surgical ward. Her diet was advanced gradually and the
abdominal drain was removed on post-operative day 8, and the chest
tube was removed 2 days later. Follow-up radiographic imaging
revealed minimal left pleural effusion without any signs of
pneumothorax. Histopathologic examination reported the presence of
Grade II chondrosarcoma with malignant invasion of the 9 and 10th
ribs. The patient was discharged on the post-operative day 14 and
referred to medical oncology for further evaluation and management.
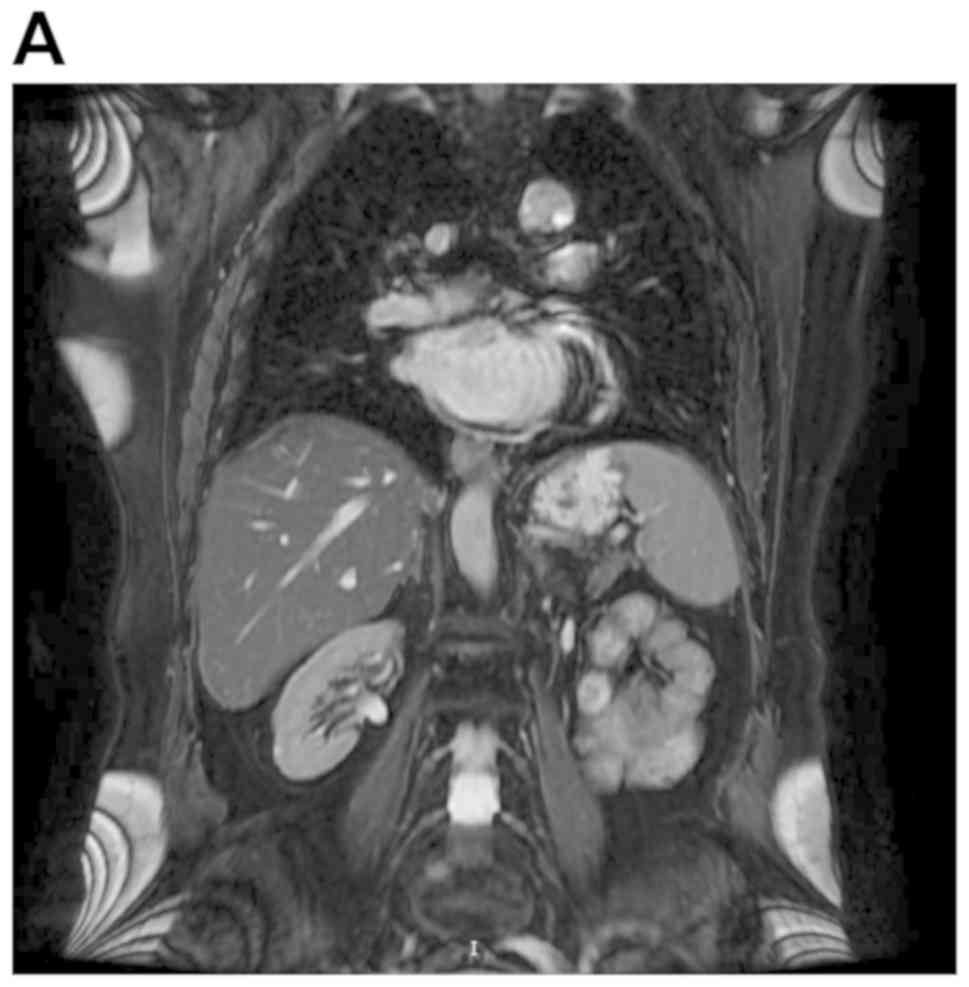
The patient remained disease-free at the 12 month follow-up, with
no evidence of disease on MRI (Fig.
2).
The present study was approved by the Ethics
Committee of Laiko General Hospital (Athens, Greece). Data analysis
was performed in accordance with the Declaration of Helsinki.
Patients who participated in this research had complete clinical
data. The signed informed consents were obtained from the patients
or the guardians.
Discussion
Soft tissue sarcomas are rare tumors of mesenchymal
origin (10). In transplant
patients, with the exception of Kaposi sarcoma-which is relatively
common with a 400-500-fold increased incidence-soft tissue sarcomas
are rarely encountered, thus the diagnostic and treatment
algorithms follow those of general population (11-13).
Chondrosarcoma is the most common primary malignancy
of the thoracic cage and is usually located in bony tissue
developing through the endochondral ossification process (1,3). These
malignant neoplasms may arise as lesions in previously healthy bone
tissues or develop in preexisting benign chondral tissue tumors,
such as enchondromas (1). In
addition, chondrosarcomas may develop centrally, in the periphery
or on the periosteal surface of the bone where they most commonly
affect the ribs, the costochondral junction and less frequently the
sternum (1,4,14).
Chondrosarcomas are reported to account for 15-48.1%
of the thoracic wall malignancies (2,4-6).
Burt et al reported that chondrosarcomas of the chest wall
correspond to 14.7% of all chondrosarcomas and most of them
originated in ribs (43%) and scapula (36%) (15). These tumors predominately affect men
with a median age of 50(1). However,
tumors have been reported in patients as young as 11 and as old as
76 (7,16). Risk factors associated with the
development of these neoplasms include prior trauma and radiation
exposure (often for the treatment of breast cancer or lymphoma),
though causation is not well established (1).
Chondrosarcoma grading is an important predictive
factor of local recurrence, distant metastasis and patient survival
(17). Conventional primary
chondrosarcomas are histologically graded as Grade I (low
cellularity, abundant matrix, rarely metastasize), Grade II
(mitoses present, less chondroid matrix) and Grade III
(undifferentiated and highly cellular tumors, with muco-myxoid
matrix and mitoses) (17).
Most patients with chest wall chondrosarcoma present
with a gradually increasing, palpable, and usually painful mass
arising from an anterior chest wall structure such as the
costochondral junction or the sternal surface (1). Tumors arising from the anterior chest
wall are usually bidirectional (growing outward and inward) and
those arising from the posterior thoracic wall tend to grow
inwardly only (16,18). Radiographic characterization of these
tumors should be the initial step to further guide the appropriate
treatment and management (19).
Patients presenting with chest wall masses should initially be
investigated with a posterior-anterior and lateral thoracic X-rays
(1). After this, CT of the chest,
including neck area and upper abdomen, is considered as the
gold-standard imaging test for accurate tumor localization and
preoperative planning (1). On CT,
chondrosarcomas characteristically appear as well-circumscribed,
lobular masses with intralesional focal, nodular or peripheral
calcifications accompanied by adjacent bone or soft tissues
invasion (19). MRI does not offer
any diagnostic benefit compared to CT and it is not routinely
performed in the investigation of suspected thoracic chondrosarcoma
(1). In paravertebral, mediastinal
or thoracic outlet tumors, where neurovascular invasion is a
concern, MRI offers additional information as it offers greater
ability to characterizing soft-tissue structures (1). Positron emission tomography (PET) is
occasionally used to identify metastatic disease and has also been
suggested as a diagnostic modality to differentiate between benign
chondral tissue tumors and chondrosarcoma (1,20).
As chondrosarcomas are resistant to radiation and
most chemotherapeutic agents, resection considered first line
treatment (7,14,16).
Surgical intervention involves tumor excision with 3-4 cm margins
in order to prevent local disease recurrence (7). However, it is not always feasible to
achieve these margins due to proximity to vital neurovascular and
visceral structures (7,16). Local tumor recurrence, metastasis,
and survival are affected by tumor grade, size, diameter, location
and positive margins (7,14,21).
Grade I chondrosarcomas have better prognosis compared to Grade II
and III tumors, with 10-years survival of 83, 64 and 29%
respectively (17). McAfee et
al reported a higher local recurrence rate in patients treated
with excision with negative margins alone compared to a 3-4 cm
margin (50 vs. 17%) (21). In
addition, 10-year survival dropped from 96% in wide margin group to
65% in local excision (21). The
prognosis is more favorable in patients with sternal tumors and
tumors measuring <6 cm, whereas survival is <2 years in
patients with Grade III tumors (21).
We present a rare case of chest wall chondrosarcoma
in a previous kidney transplant recipient, with vague symptoms due
to intra-abdominal expansion and invasion. Chest wall
chondrosarcomas are rare tumors, especially in transplant
populations, which are resistant to chemotherapy and radiotherapy
and mainly treated with wide local excision. Patients most commonly
present with insidious thoracic pain or solitary chest mass
gradually developing over months or years. Awareness of the
clinical presentation, appropriate treatment and occasionally
atypical progression of these tumors are important to avoid
unnecessary diagnostic tests and therapeutic interventions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DM and SV conceived and designed the present study.
DG, DM and SV collected the data. DG and BIS analyzed and
interpreted the data. DG, DM and BIS wrote the manuscript. DM, BIS
and SV critically revised the manuscript. All authors contributed
equally to this work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Laiko General Hospital (Athens, Greece). Data analysis
was performed in accordance with the Declaration of Helsinki.
Patients who participated in this research had complete clinical
data. The signed informed consents were obtained from the patients
or the guardians.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of all accompanying images and
data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rascoe PA, Reznik SI and Smythe WR:
Chondrosarcoma of the thorax. Sarcoma. 2011(342879)2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Waller DA and Newman RJ: Primary bone
tumours of the thoracic skeleton: An audit of the Leeds regional
bone tumour registry. Thorax. 45:850–855. 1990.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Al-Refaie RE, Amer S, Ismail MF,
Al-Shabrawy M, Al-Gamal G and Mokbel E: Chondrosarcoma of the chest
wall: Single-center experience. Asian Cardiovasc Thorac Ann.
22:829–834. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sabanathan S, Salama FD, Morgan WE and
Harvey JA: Primary chest wall tumors. Ann Thorac Surg. 39:4–15.
1985.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stanić V, Vulović T, Novaković M,
Ristanović A, Stamenović D, Cvijanović V, Stepić N and Dordević G:
Radical resection of giant chondrosarcoma of the anterior chest
wall. Vojnosanit Pregl. 65:64–68. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Noda M, Endo C, Hosaka T, Sado T, Sakurada
A, Hoshikawa Y, Okada Y and Kondo T: Dedifferentiated
chondrosarcoma of the chest wall: Reconstruction with Polypropylene
mesh using a transverse rectus abdominis myocutaneous flap. Gen
Thorac Cardiovasc Surg. 59:199–201. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fong YC, Pairolero PC, Sim FH, Cha SS,
Blanchard CL and Scully SP: Chondrosarcoma of the chest wall: A
retrospective clinical analysis. Clin Orthop Relat Res. 184–189.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Björnsson J, McLeod RA, Unni KK, Ilstrup
DM and Pritchard DJ: Primary chondrosarcoma of long bones and limb
girdles. Cancer. 83:2105–2119. 1998.PubMed/NCBI
|
|
9
|
Zavos G, Moris D, Kostakis ID, Vernadakis
S, Bokos J, Zavvos V, Lionaki S and Boletis J: De novo visceral
malignancies in renal transplant recipients: A single center
experience of 2054 recipients for more than 30 years. Exp Clin
Transplant. 13:313–318. 2015.PubMed/NCBI
|
|
10
|
Adam MA, Moris D, Behrens S, Nussbaum DP,
Jawitz O, Turner M, Lidsky M and Blazer D III: Hospital volume
threshold for the treatment of retroperitoneal sarcoma. Anticancer
Res. 39:2007–2014. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zavos G, Moris D, Vernadakis S, Bokos J,
Lionaki S, Mamarelis G, Panagiotellis K, Zavvos V and Boletis I:
Incidence and management of Kaposi sarcoma in renal transplant
recipients: The Greek experience. Transplant Proc. 46:3199–3202.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vernadakis S, Moris D, Delimpalta C, Bokos
J and Zavos G: Ovarian carcinosarcoma in a renal transplant
recipient. A unique case of a rare tumor. Hippokratia. 18:364–365.
2014.PubMed/NCBI
|
|
13
|
Vailas MG, Vernadakis S, Moris D and Zavos
G: Surgical dead end in a renal transplant recipient associated
with a rare thrombohemorrhagic syndrome. Transplant Proc.
47:2537–2540. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Le HV, Wadhwa R, Theodore P and Mummaneni
P: Excision of thoracic chondrosarcoma: Case report and review of
literature. Cureus. 8(e708)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Burt M, Fulton M, Wessner-Dunlap S, Karpeh
M, Huvos AG, Bains MS, Martini N, McCormack PM, Rusch VW and
Ginsberg RJ: Primary bony and cartilaginous sarcomas of chest wall:
Results of therapy. Ann Thorac Surg. 54:226–232. 1992.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shimoyama T, Suzuki R, Yoshiya K, Yamato Y
and Koike T: Chondrosarcoma of the rib. Jpn J Thorac Cardiovasc
Surg. 51:167–171. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gelderblom H, Hogendoorn PCW, Dijkstra SD,
van Rijswijk CS, Krol AD, Taminiau AH and Bovée JV: The clinical
approach towards chondrosarcoma. Oncologist. 13:320–329.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamamoto N, Imai S, Motohiro K, Shiota Y,
Sato N, Nakayama H, Sasaki N and Taniyama K: A case of
chondrosarcoma of the low grade malignancy originated in rib. Kyobu
Geka. 48:1141–1143. 1995.(In Japanese). PubMed/NCBI
|
|
19
|
O'Sullivan P, O'Dwyer H, Flint J, Munk PL
and Muller NL: Malignant chest wall neoplasms of bone and
cartilage: A pictorial review of CT and MR findings. Br J Radiol.
80:678–684. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Capps E, Shiller SM, Cheek S, Oza U and
Konduri K: Chest wall chondrosarcoma. Proc (Bayl Univ Med Cent).
22:362–365. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McAfee MK, Pairolero PC, Bergstralh EJ,
Piehler JM, Unni KK, McLeod RA, Bernatz PE and Payne WS:
Chondrosarcoma of the chest wall: Factors affecting survival. Ann
Thorac Surg. 40:535–541. 1985.PubMed/NCBI View Article : Google Scholar
|