Introduction
Three randomized controlled trials for high-risk
breast-cancer patients with axial lymph node metastasis and a
maximum primary-tumor diameter of 5 cm or more have shown that
radiotherapy combined with systemic therapy after mastectomy not
only reduces locoregional recurrence to one-third to one-quarter,
but also improves survival rates (1-4).
These meta-analyses have also shown decreased rates of locoregional
lymph node recurrence and increased survival rates (5).
After breast-conserving surgery (BCS), locoregional
recurrence tends to increases as axial lymph node metastases
increase (6,7). When there are more than four lymph node
metastases after BCS, improvement of local control by regional
nodal irradiation (RNI) has been reported in several retrospective
studies, but high-quality evidence is sparse (6,7).
Considering the suppressive effect of four or more lymph node
metastases at the time of mastectomy, RNI after BCS with four or
more lymph node metastases is recommended by the guidelines of both
the U.S. National Comprehensive Cancer Network (NCCN) and the
Japanese Breast Cancer Society (8,9). Few
randomized controlled trials have examined the significance of RNI
in cases with 1-3 axillary lymph node metastases after BCS with
axillary lymph-node dissection (ALND). In the MA 20 study, which
showed a beneficial effect of RNI, the proportion of lymph nodes
having from 1-3 and 0 metastases was 85 and 10%, respectively
(10). In the MA 20 study, 1,832
high-risk breast cancer patients who were either lymph node
metastasis-negative or lymph node metastasis-positive after BCS
were randomly assigned to either the group receiving WBI alone or a
group receiving WBI plus RNI. In the median follow-up (9.5 years),
the 10-year disease-free survival rates were 82.0% in the
nodal-irradiation group and 77.0% in the control group (hazard
ratio, 0.76; P=0.01), and the overall survival rate
increased from 81.8 to 82.0% (hazard ratio, 0.91; P=0.38)
(10). According to the NCCN
guideline, RNI should be strongly considered when the number of
metastasis-positive lymph nodes is between 1-3 after BCS. However,
the recurrence rate in the regional lymph nodes, including the
supraclavicular region, in patients with 1-3 metastasis-positive
lymph nodes is not high when ALND is performed (11,12). Due
to recent advances in systemic therapy, local and regional lymph
node recurrence has been decreasing with age (13). According to a retrospective study by
the M.D. Anderson Cancer Centre, for patients with 1-3 metastatic
lymph nodes, post-mastectomy radiation therapy (PMRT) has not been
effective for 5-year local and regional lymph node recurrence since
the year 2000(14). If adequate ALND
and systemic therapy is properly conducted after BCS, locoregional
recurrence seems to be lessened.
Recently, a study by the American Surgical Oncology
Group (ACOSOG) Z0011 showed that patients with micrometastatic and
macrometastatic nodular disease after BSC do not require ALND
(15). Besides, a study by the
European Cancer Research and Treatment Organization (EORTC)
10981-22023 (AMAROS) has shown that the efficacy and prevalence of
ALND and axillary RT are comparable (16). Based on these results, the number of
patients undergoing BSC and ALND is expected to continue to
decline, and it is difficult to show substantial evidence of
additional effects of adding RNI to patients with 1-3 metastases
after BCS and ALND. Therefore, we retrospectively examined the
recurrence pattern in the Aichi Cancer Centre (ACC) of patients who
had between 1-3 axillary node metastases after BCS with ALND and
whether the addition of RNI to WBI was necessary for Japanese
patients.
Patients and methods
Patient selection
The present study retrospectively analyzed local and
distant recurrences in 152 primary breast cancer patients who were
treated with BCS and ALND and who had 1-3 positive axillary nodes.
These patients were identified from among 5,164 primary breast
cancer cases seen between January 2000 and December 2014 at the
ACC. All patients received WBI and adjuvant systemic therapy with
either chemotherapy or endocrine therapy and with or without HER2
therapy. The study excluded patients with ipsilateral breast tumor
recurrence, contralateral breast cancer, neoadjuvant chemotherapy,
T4 tumors, or N2-3 nodes and distant metastasis. The study protocol
was approved by The Certified Review Board of Aichi Cancer Center
and written informed consent was obtained from all the
patients.
Adjuvant therapy
Adjuvant chemotherapy was delivered before the
radiation. Endocrine therapy with tamoxifen or an aromatase
inhibitor was administered concurrently with radiotherapy based on
several guidelines. After May 2005, trastuzumab was recommended for
patients who had HER2-positive disease. All patients were treated
with external beam radiotherapy. The dose to the breast was either
50 Gy in 25 fractions or 42.56 Gy in 16 fractions. In cases
requiring boost irradiation to the breast, irradiation was
delivered at either 60 Gy in 30 fractions or 53.2 Gy in 21
fractions.
Histopathological evaluation
The statuses of the estrogen receptor (ER),
progesterone receptor (PR) and HER2 were evaluated using
immunohistochemical (IHC) analysis. The ER and PR status are
obtained by summing the score of the percentage abundance and the
staining intensity of ER or PR-stained nuclei of tumor cells (the
so-called Allred score, ranging from 0 to 8). ER and PR positivity
were defined as >3 points using the Allred score. Positive HER2
status was defined as either 3+ or else 2+ and c-erB2 gene
amplification that used fluorescent in situ by hybridization
(FISH), and both were confirmed using IHC. The margins positive are
defined as cancer detected <5 mm from the stamp.
Statistical analysis
The survival endpoints evaluated were isolated
locoregional disease-free survival (ILDFS), DFS and overall
survival (OS). ILDFS was defined as the time from surgery to the
time of the first recurrence in the ipsilateral breast or in axial,
supraclavicular or internal mammary nodes without evidence of
distant disease. DFS was defined as the time from surgery to the
time of the first recurrence, such as relapse including ipsilateral
breast cancer recurrence; the appearance of second primary cancer,
including contralateral breast cancer; or death, whichever occurred
first. OS was defined as the time from surgery until the date of
death from any cause. ILDFS, DFS and OS functions were estimated
using the Kaplan-Meier method. All statistical analyses were
conducted using Stata® V12 software (StataCorp LP.).
Results
Patient characteristics
The total number of eligible patients who had ALND,
between 1-3 lymph node metastases and breast radiation after BSC
was 152. The median follow-up at the time of the analysis was 71
months (range: 1-176). Table I shows
the patients' characteristics. The median age was 54 (range:
29-82). The median number of axillary nodes removed was 17. The
proportions of ≤9, from 10 to <20 and from ≥20 were 7.2, 52.0
and 40.8%, respectively. In 120 patients (78.9%), the tumor
diameter was ≤2 cm and in 32 patients (21.1%), it was >2 cm.
Ninety-one patients (59.9%) had 1 metastasis to the lymph nodes, 49
(32.2%) had 2 metastases, and 12 (7.9%) had 3 metastases. The
surgical margin was positive in 22 patients (14.5%).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient
characteristic | n (%) |
|---|
| Median age (range),
year | 54 (29-82) |
| Patients who
underweight initial sentinel-lymph-node biopsy | 101 (66.4) |
| Axillary nodes
removed, median (interquartile range) | 17 (15-23) |
|
1-9 | 11 (7.2) |
|
10-19 | 79 (52.0) |
|
≥20 | 62 (40.8) |
| Tumor size, cm | |
|
≤2 | 120 (78.9) |
|
>2 | 32 (21.1) |
| Number of positive
axillary lymph nodes | |
|
1 | 91 (59.9) |
|
2 | 49 (32.2) |
|
3 | 12 (7.9) |
| Histological
grade | |
|
1 | 27 (17.8) |
|
2 | 71 (46.7) |
|
3 | 36 (23.7) |
|
Unknown | 18 (11.8) |
| ER status | |
|
Positive | 122 (80.3) |
|
Negative | 26 (17.1) |
|
Unknown | 4 (2.6) |
| Progesterone receptor
status | |
|
Positive | 105 (69.0) |
|
Negative | 43 (28.2) |
|
Unknown | 4 (2.6) |
| HER2 status -no.
(%) | |
|
Positive | 14 (9.2) |
|
Negative | 120 (78.9) |
|
Unknown | 18 (11.8) |
| Body mass index | |
|
<18.5
kg/m2 | 7 (4.6) |
|
18.5≤x<25.0
kg/m2 | 109 (71.7) |
|
25.0≤x<30.0
kg/m2 | 25 (16.4) |
|
≥30
kg/m2 | 11 (7.2) |
| Margin positive | 22 (14.5) |
Treatment characteristics
Table II shows the
treatment characteristics. Nearly 40% of the cases in the ACC
received anthracycline with taxane. Thirty-one patients (20.4%) did
not receive chemotherapy. One hundred twenty-six patients (82.9%)
received hormone treatment with either aromatase inhibitors or
tamoxifen. Boost radiation was administered to 17 (11.1%) due to
positive margins.
 | Table IITreatment characteristics. |
Table II
Treatment characteristics.
| A, Adjuvant
chemotherapy, n=121 (79.6%) |
|---|
| Treatment
characteristic | n (%) |
|---|
| Anthracycline with
taxane | 66 (43.4) |
| Anthracycline without
taxane | 12 (7.9) |
| Othera | 43 (28.2) |
| No chemotherapy | 31 (20.4) |
| B, Adjuvant endocrine
therapy, n=126 (82.9%) |
| Treatment
characteristic | n (%) |
| Aromatase
inhibitors | 66 (43.4) |
| Tamoxifen | 60 (39.5) |
| No endotherapy | 26 (17.1) |
| Boost
irradiation-number and (%) | 17 (11.1) |
Recurrence and deaths
Table III shows
statistics regarding the sites of recurrence and the deaths. The
most common site of isolated locoregional recurrence was the
breast. There were no isolated regional-only recurrences, including
axillary, supraclavicular nodal, or subclavian lymph nodal. Only
one patient had a distant recurrence at the same time as the
supraclavicular lymph node recurrence. Seven patients (4.6%) had
distant metastases, including in the lung, bone, and liver, without
local recurrence. Of the four deaths, three were caused by breast
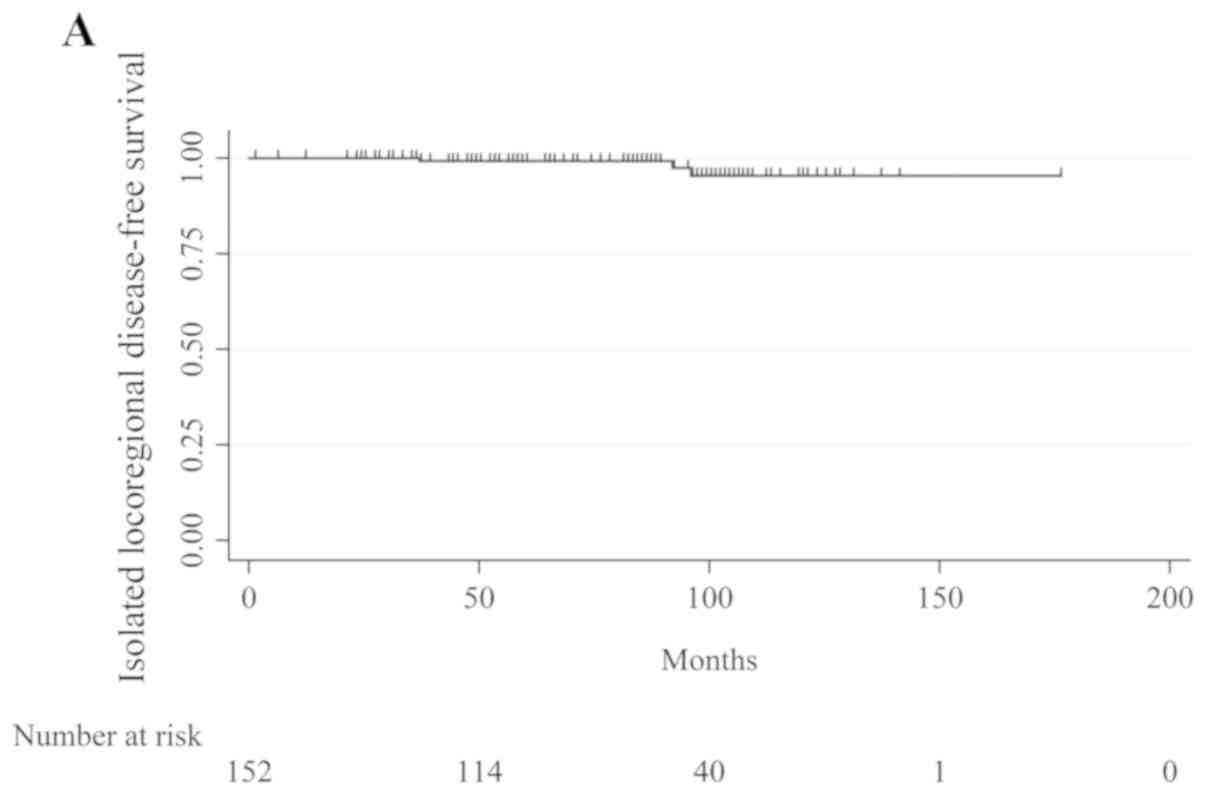
cancer. Table IV and Fig. 1 show the 5- and 10-year Kaplan-Meier
ILDFS, DFS, and OS. The recurrence rates at 5 and 10 years,
respectively, were 99.2 and 95.4% for ILDFS, 95.0 and 89.5% for
DFS, and 99.2 and 96.8% for OS.
 | Table IIIDisease recurrence or death. |
Table III
Disease recurrence or death.
| Event | n (%) |
|---|
| Isolated locoregional
recurrence | 3 (2.0) |
|
Local (in
breast) only | 3 (2.0) |
|
Regional
only | 0 (0.0) |
|
Local+regional | 0 (0.0) |
| Distant
recurrence | 1 (0.01) |
|
First or
concurrent with locoregional recurrence | 1 (0.01) |
|
After
locoregional recurrence | 0 (0.0) |
| Any recurrence or
contralateral breast cancer | 7 (4.6) |
|
Any
recurrence | 7 (4.6) |
|
Contralateral
breast cancer | 0 (0.0) |
| Death | 4 (2.6) |
|
Breast
cancer | 3 (2.0) |
|
Other
cause | 0 (0.0) |
|
Unknown | 1 (0.01) |
 | Table IVRecurrence-free survival rates at 5-
and 10-years. |
Table IV
Recurrence-free survival rates at 5-
and 10-years.
| Rate | 5 years, % | 10 years, % |
|---|
| Isolated
locoregional DFS | 99.20 | 95.41 |
| DFS | 95.00 | 89.50 |
| OS | 99.22 | 96.75 |
Discussion
The utility of RNI is in question in patients with
1-3 axillary node metastases after BCS with ALND. Therefore, the
present study examined the utility of RNI by examining the site of
recurrence in our institution, the ACC. Surprisingly, the isolated
local recurrence rate was very low (2.0%). In the MA 20 study,
isolated locoregional recurrence was observed in 62 patients (6.8%)
in the WBI group, and 39 (4.3%) in the WBI + RNI group. There were
23 (2.5%) regional-only recurrences in the WBI group and 5 (0.5%)
each in the WBI and RNI groups, indicating that RNI reduced
regional-only recurrence by 18 patients, which was 2.0% (10). Regarding the locations of
regional-only recurrences, the WBI group had axillary recurrences
in 14 patients (60.9%), and the WBI + RNI group had axillary
recurrences in 5 patients (100%). This suggests that the effect of
RNI is to suppress axillary lymph node recurrence. An analysis of
5688 women using the British Columbia Cancer Agency (BCCA) Breast
Cancer Outcomes Unit Database showed that 40 of the 68 (58.8%) who
experienced local recurrences after BCS and ALND had an axillary
node recurrence (17). In contrast,
at the ACC, local recurrence was very low and no axillary
recurrences were found. In addition, at the ACC, the 10-year
recurrence rate was 89%, which tended to be higher than those in
the MA 20 study (77.0% for WBI and 82% for WBI + RNI,
respectively). In the ACC, the 10-year isolated locoregional
recurrence rate was 95.4%, which was nearly identical to the 95.2%
for the WBI + RNI group in the MA 20 study.
Three hypotheses were formed on why the recurrence
rate was so low in the ACC. First, we considered the early clinical
stages of eligible patients. In the ACC study, the percentage of
patients who had tumors with diameters of ≤2 cm was 78.9% (120
patients), which was about half the percentage in the MA 20 study,
indicating that the ACC patients tended to have smaller tumors
diameters than those in the MA 20 study. Additionally, in the MA 20
study, about half the patients had one axillary metastasis, but in
the present study, ~60% had them. The number of axillary lymph node
metastases also tended to be smaller in the ACC than in the MA 20
study. The second hypothesis regarding the lower recurrence rates
at the ACC involved the difference in the number of lymph nodes
removed. In the ACC, ~7% of patients had <9 lymph nodes removed,
and about 40% had >20 removed. In contrast, in the MA 20 study,
about 30% of patients had ≤9 lymph nodes removed, and about half of
patients had 10 or fewer lymph nodes removed, according to an
analysis that used BCCA (10,17).
Generally, to accurately stage the axilla, it is recommended that
the number of resected axillary lymph nodes to be ≥10. Besides,
with the progress in systemic therapy and radiation therapy, there
is concern regarding the decreased quality of life (QOL) due to
axillary dissection, and the range of ALND tends to be minimized,
resulting in a decrease in the absolute number of resected lymph
nodes. However, the absolute number and ratio of positive lymph
nodes have been reported as a prognostic factor for other
carcinomas, and similar results have been reported for breast
cancer (18-21).
In the ACC, the large number of resected lymph nodes may have
resulted in a low lymph node ratio and lower local recurrence rates
and prognoses. Finally, it was hypothesized that the low body mass
index (BMI) of eligible patients had an effect. High BMI has been
reported as resulting in higher local recurrence rates and
recurrence rates (22-24).
In the ACC, the median BMI was 22.8 kg/m2, and only 11
patients (7.2%) had a BMI>30 kg/m2, which differs
significantly from the BMI distribution of Westerners, of whom 30%
are obese (25). The recurrence rate
for the ACC patients appeared to be lower because that study
targeted patients who had lower BMIs.
Considering that the addition of RNI increases
adverse events including lymphedema, dermatitis, and pneumonia, RNI
is less useful for Japanese patients because they have a very low
risk of local recurrence. Patients with young, grade III or
ER-negative tumors have been reported to have a ~15-20% risk of
locoregional recurrence despite WBI and systemic therapy (17). RNI should be considered only for
these patients.
The present study had some limitations, notably the
small number of patients and the fact that it was a single-center
retrospective analysis. In addition, the observation period was
short, with the median observation period being 71 months.
Despite these limitations, the present study's
results suggest that the addition of RNI is not effective in
patients in Japan who have 1-3 metastases after BCS and ALND. In
the future, it is expected that patients with 1-3 lymph node
metastases will undergo no ALND due to the ACOSOG Z0011 trial and
AMOROS trial. For these patients, RNI may be even more
important.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NG conceived the present study and wrote the
manuscript. MS, MH, AY, HK, YA and HI made substantial
contributions to the interpretation of data and the research
concept and design. AK, KS, NH, YO and YE helped with data
acquisition, analysis and interpretation. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by The Certified
Review Board of Aichi Cancer Center and written informed consent
was obtained from all the patients. All procedures involving human
participants were conducted in accordance with the ethical
standards of the Institutional Review Board of Aichi Cancer Centre
and with the 1964 Helsinki Declaration and its later
amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Overgaard M, Hansen PS, Overgaard J, Rose
C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen
MB and Zedeler K: Postoperative radiotherapy in high-risk
premenopausal women with breast cancer who receive adjuvant
chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N
Engl J Med. 337:949–955. 1997.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Overgaard M, Jensen MB, Overgaard J,
Hansen PS, Rose C, Andersson M, Kamby C, Kjaer M, Gadeberg CC,
Rasmussen BB, et al: Postoperative radiotherapy in high-risk
postmenopausal breast-cancer patients given adjuvant tamoxifen:
Danish Breast Cancer Cooperative Group DBCG 82c randomised trial.
Lancet (London). 353:1641–1648. 1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ragaz J, Jackson SM, Le N, Plenderleith
IH, Spinelli JJ, Basco VE, Wilson KS, Knowling MA, Coppin CM,
Paradis M, et al: Adjuvant radiotherapy and chemotherapy in
node-positive premenopausal women with breast cancer. N Engl J Med.
337:956–962. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ragaz J, Olivotto IA, Spinelli JJ,
Phillips N, Jackson SM, Wilson KS, Knowling MA, Coppin CM, Wieir L,
Gelmon K, et al: Locoregional radiation therapy in patients with
high-risk breast cancer receiving adjuvant chemotherapy: 20-year
results of the British Columbia randomized trial. J Natl Cancer
Inst. 97:116–126. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
EBCTCG (Early Breast Cancer Trialists'
Collaborative Group). McGale P, Taylor C, Correa C, Cutter D, Duane
F, Ewertz M, Gray R, Mannu G, Peto R, et al: Effect of radiotherapy
after mastectomy and axillary surgery on 10-year recurrence and
20-year breast cancer mortality: Meta-analysis of individual
patient data for 8135 women in 22 randomised trials. Lancet.
383:2127–2135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grills IS, Kestin LL, Goldstein N,
Mitchell C, Martinez A, Ingold J and Vicini FA: Risk factors for
regional nodal failure after breast-conserving therapy: Regional
nodal irradiation reduces rate of axillary failure in patients with
four or more positive lymph nodes. Int J Radiat Oncol Biol Phys.
56:658–670. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Livi L, Scotti V, Saieva C, Meattini I,
Detti B, Simontacchi G, Cardillo CD, Paiar F, Mangoni M, Marrazzo
L, et al: Outcome after conservative surgery and breast irradiation
in 5,717 patients with breast cancer: Implications for
supraclavicular nodal irradiation. Int J Radiat Oncol Biol Phys.
76:978–983. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
National Comprehensive Cancer Network
(NCCN) Clinical Practice Guidelines in Breast Cancer Version 3.
https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
Accessed March 15, 2019.
|
|
9
|
The Guidelines of the Japanese Breast
Cancer Society version 2, 2018. https://jbcs.gr.jp/guidline/2018/index/housyasen/.
Accessed March 15, 2019. (In Japanese).
|
|
10
|
Whelan TJ, Olivotto IA, Parulekar WR,
Ackerman I, Chua BH, Nabid A, Vallis KA, White JR, Rousseau P,
Fortin A, et al: Regional nodal irradiation in early-stage breast
cancer. N Engl J Med. 373:307–316. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Recht A, Pierce SM, Abner A, Vicini F,
Osteen RT, Love SM, Silver B and Harris JR: Regional nodal failure
after conservative surgery and radiotherapy for early-stage breast
carcinoma. J Clin Oncol. 9:988–996. 1991.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Taghian A, Jeong JH, Mamounas E, Anderson
S, Bryant J, Deutsch M and Wolmark N: Patterns of locoregional
failure in patients with operable breast cancer treated by
mastectomy and adjuvant chemotherapy with or without tamoxifen and
without radiotherapy: Results from five national surgical adjuvant
breast and bowel project randomized clinical trials. J Clin Oncol.
22:4247–4254. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bouganim N, Tsvetkova E, Clemons M and
Amir E: Evolution of sites of recurrence after early breast cancer
over the last 20 years: Implications for patient care and future
research. Breast Cancer Res Treat. 139:603–606. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McBride A, Allen P, Woodward W, Kim M,
Kuerer HM, Drinka EK, Sahin A, Strom EA, Buzdar A, Valero V, et al:
Locoregional recurrence risk for patients with T1,2 breast cancer
with 1-3 positive lymph nodes treated with mastectomy and systemic
treatment. Int J Radiat Oncol Biol Phys. 89:392–398.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Giuliano AE, Hunt KK, Ballman KV, Beitsch
PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM and
Morrow M: Axillary dissection vs. no axillary dissection in women
with invasive breast cancer and sentinel node metastasis: A
randomized clinical trial. JAMA. 305:569–575. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Donker M, van Tienhoven G, Straver ME,
Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH,
Klinkenbijl JH, Orzalesi L, et al: Radiotherapy or surgery of the
axilla after a positive sentinel node in breast cancer (EORTC
10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3
non-inferiority trial. Lancet Oncol. 15:1303–1310. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Truong PT, Jones SO, Kader HA, Wai ES,
Speers CH, Alexander AS and Olivotto IA: Patients with t1 to t2
breast cancer with one to three positive nodes have higher local
and regional recurrence risks compared with node-negative patients
after breast-conserving surgery and whole-breast radiotherapy. Int
J Radiat Oncol Biol Phys. 73:357–364. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
De Ridder M, Vinh-Hung V, Van Nieuwenhove
Y, Hoorens A, Sermeus A and Storme G: Prognostic value of the lymph
node ratio in node positive colon cancer. Gut.
55(1681)2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chan JK, Kapp DS, Cheung MK, Osann K, Shin
JY, Cohn D and Seid PL: The impact of the absolute number and ratio
of positive lymph nodes on survival of endometrioid uterine cancer
patients. Br J Cancer. 97:605–611. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Woodward WA, Vinh-Hung V, Ueno NT, Cheng
YC, Royce M, Tai P, Vlastos G, Wallace AM, Hortobagyi GN and Nieto
Y: Prognostic value of nodal ratios in node-positive breast cancer.
J Clin Oncol. 24:2910–2916. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim JY, Ryu MR, Choi BO, Park WC, Oh SJ,
Won JM and Chung SM: The prognostic significance of the lymph node
ratio in axillary lymph node positive breast cancer. J Breast
Cancer. 14:204–212. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bergom C, Kelly T, Bedi M, Saeed H, Prior
P, Rein LE, Szabo A, Wilson JF, Currey AD and White J: Association
of locoregional control with high body mass index in women
undergoing breast conservation therapy for early-stage breast
cancer. Int J Radiat Oncol Biol Phys. 96:65–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ewertz M, Jensen MB, Gunnarsdottir KA,
Hojris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB and Cold
S: Effect of obesity on prognosis after early-stage breast cancer.
J Clin Oncol. 29:25–31. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Warren LE, Ligibel JA, Chen YH, Truong L,
Catalano PJ and Bellon JR: Body mass index and locoregional
recurrence in women with early-stage breast cancer. Ann Surg Oncol.
23:3870–3879. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
The Organisation for Economic Co-operation
and Development (OECD): Obesity Update 2017. https://www.oecd.org/health/obesity-update.htm.
Accessed March 15, 2019.
|