Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is
one of the most common side effects of chemotherapy (1). Approximately ≥70% of patients with
ovarian cancer undergoing chemotherapy, particularly paclitaxel,
develop CIPN (2). The predominant
symptoms of CIPN are sensory disorder, numbness, tingling sensation
and pain. Occasionally, CIPN leads to motor dysfunction. The
factors affecting the development of CIPN include the type of
chemotherapy, such as platinum agents, taxanes and vinca alkaloids,
administration method, patient age, and pre-existing peripheral
neuropathy, such as diabetes mellitus (3). One of the critical problems in CIPN is
the dose-limiting toxicity of chemotherapy. Some patients who are
responsive to chemotherapy are unable to continue the treatment due
to CIPN. Furthermore, CIPN may be a persistent side effect. The
probability of persistent CIPN 6 months after completion of
chemotherapy is 15%, and the probability at 2 years after
completion of chemotherapy is 11% (4). The treatment and prevention of CIPN are
not yet clearly established.
CIPN can be evaluated using various methods, but
there are currently no standard methods for the evaluation of CIPN,
which hinders early treatment of this condition (5). The diagnosis of CIPN is mainly
clinical. The National Cancer Institute Common Terminology Criteria
for Adverse Events (NCI-CTCAE) (6)
version 4.0, the scale most commonly used for CIPN grading, is
evaluated by physicians, nurses and other clinical staff (7). The European Organisation for Research
and Treatment of Cancer quality of life questionnaire
(EORTC-QLQ)-CIPN20 and THE Functional Assessment of Cancer
Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx) are
commonly used as patient-based assessments (8,9).
However, the assessment by physicians and patients is
subjective.
There are few reports regarding the instrumentation
required for quantitative evaluation (7,10-13).
Intraepidermal electrical stimulation (IES) has been reported as a
non-invasive and quantitative measurement method for the evaluation
of diabetic neuropathy (14-16).
The aim of the present study was to evaluate CIPN by using IES
instruments.
Materials and methods
Subjects
This was a retrospective study that enrolled 57
patients who underwent taxane-based chemotherapy for gynecological
cancer at Hirosaki University Hospital between April 2017 and April
2018. The median age of the patients was 63 years (range, 40-86
years). The criteria for eligibility included i) current or
previous history of taxane-based chemotherapy; ii) Eastern
Cooperative Oncology Group performance status of 0-3; iii) consent
to participate in this study. The exclusion criteria included
pre-existing peripheral neuropathies, such as diabetic and
alcoholic neuropathy, and rheumatoid arthritis. Conventional
paclitaxel dose (175 mg/m2) and a platinum-based
chemotherapy (carboplatin; area under the blood concentration
curve=6; cisplatin, 75 mg/m2) with or without
bevacizumab (15 mg/kg) were administered every 3 weeks. The weekly
dose of paclitaxel was 80 mg/m2. The protocol of the
present study was approved by the Institutional Review Board (IRB)
of the Hirosaki University Graduate School of Medicine (IRB
approval no. 2016-263). Informed consent was obtained from all the
patients.
Evaluation of CIPN
CIPN was clinically evaluated by using CTCAE version
4.0. Simultaneously, CIPN was evaluated by using IES. IES
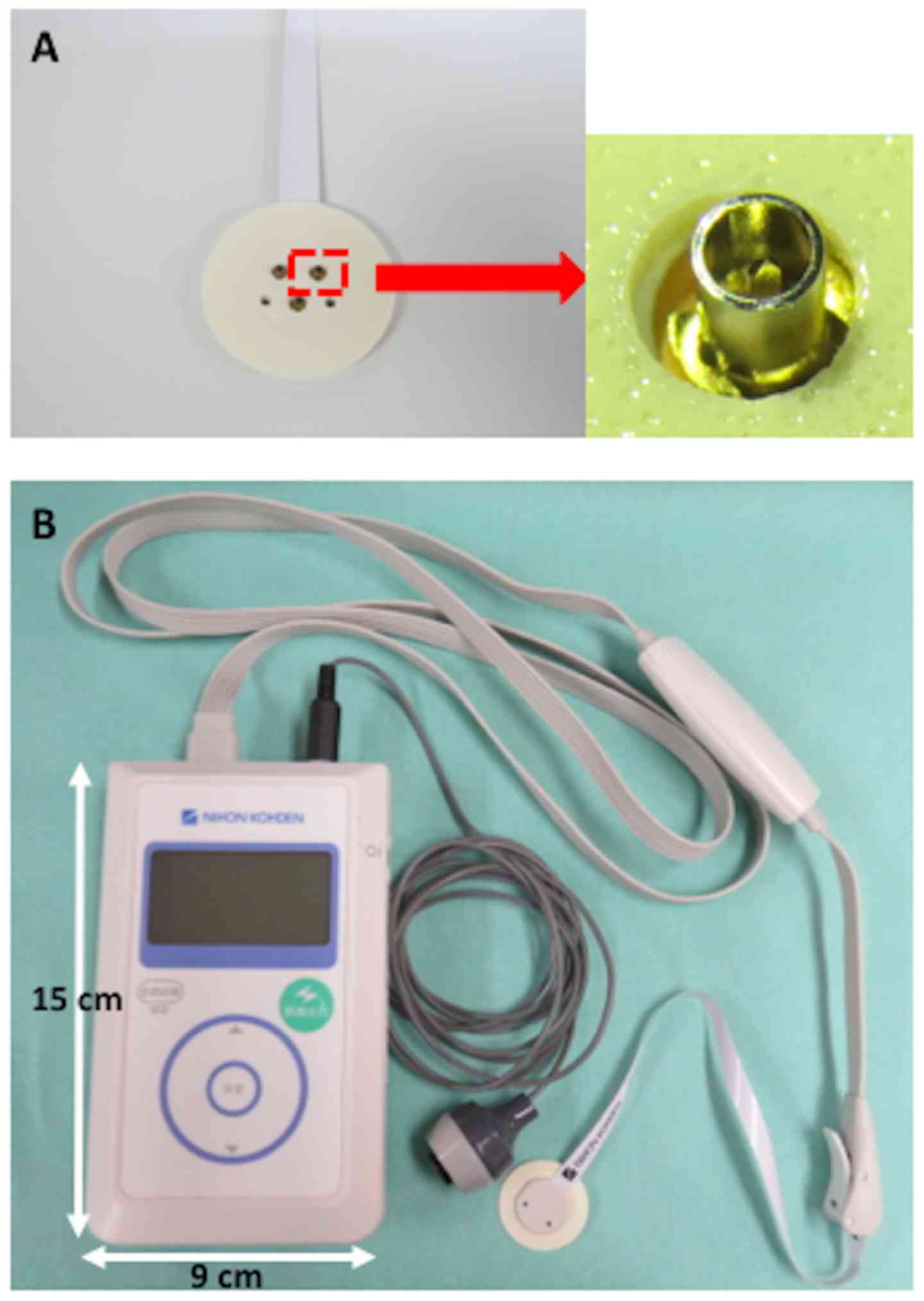
instruments were used as previously described (16). Briefly, a disposable, concentric
bipolar needle electrode was used for IES (NM-983W; Nihon Kohden
Corp.), which consists of three concentric bipolar needle
electrodes with an outer ring (1.3 mm in diameter), and the cathode
is an inner needle that protrudes 0.2 mm from the outer ring
(Fig. 1A). By gently pressing the
electrode against the skin, the needle tip was inserted into the
epidermis where the nociceptors are located, while the outer ring
remained attached to the skin surface. The IES electrode was placed
onto the skin of the dorsum of the hand. The temperature of the
skin was maintained at ≥30˚C. Subsequently, a stimulator (PNS-7000;
Nihon Kohden Corp.) was used for IES (Fig. 1B) (14). When a button was pushed, a weak
electric stimulation was delivered to the attached skin, and the
patients pushed the hand switch button as soon as they felt a
sensation. The stimulation was started at an intensity of 0.5 mA.
Subsequently, the intensity was gradually decreased by 0.05 mA
until the patients no longer felt the sensation. When the patients
felt the sensation thrice at the same current intensity, this
intensity was defined as the pain threshold. The evaluation was
performed prior to chemotherapy and for each of the three cycles.
The mean number of chemotherapy cycles administered to the patients
was 7. During admission or in the outpatient clinic, the
measurement was performed when the patients experienced symptoms of
CIPN, such as the severe sensory disorder, numbness, tingling and
pain. Even if the patients did not report CIPN symptoms, the
evaluation of CIPN was performed at least every 3 cycles. The
endpoint of the study was confirming the correlations between CIPN
grade and the pain threshold by using PNS-7000 and CTCAE version
4.0.
Statistical analysis
Kruskal-Wallis test followed by Dunn's multiple
comparison test was used for the measurement data from each group.
P<0.05 was considered to indicate statistically significant
differences.
Results
Patient background
A total of 57 patients were enrolled in the present
study, and the number of IES measurements was 151. The
characteristics of the patients are summarized in Table I. The median age was 63 years (range,
40-86 years). The type of cancer was ovarian in 24 cases, uterine
endometrial in 20, peritoneal in 5, uterine cervical in 4,
fallopian tube in 2, and primary unknown cancer in 2 cases.
Chemotherapy was performed as initial treatment in 42 cases and as
treatment for recurrence in 15 cases. Paclitaxel was included in
all chemotherapy regimens (Table
II). The number of chemotherapy cycles and the type of
chemotherapy regimens are shown in Table II. Only first-line regimen was
performed in most cases. The number of patients with CIPN was 54
(94.7%) (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | No. |
|---|
| Median age, years
(range) | 63 (40-86) |
| Primary site of
cancer | |
|
Ovary | 24 |
|
Uterine
endometrium | 20 |
|
Peritoneum | 5 |
|
Uterine
cervix | 4 |
|
Fallopian
tube | 2 |
|
Primary
unknown | 2 |
| Purpose of
chemotherapy | |
|
Initial
treatment | 42 |
|
Recurrence | 15 |
| Chemotherapy-induced
peripheral neuropathy | |
|
Yes | 54 |
|
No | 3 |
 | Table IINumber and type of chemotherapy
regimen. |
Table II
Number and type of chemotherapy
regimen.
| Line of
chemotherapy | |
|---|
| 1st | 2nd | 3rd | 4th | No. of patients |
|---|
| TC | None | None | None | 45 |
| TC | DC | None | None | 2 |
| TC | PLD | None | None | 2 |
| TC | IAP | None | None | 1 |
| TC | CPT-11 | None | None | 1 |
| TC | EC | DC | None | 2 |
| TC | DC | TC | None | 2 |
| TC | CPT-11 + PLD | T | GEM | 2 |
The characteristics of patients with CIPN are listed
in Table III. The number of
patients with CIPN grades 1 and >2 was 31 and 23, respectively.
No significant differences were found for age, type of cancer,
cumulative dose of paclitaxel and treatment for CIPN between the
two groups. The onset of CIPN occurred in 34 patients (63.0%) after
1 treatment cycle. Persistence of CIPN was observed in 21 (53.8%)
of the 39 patients who completed chemotherapy. There was no
significant correlation on between CTCAE grade and paclitaxel dose
or type of chemotherapy regimen by Pearson's correlation
coefficient test.
 | Table IIICharacteristics of patients with
CIPN. |
Table III
Characteristics of patients with
CIPN.
| Characteristics | Grade1 (n=31) | Grade ≥2 (n=23) |
|---|
| Age (years) ±
standard deviation | 63±10.6 | 59±9.7 |
| Primary site of
cancer, n | | |
|
Ovary | 12 | 9 |
|
Uterine
cervix | 2 | 2 |
|
Uterine
endometrium | 14 | 6 |
|
Fallopian
tube | 0 | 2 |
|
Peritoneum | 2 | 3 |
|
Primary
unknown | 1 | 1 |
| Total dose of
paclitaxel, mg | 1,500±1,110.4 | 1,440±1,064.4 |
| Treatment for CIPN
(Plural treatment was used for CIPN in some cases), n |
|
Herbal
medicine | 30 | 23 |
|
Pregabalin | 18 | 16 |
|
NSAIDs | 1 | 10 |
|
Other | 0 | 1 |
| Onset of CIPN, n | | |
|
After 1
cycle | 18 | 16 |
|
After 2
cycles | 9 | 4 |
|
After 3
cycles | 3 | 3 |
|
After 4
cycles | 1 | 0 |
Measurement of pain threshold by using
IES
CIPN measurement was performed for a mean of 1.75
times per patient. The distribution of CIPN measurements was as
follows: 1 time in 32 patients, 2 times in 13 patients, 3 times in
7 patients, 4 times in 4 patients, and 5 times in 1 patient.
The association between pain threshold and clinical
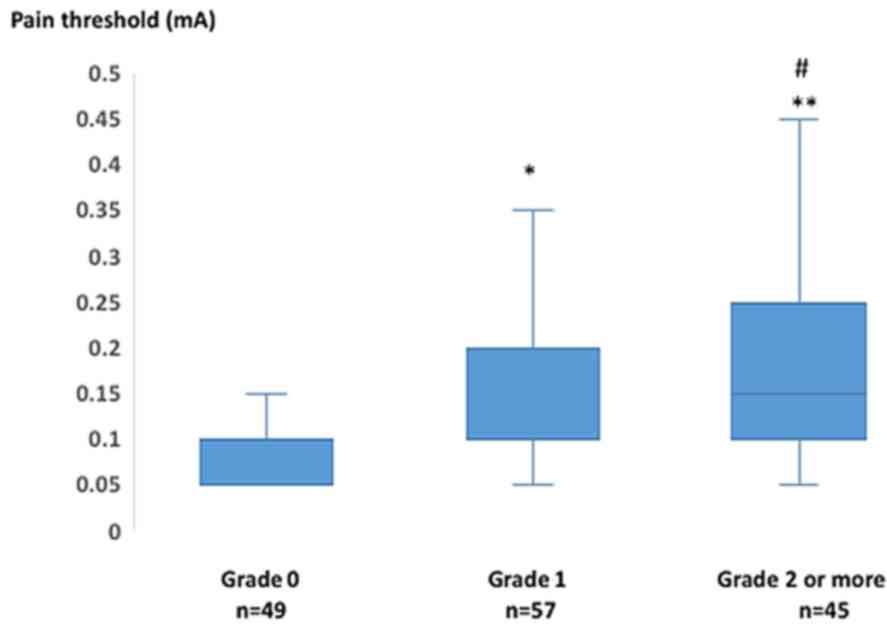
grading scale (NCI-CTCAE version 4.0) in 151 measurements is shown
in Fig. 2. The number of
measurements with clinical grades of 0, 1 and ≥2 was 49, 57 and 45,
respectively. The mean pain threshold in grade 1 was significantly
increased compared with that in grade 0 (0.1±0.07 vs. 0.14±0.05 mA,
respectively; P=0.024). Similarly, the mean pain threshold in grade
≥2 was significantly increased compared with that in grade 0
(0.18±0.12 vs. 0.1±0.05 mA, respectively; P=0.000). The mean pain
threshold in grade ≥2 was increased, with a marginal significance
compared with that in grade 1 (0.18±0.12 vs. 0.14±0.07 mA,
respectively; P=0.098). Therefore, the mean pain threshold
gradually increased with the progression of clinical grading
scale.
Only 2 cases suffered from grade 3 CIPN and are
briefly presented below:
Case 1
A 68-year-old woman who was treated with 3 cycles of
combined paclitaxel and carboplatin (TC) for stage IIIb
(International Federation of Gynecology and Obstetrics) ovarian
carcinosarcoma developed grade 3 CIPN after 3 cycles of TC, and the
pain threshold was 0.45 mA at that time. A computed tomography scan
revealed a pelvic mass 3 months after the initial surgery. The
patient was treated with ifosfamide + adriamycin + cisplatin
chemotherapy. The pain threshold gradually decreased by 0.05 mA and
the patient displayed no CIPN at 4 months after the change in
chemotherapy regimen.
Case 2
A 70-year-old woman who was treated with 2 cycles of
TC as adjuvant chemotherapy for uterine endometrial cancer
experienced severe numbness and tingling of her fingertips and the
tips of her toes. The patient was diagnosed with grade 3 CIPN and
the pain threshold at the time was 0.20 mA. TC chemotherapy was
switched to docetaxel and carboplatin (DC). However, the patient
suffered from ileus and severe constipation, likely caused by the
DC regimen. After that, chemotherapy was discontinued due to the
CIPN and those adverse effects. The symptoms subsided but became
less serious after chemotherapy discontinuation.
Discussion
The frequency of CIPN in the present study was
94.7%, which is higher compared with that reported previously
(17). In the present study, all
regimens were paclitaxel-based. However, the results may depend on
the CIPN evaluation method. Therefore, the method for evaluation is
crucial. Persistence of CIPN was seen in 21 (53.8%) of 39 patients
who completed chemotherapy. This percentage is markedly higher
compared with that reported previously (18,19).
Therefore, it is urgent to establish a standard method for the
evaluation of CIPN.
CIPN has been subjectively evaluated to date.
Physician-based grading scales are widely used in the clinical
practice and trials, but the evaluation methods differ among
physicians. Thus, the number of patient-reported evaluation
methods, including EORTC-QLQ-CIPN20 and FACT/GOG-Ntx, have
increased recently. However, there are several issues in the
evaluation of CIPN. Some patients do not fully understand the
content of the questionnaire, and a significant discordance was
observed between physician- and patient-reported CIPN (5).
Therefore, a consensus-based standardized CIPN
evaluation method is urgently required. One of the objective
assessment methods includes the use of a nerve conduction device
(11). This device demonstrated that
the progression of CIPN was associated with a significant decrease
in sensory nerve action potential. However, this method is not
prevalent worldwide. Additional objective assessment methods
include quantitative pain measurement, IES and Pain
Vision® (7,10,12,13).
These devices quantify the degree of pain by measuring the lowest
perceptible current and the current at which pain is perceived. The
benefits of both devices include that they are non-invasive,
painless, are associated with low discomfort, and do not require
special skills to operate. The disadvantages include the condition
being more costly compared with subjective assessments and the
necessity of an examination room for operating the devices.
The PNS-7000 was employed to evaluate CIPN. This
device was used for the quantitative measurement of diabetic
neuropathy, and the results demonstrated that the mean pain
threshold by PNS-7000 gradually increased with the progression of
clinical grading scale. To the best of our knowledge, this is the
first study to employ PNS-7000 for the assessment of CIPN. PNS-7000
may prove to be a non-invasive and convenient tool to evaluate
CIPN. The size of PNS-7000 is 15x9 cm, and its weight is 290 g,
making it easy to carry and the evaluation may be performed
anywhere, including at the bedside. The cost of PNS-7000 is
relatively lower compared with that of other devices used for the
measurement of CIPN. As regards objective assessment, the study
sample was the largest to date.
Of note, PNS-7000 precisely clarified the clinical
grading scale in the present study. Pain Vision® is a
similar device, and no difference in pain degree was observed
between grades 1 and 2. These results may depend on the difference
among the nerve fibers being stimulated. Pain is transmitted by
three types of nerve fibers, depending on its properties. Aβ fibers
transmit the senses of touch and pressure; Aδ fibers transmit
mechanical irritation, temperature and momentary pain; and C fibers
transmit burning pain sensation. Pain Vision® mainly
stimulates Aβ fibers. On the contrary, PNS-7000 selectively
stimulates Aδ and C fibers (12,14).
This distinction of the type of nerve fiber may cause differences
in the results. In the present study, the change in pain threshold
of each case was manifested. Thus, the clinical relevance of this
study is that we can determine objective CIPN.
There were several limitations to the present study.
First, this study was conducted in a single institution. Although
this was the largest objective study on the measurement on CIPN to
date, a larger patient sample is required, and the study must be
performed in a similar manner at multiple institutions. Second, the
result of IES were compared with clinical grading score using CTCAE
version 4.0. Third, only one CIPN measurement was performed in 32
patients. This may reflect a low compliance with the study
protocol, and may add uncertainty to the study results. In future
studies, we aim to compare the results with patient-reported
outcomes. PNS-7000 may help physicians to confirm the grade of
CIPN, which may allow for early intervention. A large randomized
validation study is urgently needed to verify the observations of
the present study.
In conclusion, the measurement of pain threshold by
using IES may prove to be a reliable indicator for quantitative
evaluation of CIPN.
Acknowledgements
The authors would like to thank all members of the
Gynecologic Oncology group of Hirosaki Graduate School of Medicine
for their helpful discussion and advice regarding the present
study.
Funding
The present study was supported by a Grant-in-Aid
for Cancer Research from the Ministry of Education, Culture,
Sports, Science and Technology, Tokyo, Japan (grant no.
17K11263).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
FO, MF and YY designed the project and experiments.
HO, AT, AA, TK, MM, MK, MO, RM and HH measured CIPN by using IES
clinically and also evaluated the CIPN grading of the patients. FO
and MF analyzed the data obtained in the present study and
generated the figures. FO and YY wrote the manuscript. All the
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of our institution (IRB approval no. 2016-263).
Informed consent was obtained from all the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wolf S, Barton D, Kottschade L, Grothey A
and Loprinzi C: Chemotherapy-induced peripheral neuropathy:
Prevention and treatment strategies. Eur J Cancer. 44:1507–1515.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gaballah A, Shafik A, Elhusseiny K and
Ashraf M: Chemotherapy-induced peripheral neuropathy in Egyptian
patients: Single institution retrospective analysis. Asian Pac J
Cancer Prev. 19:2223–2227. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Miltenburg NC and Boogerd W:
Chemotherapy-induced neuropathy: A comprehensive survey. Cancer
Treat Rev. 40:872–882. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pignata S, De Placido S, Biamonte R,
Scambia G, Di Vagno G, Colucci G, Febbraro A, Marinaccio M,
Lombardi AV, Manzione L, et al: Residual neurotoxicity in ovarian
cancer patients in clinical remission after first-line chemotherapy
with carboplatin and paclitaxel: The Multicenter Italian Trial in
Ovarian cancer (MITO-4) retrospective study. BMC Cancer.
6(5)2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Park SB, Kwok JB, Asher R, Lee CK, Beale
P, Selle F and Friedlander M: Clinical and genetic predictors of
paclitaxel neurotoxicity based on patient-versus clinician-reported
incidence and severity of neurotoxicity in the ICON7 trial. Ann
Oncol. 28:2733–2740. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
National Cancer Institute Division of
Cancer Treatment and Diagnosis: Common Terminology Criteria for
Adverse Events (CTCAE) v4.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
Accessed July 15, 2016.
|
|
7
|
Griffith KA, Couture DJ, Zhu S, Pandya N,
Johantgen ME, Cavaletti G, Davenport JM, Tanguay LJ, Choflet A,
Milliron T, et al: Evaluation of chemotherapy-induced peripheral
neuropathy using current perception threshold and clinical
evaluations. Support Care Cancer. 22:1161–1169. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wolf SL, Barton DL, Qin R, Wos EJ, Sloan
JA, Liu H, Aaronson NK, Satele DV, Mattar BI, Green NB and Loprinzi
CL: The relationship between numbness, tingling, and
shooting/burning pain in patients with chemotherapy-induced
peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20
instrument, N06CA. Support Care Cancer. 20:625–632. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Haryani H, Fetzer SJ, Wu CL and Hsu YY:
Chemotherapy-induced peripheral neuropathy assessment tools: A
systematic review. Oncol Nurs Forum. 44:E111–E123. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Doi D, Ota Y, Konishi H, Yoneyama K and
Araki T: Evaluation of the neurotoxicity of paclitaxel and
carboplatin by current perception threshold in ovarian cancer
patients. J Nippon Med Sch. 70:129–134. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Matsuoka A, Mitsuma A, Maeda O, Kajiyama
H, Kiyoi H, Kodera Y, Nagino M, Goto H and Ando Y: Quantitative
assessment of chemotherapy-induced peripheral neurotoxicity using a
point-of-care nerve conduction device. Cancer Sci. 107:1453–1457.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sato J, Mori M, Nihei S, Takeuchi S,
Kashiwaba M and Kudo K: Objective evaluation of
chemotherapy-induced peripheral neuropathy using quantitative pain
measurement system (Pain Vision®), a pilot study. J
Pharm Health Care Sci. 3(21)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gewandter JS, Chaudari J, Ibegbu C, Kitt
R, Serventi J, Burke J, Culakova E, Kolb N, Sluka KA, Tejani MA and
Mohile NA: Wireless transcutaneous electrical nerve stimulation
device for chemotherapy-induced peripheral neuropathy: An
open-label feasibility study. Support Care Cancer. 27:1765–1774.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Inui K and Kakigi R: Pain perception in
humans: Use of intraepidermal electrical stimulation. J Neurol
Neurosurg Psychiatry. 83:551–556. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kukidome D, Nishikawa T, Sato M, Igata M,
Kawashima J, Shimoda S, Matsui K, Obayashi K, Ando Y and Araki E:
Measurement of small fibre pain threshold values for the early
detection of diabetic polyneuropathy. Diabet Med. 33:62–69.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Suzuki C, Kon T, Funamizu Y, Ueno T, Haga
R, Nishijima H, Arai A, Tomiyama M and Baba M: Elevated pain
threshold in patients with asymptomatic diabetic neuropathy: An
intraepidermal electrical stimulation study. Muscle Nerve.
54:146–149. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vasey PA, Jayson GC, Gordon A, Gabra H,
Coleman R, Atkinson R, Parkin D, Paul J, Hay A and Kaye SB:
Scottish Gynaecological Cancer Trials Group. Phase III randomized
trial of docetaxel-carboplatin versus paclitaxel-carboplatin as
first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst.
96:1682–1691. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ewertz M, Qvortrup C and Eckhoff L:
Chemotherapy-induced peripheral neuropathy in patients treated with
taxanes and platinum derivatives. Acta Oncol. 54:587–591.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eckhoff L, Knoop A, Jensen MB and Ewertz
M: Persistence of docetaxel-induced neuropathy and impact of
quality of life among breast cancer survivors. Eur J Cancer.
51:292–300. 2015.PubMed/NCBI View Article : Google Scholar
|