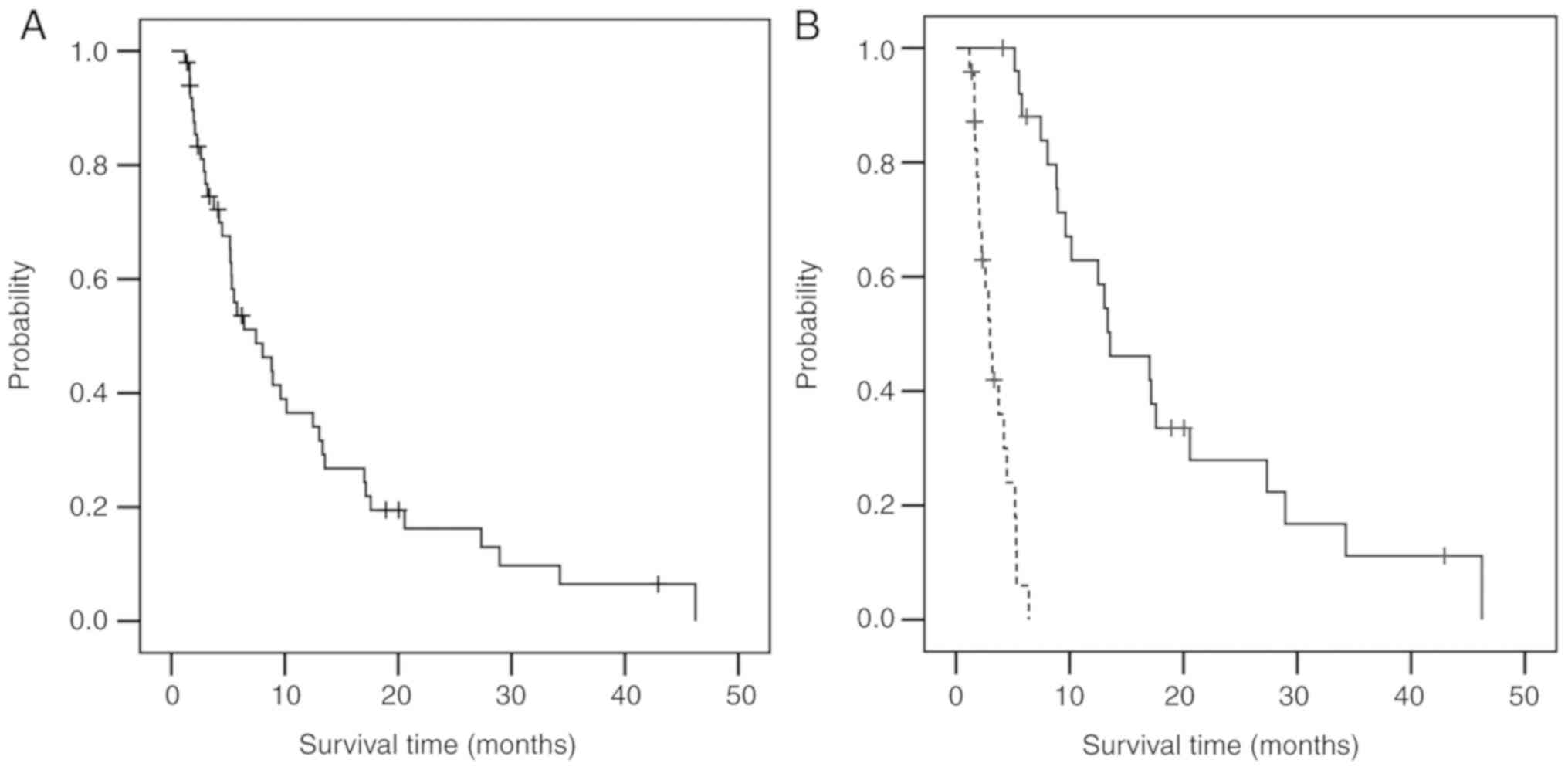
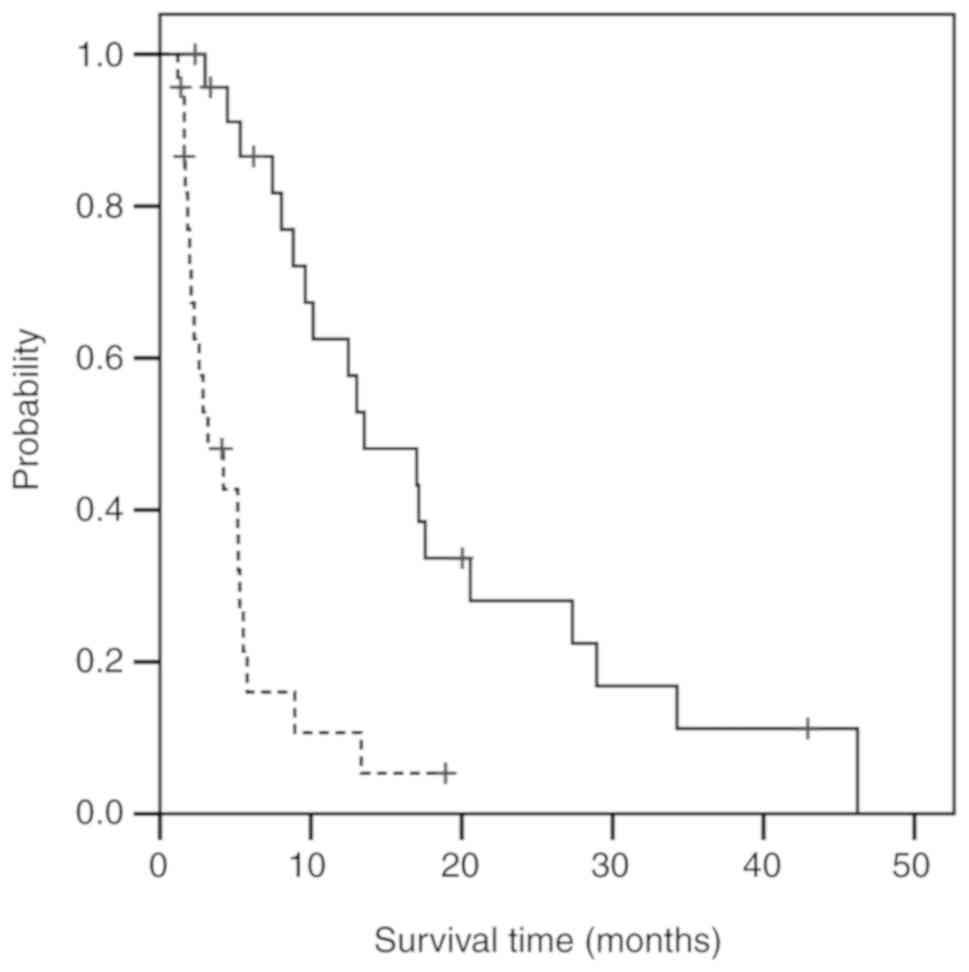
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yamaguchi Y, Ohshita A, Kawabuchi Y, Ohta
K, Shimizu K, Minami K, Hihara J, Miyahara E and Toge T: Adoptive
immunotherapy of cancer using activated autologous
lymphocytes-current status and new strategies. Hum Cell.
16:183–189. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yamaguchi Y, Ohta K, Kawabuchi Y, Ohshita
A, Okita R, Okawaki M, Hironaka K, Matsuura K and Toge T:
Feasibility study of adoptive immunotherapy for metastatic lung
tumors using peptide-pulsed dendritic cell-activated killer (PDAK)
cells. Anticancer Res. 25:2407–2415. 2005.PubMed/NCBI
|
|
4
|
Takayama T, Sekine T, Makuuchi M, Yamasaki
S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi
Y and Kakizoe T: Adoptive immunotherapy to lower postsurgical
recurrence rates of hepatocellular carcinoma: A randomised trial.
Lancet. 356:802–807. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kimura H and Yamaguchi Y: A phase III
randomized study of interleukin-2 lymphokine-activated killer cell
immunotherapy combined with chemotherapy or radiotherapy after
curative or noncurative resection of primary lung carcinoma.
Cancer. 80:42–49. 1997.PubMed/NCBI
|
|
6
|
Nagamine I, Yamaguchi Y, Ohara M, Ikeda T
and Okada M: Induction of gamma delta T cells using zoledronate
plus interleukin-2 in patients with metastatic cancer. Hiroshima J
Med Sci. 58:37–44. 2009.PubMed/NCBI
|
|
7
|
Braza MS and Klein B: Anti-tumour
immunotherapy with Vγ9Vδ2 T lymphocytes: From the bench to the
bedside. Br J Haematol. 160:123–132. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brandes M, Willimann K and Moser B:
Professional antigen-presentation function by human gammadelta T
cells. Science. 309:264–268. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sakamoto M, Nakajima J, Murakawa T, Fukami
T, Yoshida Y, Murayama T, Takamoto S, Matsushita H and Kakimi K:
Adoptive immunotherapy for advanced non-small cell lung cancer
using zoledronate-expanded γδTcells: A phase I clinical study. J
Immunother. 34:202–211. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Noguchi A, Kaneko T, Kamigaki T, Fujimoto
K, Ozawa M, Saito M, Ariyoshi N and Goto S: Zoledronate-activated
Vγ9γδ T cell-based immunotherapy is feasible and restores the
impairment of γδ T cells in patients with solid tumors.
Cytotherapy. 13:92–97. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bacik J, Mazumdar M, Murphy BA, Fairclough
DL, Eremenco S, Mariani T, Motzer RJ and Cella D: The functional
assessment of cancer therapy-BRM (FACT-BRM): A new tool for the
assessment of quality of life in patients treated with biologic
response modifiers. Qual Life Res. 13:137–154. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yamaguchi Y, Katata Y, Okawaki M, Sawaki A
and Yamamura M: A prospective observational study of adoptive
immunotherapy for cancer using zoledronate-activated killer (ZAK)
cells-an analysis for patients with incurable pancreatic cancer.
Anticancer Res. 36:2307–2313. 2016.PubMed/NCBI
|
|
14
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Koizumi W, Kim YH, Fujii M, Kim HK,
Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY, et al:
Addition of docetaxel to S-1 without platinum prolongs survival of
patients with advanced gastric cancer: A randomized study (START).
J Cancer Res Clin Oncol. 140:319–328. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qiao G, Wang X, Zhou L, Zhou X, Song Y,
Wang S, Zhao L, Morse MA, Hobeika A, Song J, et al: Autologous
dendritic cell-cytokine induced killer cell immunotherapy combined
with S-1 plus cisplatin in patients with advanced gastric cancer: A
prospective study. Clin Cancer Res. 25:1494–1504. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kono K, Ichihara F, Iizuka H, Sekikawa T
and Matsumoto Y: Expression of signal transducing T-cell receptor
zeta molecules after adoptive immunotherapy in patients with
gastric and colon cancer. Int J Cancer. 78:301–305. 1998.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomized,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Iwasaki M, Tanaka Y, Kobayashi H,
Murata-Hirai K, Miyabe H, Sugie T, Toi M and Minato N: Expression
and function of PD-1 in human γδ T cells that recognize
phosphoantigens. Eur J Immunol. 41:345–355. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao W, Jia L, Zhang M, Huang X, Qian P,
Tang Q, Zhu J and Feng Z: The killing effect of novel bi-specific
Trop2/PD-L1 CAR-T cell targeted gastric cancer. Am J Cancer Res.
9:1846–1856. 2019.PubMed/NCBI
|
|
22
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al: Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hirahara T, Arigami T, Yanagita S,
Matsushita D, Uchikado Y, Kita Y, Mori S, Sasaki K, Omoto I,
Kurahara H, et al: Combined neutrophil-lymphocyte ratio and
platelet-lymphocyte ratio predicts chemotherapy response and
prognosis in patients with advanced gastric cancer. BMC Cancer.
19(672)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yuan SQ, Nie RC, Chen YM, Qiu HB, Li XP,
Chen XJ, Xu LP, Yang LF, Sun XW, Li YF, et al: Glasgow prognostic
score is superior to ECOG PS as a prognostic factor in patients
with gastric cancer with peritoneal seeding. Oncol Lett.
15:4193–4200. 2018.PubMed/NCBI View Article : Google Scholar
|