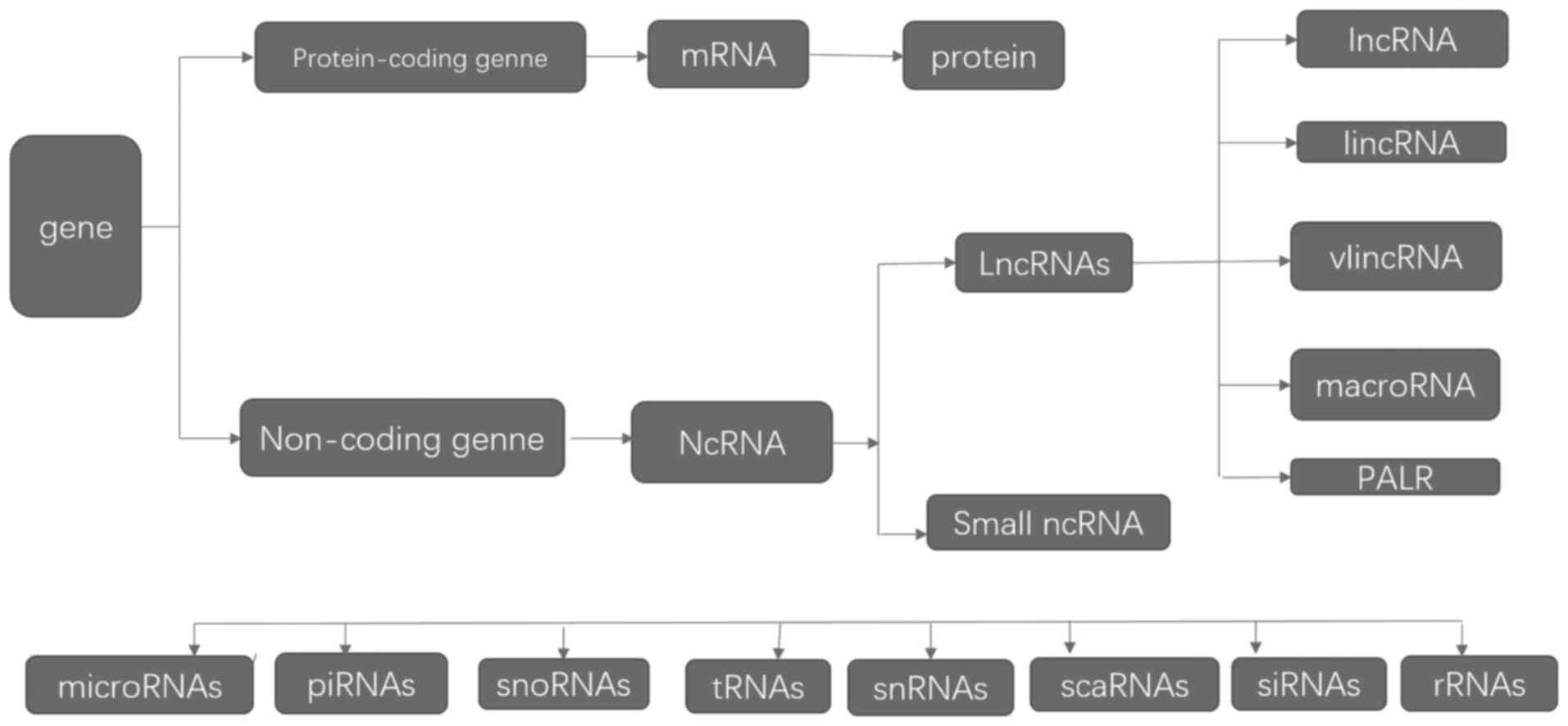
Prostate cancer (PCa) is the second most common
malignancy in Western countries and accounts for 10% of
cancer-related deaths (1).
Approximately 26,730 deaths occurred in 2017 due to PCa in the
United States. The causes of this disease include age, functional
testicles, and family heredity. Most cases of PCa undergo a series
of processes from androgen sensitivity neoplasia to metastatic
castration-resistant PCa (mCRPC), which is presently incurable.
Currently available means for diagnosis depend on prostate specific
antigen (PSA) and pathological biopsy. Since PSA testing was
introduced, the incidence of localized PCa has increased
significantly; PSA testing can predict cancer risk and treatment
outcome (2). According to the
literature, almost 240,000 individuals developed PCa in the United
States yearly; however, <15% of these patients eventually died;
mortality is largely dependent upon PSA testing and reasonable
treatment of PCa at an early stage (3-6).
However, serum PSA is not particular to PCa, and levels can be
enhanced in benign prostatic hyperplasia (BPH) (7) and prostatitis (8) even after a digital rectal examination.
Therefore, the lack of specificity limits its further development.
Low specificity has caused unnecessary biopsies, thereby leading to
the overtreatment of indolent cancers. Pathological biopsy is the
standard in diagnosing PCa, but it is an invasive examination,
which will cause hemorrhage, infection, and even blood poisoning.
Hence, a novel biomarker for PCa diagnosis and therapy should is
urgently needed.
RNA plays a crucial part in the regulation of gene
expression and genome organization (9,10). RNA
serves as a template for protein synthesis and exerts many
functions (11). The current
research has concluded that only 10% of the genome is made up of
protein-coding genes. A large part of the genome (~70%) is actively
transcribed, which indicates that noncoding RNAs (ncRNAs) account
for an overwhelming percentage of the human transcriptome (Fig. 1). At the start, NcRNAs, which is
viewed as ‘noisy RNAs,’ have no transcriptional function and
account for almost 90% RNAs in humans (12). ncRNAs can be divided into two major
groups according to their sizes, as follows: Long (lncRNA, >200
bp) and small ncRNAs (<200 bp) (13). Small ncRNAs include
termini-associated short, transcription initiation, splice site,
and antisense termini-associated short RNAs (14). Most lncRNAs are generated similar to
other mRNAs, as emphasized by RNA polymerase II activity and
histone modifications associated with transcription initiation and
elongation (15). lncRNAs can be
divided into intragenic and intergenic lncRNAs according to their
locations in the genome relative to protein coding genes (16). lncRNAs also play a role as decoys
for transcription factors (17) and
regulate protein activity (18,19).
lncRNAs are aberrantly expressed in a several human diseases,
including kidney cancer (20),
colorectal cancer (21),
endometrial cancer (22),
testicular cancer (23,24), breast cancer (25), and hematological cancers (26,27).
Bussemakers (28) first found
DD3PCA3, which is a lncRNA and is a potential diagnostic
biomarker for PCa in 1999. This discovery started the research on
the involvement of lncRNAs in PCa. We summarized the viewpoints, as
follows (Fig. 1).
The AR, as a protein coding gene, is situated on the
X chromosome, is approximately 110 kD and made up of four
functional regions, namely, (1) the
hinge region, (2) ligand-binding
domain, (3) N-terminal
transactivation domain, and (4)
DNA-binding domain (29-31).
The AR is a nuclear transcription factor required for normal
prostate development and PCa; thus, it plays key roles in PCa
initiation and progression (32,33).
PCa undergoes progression from androgen-sensitive to resistance to
castration. Androgen deprivation therapy (ADT) is the frontline
treatment for PCa at the late stages. However, after 12 to 24
months of androgen deprivation, PCa will eventually progress to the
lethal form of the disease known as castration-resistant PCa
(CRPC), which is eventually fatal for patients with PCa. The
reactivation of the AR is central to the progression of CRPC, and
treatment mechanisms may also be mediated by the AR signaling axis.
AR-dependent resistance mechanisms include AR enhancement, AR
single-base substitution, changed intratumoral androgen
biosynthesis, and the expression of constitutively active AR splice
variants (34-37).
lncRNAs can function as oncogenic and tumor suppressor in PCa
through AR signaling axis (Table
I).
PCGEM1, as the earliest prostate-specific lncRNA, is
located on chromosome 2q32 and overexpressed in nearly 84% of
patients with PCa. Recent studies revealed the PRNCR1 binding site
to AR 549-623 location and the PCGEM1 combining site to the
N-terminal location of AR. PCGEM1 and PRNCR1 interact with AR
(38) in a recently published
report. In various PCa cells, lncRNA cannot be detected in AR-null
cell lines, such as DU145 and PC3 (39,40).
The coalition of PRNCR1 and AR leads to enrollment of DOT1L
methyltransferase methylating AR and allows the subsequent
interaction of PCGEM1 with the methylated AR. In turn, PCGEM1
recruits PYGO2 (Pygopus 2), thereby allowing the binding of AR to
H3K4me3 chromatin marks to the promoter regions of AR-regulated
genes and leading to their activation (38).
A positive correlation of PCGEM1 with AR3, which is
one of the most important splice variants that play a key role in
castration resistance, was observed (41-43).
This AD-PCGEM1-AR3 axis can explain several reasons why the
effectiveness of ADT can only be sustained for a short time.
Heterogeneous nuclear ribonucleoprotein A1 (HnRNP A1) and U2AF65,
as splicing factors, play key roles in AR3 expression. When hnRNP
A1 combines with PCGEM1, the coalition activity of hnRNP A1 to AR
pre-mRNA is weakened. By contrast, the binding activity of U2AF65
to AR pre-mRNA is enhanced. A specific molecular mechanism does not
explain why interaction between U2AF65 and PCGEM1 is dominant. The
binding of PCGEM1 to U2AF65 is more competitive than that of hnRNP
A1 to AR pre-mRNA (44).
HOTAIR lncRNA is a 2.2 kb-long transcript and
overexpressed in a variety of cancer types, such as breast cancer,
colorectal cancer, lung cancer, and pancreatic cancer (45-48).
HOTAIR is sensitive to androgen and inhibited by androgen severely,
and its expression inhibits AR ubiquitination and avoids AR protein
degradation (49). HOTAIR lncRNA is
entirely abolished after AR target gene is knocked down via RNA
interference (49). Zhang et
al (49) concluded that HOTAIR
overexpression enhances aggressivity in PCa and upregulates in
enzalutamide-resistant PCa cells. Therefore, attention should be
paid to HOTAIR as a potential therapy target in
enzalutamide-resistant patients with PCa in the future.
C-Terminal binding protein 1 antisense (CTBP1-AS)
CTBP1-AS, which is situated in the AS region of C-terminal binding
protein 1 (CTBP1), is related to AR signaling pathway and is
overexpressed in both local PCa patients and metastatic PCa
patients, but not in Benign Prostatic Hyperplasia (BPH). It is
recruited to AR-binding sites. The CTBP1-AS lncRNA directly
inhibited the expression of CTBP1(50), which acted as the corepressor of AR
by recruiting the RNA binding transcriptional repressor
PTB-associated splicing factor and histone deacetylases. Thus,
CTBP1-AS can enhance AR transcriptional activity. Takayama et
al (51) have reported that
upregulation of CTBP1-AS and downregulation of CTBP1 in PCa.
CTBP1-AS knockdown inhibited cell proliferation in hormone-depleted
condition in both cell lines; in contrast, CTBP1-AS overexpression
induced tumor growth after castration (51).
PCA3 is a lncRNA that was initially named as DD3 and
is located on chromosome 9q21-22 in antisense direction within the
intron 6 of the Prune homolog 2 gene (PRUNE2 or BMCC1) (52). PCA3 is overexpressed in PCa cell
lines (53,54) and modulates PCa cell survival partly
according to the AR pathway, which is involved in the oncogenesis
of PCa. Meanwhile, the positive rates of its sensitivity and
specificity are 82.3 and 89.0%, respectively, compared with PSA,
which showed only 57.4 and 53.8% (55). Lemos et al (56) reported that PCA3 may regulate AR
signal pathway through AR cofactors (57), such as ARA 54, ARA 70, CBP, and
P300, when PCA3 and ERK are silenced, and Akt protein
phosphorylation levels stayed the same. Thus, the preferred method
should be activate AR. Both AR cofactors, including coactivators
and corepressors, were upregulated, which indicated that PCA3 may
be a negative modulator to AR and aberrant cofactor activity
because altered or changed expression levels may be factors to the
progression to mCRPC. A future potential therapy for PCa patients,
especially mCRPC, is the application of PCA3.
PI3K/Akt pathway is one of the key signal
transduction pathways regulating cell proliferation. PI3K/AKT/mTOR
signaling, PTEN/PI3K/AKT pathway, and PI3K/AKT/NF-kappaB/BMP-2-Smad
axis play important roles in cancer progression and development
(58). PI3K enzymes regulate
cellular signal transduction. The PI3K/Akt pathway mainly includes
PI3K activation, recruiting pleckstrin homology (PH)
domain-containing proteins, phosphorylation, activating AKT, and
activating necessary downstream targets (59). PI3K/AKT/mTOR is overexpressed in
30-50% of all prostate cancers (60), and its signal is regulated in PCa
cellular proliferation (61),
apoptosis (62), invasion, and
migration (63).
Genome instability is the main factor in the
promotion of cancer. The aberrant expression of lncRNAs is related
to the development and progression of PCa and plays an important
role in tumorigenesis or tumor-inhibiting in patients with PCa.
Several lncRNAs are upregulated as oncogenes, whereas others are
downregulated in cancer.
PCAT-1, which is located in the 8q24.21 gene desert
with nearly 725 kb upstream of the c-MYC oncogene (70), is overexpressed in patients with PCa
(71). C-MYC protein is upregulated
by PCAT-1, thereby resulting in specific gene expression programs
and cell proliferation (72). When
PCAT-1 is knocked down in LNCaP cells, cellular proliferation is
diminished, thereby indicating that it is a potential novel
biomarker for colorectal cancer metastasis.
The lncRNA NEAT2 is 7 kb long and is also known as
MALAT1. NEAT2 is highly overexpressed in a series of cancers,
including prostate cancer (77),
osteosarcoma (78), pancreatic
cancer (79), breast cancer
(80), bladder cancer (81), and esophageal cancer (82,83).
When NEAT2 is knocked down, cell hyperproliferation and metastasis
are inhibited in a PCa cell, thereby leading to cell cycle block in
the G0/G1 phases (77). NEAT2 promotes the activation of PRC2
by connecting to the polycomb protein enhancer of zeste homolog 2
(EZH2) and enhances the EZH2-mediated inhibition of
polycomb-dependent target gene E-cadherin in clear renal cancer
(84). Meanwhile, NEAT2 controls
cell cycle progression by regulating the oncogenic transcription
factor B-MYB (Mybl2) (85).
The H19 gene, which is transcribed from H19/Igf2
gene cluster, is located on human chromosome 11p15.5. H19 can acts
as a tumor suppressor gene. Zhu et al (86) found that the decreased H19
expression is significant in metastatic prostate cell compared with
local prostate epithelial cell. Hence, H19/miR-675 axis inhibits
PCa metastasis according to TGFBI downregulation.
Double-strand DNA breaks (DSBs) are potentially
lethal DNA lesions. Homologous recombination (HR) is an effective
pathway for eliminating DSBs and repairing injured DNA replication
forks. RAD51 is the core recombinase involved in HR, and increased
RAD51 levels may cause tumorigenesis. Prensner et al
(87) found that lncRNA PCAT1 is
involved in the DSB repair process in PCa. TODRA is a novel lncRNA
that is also known as RAD51 antisense RNA 1 and is located on
15q15.1. TODRA plays a role in RAD51 regulation. Gazy et al
(88) reported that the
overexpression of TODRA causes DSB repair by HR and also enhances
the fraction of RAD51 foci formed after DNA damage.
Recent studies indicated that lncRNAs can recognize
miRNA elements that can be targeted by miRNAs (89). A Zebrafish model where miR-125b
regulates 7sl lncRNA expression is a typical example of the
miRNA-lncRNA interaction (90).
Meanwhile, HOTAIR downregulation is targeted by the tumor
suppressor miR-34a, thereby inhibiting CRPC cell growth (91).
lncRNAs has multiple functions and high cell-type
specificity. Thus, lncRNAs can provide an avenue for PCa diagnosis,
prognosis, and therapy. Currently, the use of lncRNA for PCa
patients is being explored.
The RNAi technology, which interferes with RNA
expression through antisense technologies, can be widely used for
the weak expression levels of lncRNAs with oncology potential
(92). The key cancer-associated
genes with therapeutic siRNAs have been suppressed in clinical
trials.
Another method for inhibiting cancer-associated RNA
is by using catalytic nucleic acids, including antisense
oligonucleotides (ASOs) or by using small molecule inhibitors that
can also be used to modulate lncRNAs. The small molecule inhibitors
prevent the interaction of HOTAIR with LSD1 or PRC2 complexes,
thereby restricting the metastatic potential of breast cancer
(93).
Currently, CRISPER/Cas (clustered regularly
interspaced short palindromicrepeats/CRISPER-associated system)
take advantage of knocking out targeting gene for treating PCa
patients. Meanwhile, Shechner et al (94) have ever reported of using
CRISPER/Cas system successfully.
lncRNAs are potential novel biomarkers as
therapeutic targets for patients with PCa. Considering the multiple
and varied processes of PCa, its treatment should be planned
precisely for each patient. At the same time, when modern
technology is used to kill tumors, the safety of normal tissues
should be ensured. However, in spite of its advantage over other
therapeutic options, many questions need to be addressed.
The biggest challenge is that further research and
large-scale validation studies are imperative before the successful
application to clinical trials, because the molecular mechanisms of
lncRNAs and pathogenesis of PCa have not been thoroughly
understood. Nevertheless, the clearer the lncRNA functions are, the
better their field of therapeutic usage will be. The next challenge
is that the current lncRNA marker candidates are mostly based on a
small sample, and the lack of validity limits further development.
Thus, the effectiveness of lncRNA markers have to be prospectively
verified in large and varied datasets. Research in animal models
and clinical trials is needed to evaluate the potential side
effects, including toxicity, body distribution, pharmacokinetics,
and pharmacodynamics data.
Hence, lncRNAs are intriguing targets in treating
patients with PCa, and their potential in therapy can be
remarkable.
The authors would like to thank Miss Chen Gao
(Department of Surgery, Peking University Shenzhen Hospital,
Shenzhen, Guangdong, China) for assistance.
The present study was supported by funding from
Science and Technology Plan Project of Guangzhou of China (grant
no. 201510010177), Medical Science and Technology Program of
Guangzhou of China (grant nos. 20171A011329 and 20171A010325),
Youth Scientific Research Project of Guangzhou Medical University
of China (grant no. 2015A14) and Medical Research Foundation of
Guangdong Province of China (grant no. A2018093).
All data generated or analyzed during the present
study are included in this article.
GJ, ZS, XL, YH, ZL and XJ conceived and designed the
study. GJ and ZS wrote the manuscript. GJ, ZS, XL, YH, ZL and XJ
collected the data. ZS and XJ reviewed and edited the manuscript.
All authors read and approved the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Carter HB and Pearson JD: PSA velocity for
the diagnosis of early prostate cancer. A new concept. Urol Clin
North Am. 20:665–670. 1993.PubMed/NCBI
|
|
3
|
Gandellini P, Folini M, Longoni N, Pennati
M, Binda M, Colecchia M, Salvioni R, Supino R, Moretti R, Limonta
P, et al: miR-205 Exerts tumor-suppressive functions in human
prostate through down-regulation of protein kinase Cepsilon. Cancer
Res. 69:2287–2295. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tucci P, Agostini M, Grespi F, Markert EK,
Terrinoni A, Vousden KH, Muller PA, Dotsch V, Kehrloesser S, Sayan
BS, et al: Loss of p63 and its microRNA-205 target results in
enhanced cell migration and metastasis in prostate cancer. Proc
Natl Acad Sci USA. 109:15312–15317. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gandellini P, Profumo V, Casamichele A,
Fenderico N, Borrelli S, Petrovich G, Santilli G, Callari M,
Colecchia M, Pozzi S, et al: miR-205 regulates basement membrane
deposition in human prostate: Implications for cancer development.
Cell Death Differ. 19:1750–1760. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bonci D, Coppola V, Musumeci M, Addario A,
Giuffrida R, Memeo L, D'Urso L, Pagliuca A, Biffoni M, Labbaye C,
et al: The miR-15a-miR-16-1 cluster controls prostate cancer by
targeting multiple oncogenic activities. Nat Med. 14:1271–1277.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Park TY, Chae JY, Kim JW, Kim JW, Oh MM,
Yoon CY and Moon du G: Prostate-specific antigen mass and free
prostate-specific antigen mass for predicting the prostate volume
of korean men with biopsy-proven benign prostatic hyperplasia.
Korean J Urol. 54:609–614. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Hu HL, Liu ZF, Sun WZ, Chen XX and
Wu CL: Diagnosis and treatment of xanthogranulomatous prostatitis:
A case report and review of the literature. Zhonghua Nan Ke Xue.
19:149–152. 2013.PubMed/NCBI(In Chinese).
|
|
9
|
ENCODE Project Consortium. An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ma W, Ay F, Lee C, Gulsoy G, Deng X, Cook
S, Hesson J, Cavanaugh C, Ware CB, Krumm A, et al: Fine-scale
chromatin interaction maps reveal the cis-regulatory landscape of
human lincRNA genes. Nat Methods. 12:71–78. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ling H, Vincent K, Pichler M, Fodde R,
Berindan-Neagoe I, Slack FJ and Calin GA: Junk DNA and the long
non-coding RNA twist in cancer genetics. Oncogene. 34:5003–5011.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kapranov P, Cheng J, Dike S, Nix DA,
Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermuller J,
Hofacker IL, et al: RNA maps reveal new RNA classes and a possible
function for pervasive transcription. Science. 316:1484–1488.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brosnan CA and Voinnet O: The long and the
short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10(38)2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mercer TR and Mattick JS: Structure and
function of long noncoding RNAs in epigenetic regulation. Nat
Struct Mol Biol. 20:300–307. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Noncoding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3(ra8)2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mallory AC and Shkumatava A: lncRNAs in
vertebrates: Advances and challenges. Biochimie. 117:3–14.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yeh E, Cunningham M, Arnold H, Chasse D,
Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida
T, et al: A signalling pathway controlling c-Myc degradation that
impacts oncogenic transformation of human cells. Nat Cell Biol.
6:308–318. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Seles M, Hutterer GC, Kiesslich T, Pummer
K, Berindan-Neagoe I, Perakis S, Schwarzenbacher D, Stotz M, Gerger
A and Pichler M: Current insights into long non-coding RNAs in
renal cell carcinoma. Int J Mol Sci. 17(573)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Smolle M, Uranitsch S, Gerger A, Pichler M
and Haybaeck J: Current status of long non-coding RNAs in human
cancer with specific focus on colorectal cancer. Int J Mol Sci.
15:13993–14013. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Smolle MA, Bullock MD, Ling H, Pichler M
and Haybaeck J: Long non-coding RNAs in endometrial carcinoma. Int
J Mol Sci. 16:26463–26472. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bezan A, Gerger A and Pichler M: MicroRNAs
in testicular cancer: Implications for pathogenesis, diagnosis,
prognosis and therapy. Anticancer Res. 34:2709–2713.
2014.PubMed/NCBI
|
|
24
|
Ling H, Krassnig L, Bullock MD and Pichler
M: MicroRNAs in testicular cancer diagnosis and prognosis. Urol
Clin North Am. 43:127–134. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cerk S, Schwarzenbacher D, Adiprasito JB,
Stotz M, Hutterer GC, Gerger A, Ling H, Calin GA and Pichler M:
Current status of long Non-coding RNAs in human breast cancer. Int
J Mol Sci. 17(1485)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zebisch A, Hatzl S, Pichler M, Wolfler A
and Sill H: Therapeutic resistance in acute myeloid leukemia: The
role of non-coding RNAs. Int J Mol Sci. 17(2080)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Troppan K, Wenzl K, Deutsch A, Ling H,
Neumeister P and Pichler M: MicroRNAs in diffuse large B-cell
lymphoma: Implications for pathogenesis, diagnosis, prognosis and
therapy. Anticancer Res. 34:557–564. 2014.PubMed/NCBI
|
|
28
|
Bussemakers MJ, van Bokhoven A, Verhaegh
GW, Smit FP, Karthaus HF, Schalken JA, Debruyne FM, Ru N and Isaacs
WB: DD3: A new prostate-specific gene, highly overexpressed in
prostate cancer. Cancer Res. 59:5975–5979. 1999.PubMed/NCBI
|
|
29
|
Gelmann EP: Molecular biology of the
androgen receptor. J Clin Oncol. 20:3001–3015. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Claessens F, Denayer S, Van Tilborgh N,
Kerkhofs S, Helsen C and Haelens A: Diverse roles of androgen
receptor (AR) domains in AR-mediated signaling. Nucl Recept Signal.
6(e008)2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Heemers HV and Tindall DJ: Androgen
receptor (AR) coregulators: A diversity of functions converging on
and regulating the AR transcriptional complex. Endocr Rev.
28:778–808. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chang CS, Kokontis J and Liao ST:
Molecular cloning of human and rat complementary DNA encoding
androgen receptors. Science. 240:324–326. 1988.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Heinlein CA and Chang C: Androgen receptor
in prostate cancer. Endocr Rev. 25:276–308. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jentzmik F, Azoitei A, Zengerling F,
Damjanoski I and Cronauer MV: Androgen receptor aberrations in the
era of abiraterone and enzalutamide. World J Urol. 34:297–303.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mitsiades N: A road map to comprehensive
androgen receptor axis targeting for castration-resistant prostate
cancer. Cancer Res. 73:4599–4605. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Scher HI and Sawyers CL: Biology of
progressive, castration-resistant prostate cancer: Directed
therapies targeting the androgen-receptor signaling axis. J Clin
Oncol. 23:8253–8261. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Karantanos T, Corn PG and Thompson TC:
Prostate cancer progression after androgen deprivation therapy:
Mechanisms of castrate resistance and novel therapeutic approaches.
Oncogene. 32:5501–5511. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang L, Lin C, Jin C, Yang JC, Tanasa B,
Li W, Merkurjev D, Ohgi KA, Meng D, Zhang J, et al:
lncRNA-dependent mechanisms of androgen-receptor-regulated gene
activation programs. Nature. 500:598–602. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Srikantan V, Zou Z, Petrovics G, Xu L,
Augustus M, Davis L, Livezey JR, Connell T, Sesterhenn IA, Yoshino
K, et al: PCGEM1, a prostate-specific gene, is overexpressed in
prostate cancer. Proc Natl Acad Sci USA. 97:12216–12221.
2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Parolia A, Crea F, Xue H, Wang Y, Mo F,
Ramnarine VR, Liu HH, Lin D, Saidy NR, Clermont PL, et al: The long
non-coding RNA PCGEM1 is regulated by androgen receptor activity in
vivo. Mol Cancer. 14(46)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hu R, Dunn TA, Wei S, Isharwal S, Veltri
RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, et al:
Ligand-independent androgen receptor variants derived from splicing
of cryptic exons signify hormone-refractory prostate cancer. Cancer
Res. 69:16–22. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hu R, Lu C, Mostaghel EA, Yegnasubramanian
S, Gurel M, Tannahill C, Edwards J, Isaacs WB, Nelson PS, Bluemn E,
et al: Distinct transcriptional programs mediated by the
ligand-dependent full-length androgen receptor and its splice
variants in castration-resistant prostate cancer. Cancer Res.
72:3457–3462. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Khurana N, Kim H, Chandra PK, Talwar S,
Sharma P, Abdel-Mageed AB, Sikka SC and Mondal D: Multimodal
actions of the phytochemical sulforaphane suppress both AR and
AR-V7 in 22Rv1 cells: Advocating a potent pharmaceutical
combination against castration-resistant prostate cancer. Oncol
Rep. 38:2774–2786. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang Z, Zhou N, Huang J, Ho TT, Zhu Z,
Qiu Z, Zhou X, Bai C, Wu F, Xu M and Mo YY: Regulation of androgen
receptor splice variant AR3 by PCGEM1. Oncotarget. 7:15481–15491.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kogo R, Shimamura T, Mimori K, Kawahara K,
Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al:
Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nakagawa T, Endo H, Yokoyama M, Abe J,
Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T and Satoh K: Large
noncoding RNA HOTAIR enhances aggressive biological behavior and is
associated with short disease-free survival in human non-small cell
lung cancer. Biochem Biophys Res Commun. 436:319–324.
2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang A, Zhao JC, Kim J, Fong KW, Yang YA,
Chakravarti D, Mo YY and Yu J: lncRNA HOTAIR Enhances the
Androgen-receptor-mediated transcriptional program and drives
castration-resistant prostate cancer. Cell Rep. 13:209–221.
2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Takayama K, Tsutsumi S, Katayama S,
Okayama T, Horie-Inoue K, Ikeda K, Urano T, Kawazu C, Hasegawa A,
Ikeo K, et al: Integration of cap analysis of gene expression and
chromatin immunoprecipitation analysis on array reveals genome-wide
androgen receptor signaling in prostate cancer cells. Oncogene.
30:619–630. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Takayama K, Horie-Inoue K, Katayama S,
Suzuki T, Tsutsumi S, Ikeda K, Urano T, Fujimura T, Takagi K,
Takahashi S, et al: Androgen-responsive long noncoding RNA CTBP1-AS
promotes prostate cancer. EMBO J. 32:1665–1680. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Clarke RA, Zhao Z, Guo AY, Roper K, Teng
L, Fang ZM, Samaratunga H, Lavin MF and Gardiner RA: New genomic
structure for prostate cancer specific gene PCA3 within BMCC1:
Implications for prostate cancer detection and progression. PLoS
One. 4(e4995)2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
de Kok JB, Verhaegh GW, Roelofs RW,
Hessels D, Kiemeney LA, Aalders TW, Swinkels DW and Schalken JA:
DD3(PCA3), a very sensitive and specific marker to detect prostate
tumors. Cancer Res. 62:2695–2698. 2002.PubMed/NCBI
|
|
54
|
van Bokhoven A, Varella-Garcia M, Korch C,
Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ and Lucia
MS: Molecular characterization of human prostate carcinoma cell
lines. Prostate. 57:205–225. 2003.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yang Z, Yu L and Wang Z: PCA3 and
TMPRSS2-ERG gene fusions as diagnostic biomarkers for prostate
cancer. Chin J Cancer Res. 28:65–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lemos AE, Ferreira LB, Batoreu NM, de
Freitas PP, Bonamino MH and Gimba ER: PCA3 long noncoding RNA
modulates the expression of key cancer-related genes in LNCaP
prostate cancer cells. Tumour Biol. 37:11339–11348. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sung YY and Cheung E: Androgen receptor
co-regulatory networks in castration-resistant prostate cancer.
Endocr Relat Cancer. 21:R1–R11. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lui GY, Kovacevic Z, Richardson V, Merlot
AM, Kalinowski DS and Richardson DR: Targeting cancer by binding
iron: Dissecting cellular signaling pathways. Oncotarget.
6:18748–18779. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Morgan TM, Koreckij TD and Corey E:
Targeted therapy for advanced prostate cancer: Inhibition of the
PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 9:237–249.
2009.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gao N, Zhang Z, Jiang BH and Shi X: Role
of PI3K/AKT/mTOR signaling in the cell cycle progression of human
prostate cancer. Biochem Biophys Res Commun. 310:1124–1132.
2003.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kim SM, Park JH, Kim KD, Nam D, Shim BS,
Kim SH and Ahn KS, Choi SH and Ahn KS: Brassinin induces apoptosis
in PC-3 human prostate cancer cells through the suppression of
PI3K/Akt/mTOR/S6K1 signaling cascades. Phytother Res. 28:423–431.
2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Vo BT, Morton D Jr, Komaragiri S, Millena
AC, Leath C and Khan SA: TGF-β effects on prostate cancer cell
migration and invasion are mediated by PGE2 through activation of
PI3K/AKT/mTOR pathway. Endocrinology. 154:1768–1779.
2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Xu S, Yi XM, Tang CP, Ge JP, Zhang ZY and
Zhou WQ: Long non-coding RNA ATB promotes growth and
epithelial-mesenchymal transition and predicts poor prognosis in
human prostate carcinoma. Oncol Rep. 36:10–22. 2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Xue M, Chen W and Li X: Urothelial cancer
associated 1: A long noncoding RNA with a crucial role in cancer. J
Cancer Res Clin Oncol. 142:1407–1419. 2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ghiam AF, Taeb S, Huang X, Huang V, Ray J,
Scarcello S, Hoey C, Jahangiri S, Fokas E, Loblaw A, et al: Long
non-coding RNA urothelial carcinoma associated 1 (UCA1) mediates
radiation response in prostate cancer. Oncotarget. 8:4668–4689.
2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wu J, Cheng G, Zhang C, Zheng Y, Xu H,
Yang H and Hua L: Long noncoding RNA LINC01296 is associated with
poor prognosis in prostate cancer and promotes cancer-cell
proliferation and metastasis. Onco Targets Ther. 10:1843–1852.
2017.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wang J, Cheng G, Li X, Pan Y, Qin C, Yang
H, Hua L and Wang Z: Overexpression of long non-coding RNA
LOC400891 promotes tumor progression and poor prognosis in prostate
cancer. Tumour Biol. 37:9603–9613. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Thorne H, Mitchell G and Fox S: kConFab: A
familial breast cancer consortium facilitating research and
translational oncology. J Natl Cancer Inst Monogr. 2011:79–81.
2011.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Du Z, Fei T, Verhaak RG, Su Z, Zhang Y,
Brown M, Chen Y and Liu XS: Integrative genomic analyses reveal
clinically relevant long noncoding RNAs in human cancer. Nat Struct
Mol Biol. 20:908–913. 2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Prensner JR, Chen W, Han S, Iyer MK, Cao
Q, Kothari V, Evans JR, Knudsen KE, Paulsen MT, Ljungman M, et al:
The long non-coding RNA PCAT-1 promotes prostate cancer cell
proliferation through cMyc. Neoplasia. 16:900–908. 2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Prensner JR, Zhao S, Erho N, Schipper M,
Iyer MK, Dhanasekaran SM, Magi-Galluzzi C, Mehra R, Sahu A,
Siddiqui J, et al: RNA biomarkers associated with metastatic
progression in prostate cancer: A multi-institutional
high-throughput analysis of SChLAP1. Lancet Oncol. 15:1469–1480.
2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Mehra R, Udager AM, Ahearn TU, Cao X, Feng
FY, Loda M, Petimar JS, Kantoff P, Mucci LA and Chinnaiyan AM:
Overexpression of the long Non-coding RNA SChLAP1 independently
predicts lethal prostate cancer. Eur Urol. 70:549–552.
2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Mehra R, Shi Y, Udager AM, Prensner JR,
Sahu A, Iyer MK, Siddiqui J, Cao X, Wei J, Jiang H, et al: A novel
RNA in situ hybridization assay for the long noncoding RNA SChLAP1
predicts poor clinical outcome after radical prostatectomy in
clinically localized prostate cancer. Neoplasia. 16:1121–1127.
2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Prensner JR, Iyer MK, Sahu A, Asangani IA,
Cao Q, Patel L, Vergara IA, Davicioni E, Erho N, Ghadessi M, et al:
The long noncoding RNA SChLAP1 promotes aggressive prostate cancer
and antagonizes the SWI/SNF complex. Nat Genet. 45:1392–1398.
2013.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F,
Wei M, Shen J, Hou J, Gao X, et al: Long noncoding RNA MALAT-1 is a
new potential therapeutic target for castration resistant prostate
cancer. J Urol. 190:2278–2287. 2013.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Viazzi F, Bonino B, Ratto E, Desideri G
and Pontremoli R: Hyperuricemia, diabetes and hypertension. G Ital
Nefrol. 32 (Suppl 62)(gin/32.S62.10)2015.PubMed/NCBI(In Italian).
|
|
79
|
Mei YH, Yu JP and Li G: An extramedullary
plasmacytoma in the kidney of a 14-year-old girl: Case report and
review of the literature. Medicine (Baltimore).
96(e6092)2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zhao Z, Chen C, Liu Y and Wu C:
17β-Estradiol treatment inhibits breast cell proliferation,
migration and invasion by decreasing MALAT-1 RNA level. Biochem
Biophys Res Commun. 445:388–393. 2014.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ying L, Chen Q, Wang Y, Zhou Z, Huang Y
and Qiu F: Upregulated MALAT-1 contributes to bladder cancer cell
migration by inducing epithelial-to-mesenchymal transition. Mol
Biosyst. 8:2289–2294. 2012.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y
and Yang K: Up-regulation of long noncoding RNA MALAT1 contributes
to proliferation and metastasis in esophageal squamous cell
carcinoma. J Exp Clin Cancer Res. 34(7)2015.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Wang X, Li M, Wang Z, Han S, Tang X, Ge Y,
Zhou L, Zhou C, Yuan Q and Yang M: Silencing of long noncoding RNA
MALAT1 by miR-101 and miR-217 inhibits proliferation, migration,
and invasion of esophageal squamous cell carcinoma cells. J Biol
Chem. 290:3925–3935. 2015.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Hirata H, Hinoda Y, Shahryari V, Deng G,
Nakajima K, Tabatabai ZL, Ishii N and Dahiya R: Long noncoding RNA
MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and
interacts with miR-205. Cancer Res. 75:1322–1331. 2015.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Joaquin M and Watson RJ: Cell cycle
regulation by the B-Myb transcription factor. Cell Mol Life Sci.
60:2389–2401. 2003.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Zhu M, Chen Q, Liu X, Sun Q, Zhao X, Deng
R, Wang Y, Huang J, Xu M, Yan J and Yu J: lncRNA H19/miR-675 axis
represses prostate cancer metastasis by targeting TGFBI. FEBS J.
281:3766–3775. 2014.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Prensner JR, Chen W, Iyer MK, Cao Q, Ma T,
Han S, Sahu A, Malik R, Wilder-Romans K, Navone N, et al: PCAT-1, a
long noncoding RNA, regulates BRCA2 and controls homologous
recombination in cancer. Cancer Res. 74:1651–1660. 2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Gazy I, Zeevi DA, Renbaum P, Zeligson S,
Eini L, Bashari D, Smith Y, Lahad A, Goldberg M, Ginsberg D and
Levy-Lahad E: TODRA, a lncRNA at the RAD51 locus, is oppositely
regulated to RAD51, and enhances RAD51-dependent DSB (Double Strand
Break) repair. PLoS One. 10(e0134120)2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Jalali S, Bhartiya D, Lalwani MK,
Sivasubbu S and Scaria V: Systematic transcriptome wide analysis of
lncRNA-miRNA interactions. PLoS One. 8(e53823)2013.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Chiyomaru T, Yamamura S, Fukuhara S,
Yoshino H, Kinoshita T, Majid S, Saini S, Chang I, Tanaka Y,
Enokida H, et al: Genistein inhibits prostate cancer cell growth by
targeting miR-34a and oncogenic HOTAIR. PLoS One.
8(e70372)2013.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Salehi S, Taheri MN, Azarpira N, Zare A
and Behzad-Behbahani A: State of the art technologies to explore
long non-coding RNAs in cancer. J Cell Mol Med. 21:3120–3140.
2017.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Tsai MC, Spitale RC and Chang HY: Long
intergenic noncoding RNAs: New links in cancer progression. Cancer
Res. 71:3–7. 2011.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Shechner DM, Hacisuleyman E, Younger ST
and Rinn JL: Multiplexable, locus-specific targeting of long RNAs
with CRISPR-display. Nat Methods. 12:664–670. 2015.PubMed/NCBI View Article : Google Scholar
|
|
95
|
St Laurent G, Wahlestedt C and Kapranov P:
The Landscape of long noncoding RNA classification. Trends Genet.
31:239–251. 2015.PubMed/NCBI View Article : Google Scholar
|