Introduction
Cancer has become one of the world's major disease
burdens. Its incidence, and the mortality due to the disease, are
increasing rapidly. In 2018 the cancer with the highest death rate
globally was lung cancer for both sexes, followed by colorectal
cancer, stomach cancer, liver cancer, female breast cancer and
oesophageal cancer (1,2). Cancer incidence and mortality in China
are about 50% of those in Asia. The five leading types of cancer
death of China in 2013, 2015 and 2018 were lung cancer, liver
cancer, stomach cancer, oesophageal cancer, and colorectal cancer
(3-5).
The main treatments used for cancer are surgery, radiotherapy,
chemotherapy, targeted therapy, and immunotherapy. The genes and
proteins which are altered in cancer cells, immune cells and other
cells in the tumour microenvironment can be targeted for treatment
(6), and the selenoproteome may be
targeted in both cancer cells and immune cells for therapy, due to
the important role of selenium in cancer and immunity (7-10).
It has been demonstrated that selenoproteins,
including selenoprotein P (SELP, SELENOP), glutathione peroxidases
(GPX), thioredoxin reductases (TXNRD) and selenoprotein F (SEP15,
SELENOF), can regulate tumourigenesis and progression through their
effects on cancer-related signalling pathways (11). The relationship between single
nucleotide polymorphisms in selenoprotein genes and cancer risk has
been studied for SELENOP and GPX (12), as well as TXNRD, selenoprotein N
(SEPN1, SELENON), selenoprotein S (VIMP, SELENOS), and
selenoprotein W (SEPW1, SELENOW) (13). It has been suggested that SELENOP is
decreased in various cancers, except for metastatic melanoma, in
which it is elevated (11), and low
concentrations of SELENOP are related to poor survival in renal
cancer (14). TXNRD has been shown
to be overexpressed in aggressive tumours, including breast cancer
and melanomas (15), but whether
there is a relationship between TXNRD levels and prognosis is
unknown. Methylation of GPX1, GPX3, selenium binding protein 1
(SELENBP1) and methionine sulfoxide reductase B1 (MSRB1, SEPX1,
SELENOR) have been observed in human cancers and human cancer cell
lines, and GPX3 methylation can be a tumour biomarker in prostate
cancer (16).
Previous studies have focused primarily on the link
between a single selenoprotein and a specific cancer risk,
including changes in gene expression, single nucleotide
polymorphisms, and methylation. However, the selenoproteome contain
25 selenoproteins and seven proteins related to selenoprotein
synthesis, and changes in selenoproteome expression levels and
methylation, may be valuable for tumour identification and
prognosis. The Gene Set Cancer Analysis website GSCALite
(http://bioinfo.life.hust.edu.cn/web/GSCALite/)
(17) is a useful resource for the
analysis of the roles of selenoproteomes in various cancers.
In the present study, GSCALite was used to evaluate
differential expression and survival analysis of the selenoproteome
in the five leading types of cancer. Single Nucleotide Variations
(SNVs) and overall survival (OS) affected by SNVs were analysed.
Copy Number Variation (CNV), methylation and the relationship
between CNV, methylation and gene expression, as well as the
effects of methylation on OS were also studied. Finally, the
pathways involved in cancer development, growth, and progression
were evaluated.
Materials and methods
Selenoproteome gene set
collection
The HUGO Gene Nomenclature Committee (HGNC) symbol
gene set for transcription and translation of selenoprotein was
accessed. The selenoprotein gene set included iodothyronine
deiodinase 1 (DIO1), DIO2, DIO3, GPX1, GPX2, GPX3, GPX4, GPX6,
SELENOR, SELENOF, selenoprotein H (C11orf31, SELENOH),
selenoprotein I (EPT1, SELENOI), selenoprotein K (SELK, SELENOK),
selenoprotein M (SELM, SELENOM), SELENON, selenoprotein O (SELO,
SELENOO), SELENOP, SELENOS, selenoprotein T (SELT, SELENOT),
selenoprotein V (SELV, SELENOV), SELENOW, TXNRD1, TXNRD2, TXNRD3
and selenophosphate synthetase 2 (SEPHS2). The selenoprotein
expression-related gene set included selenocysteine lyase (SCLY),
SEPHS1, SELENBP1, selenocysteine insertion sequence-binding protein
2 (SECISBP2), tRNA selenocysteine associated protein (SECp43), Sep
(O-phosphoserine) tRNA:Sec (selenocysteine) tRNA synthase (SEPSECS)
and tRNA selenocysteine 1 associated protein 1 (TRNAU1AP). In
addition, homologues of selenium-containing GPX, including GPX5,
GPX7, GPX8, and homologues of SELENOR, such as MSRB2 and MSRB3,
were also contained in the gene set.
Selenoproteome gene set analysis in
cancer
Selenoproteome gene set analysis in cancer was
performed using the GSCALite website. Cancer genomic and normal
tissue data were downloaded from The Cancer Genome Atlas (TCGA) and
the Genotype-Tissue Expression (GTEx) database in the GSCALite
website. To access these data, we entered the gene set into the
search box at the top of the web page, then selected the databases
of colon adenocarcinoma (COAD), oesophageal carcinoma (ESCA), liver
hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung
squamous cell carcinoma (LUSC) and stomach adenocarcinoma (STAD)
from TCGA and the normal tissues of colon, oesophagus, liver, lung
and stomach from GTEx in the left search box. Finally, mRNA
Expression, SNV, CNV, Methylation, Pathway Activity and GTEx
Expression were selected in the right search box followed by
clicking the button Start Gene Set Analysis. A detailed description
of the method can be seen on the website (http://bioinfo.life.hust.edu.cn/web/GSCALite/).
For survival analysis, the OS in lung and liver were further
verified using Kaplan-Meier plots (http://kmplot.com/analysis/) (18,19).
Statistical analysis
The differential expression of the selenoproteome
was analysed using GSCALite. For mRNA Expression, the significant
conditions used were fold change (FC) >2 and FDR <0.05. For
survival analysis, genes with a Kaplan-Meier log-rank test P-value
<0.05 were used. For Methylation and Pathway activity, FDR ≤0.05
was considered significant.
Results
Selenoproteome mRNA and survival
Selenoproteome expression levels in the normal
tissues were analysed. The results shown in Fig. S1 suggested that the expression of
the selenoproteome was tissue specific. The three most highly
expressed genes in the colon were GPX3, SELENBP1 and SELENOW; in
the oesophagus they were GPX3, SELENOM and SELENOW; in the liver
were GPX3, GPX2 and GPX1; in the lung were GPX3, GPX1 and GPX4; and
in the stomach were GPX3, GPX2 and SELENOM. The differing
expression levels of selenoproteins between normal tissues and
tumours were analysed (Fig. 1A),
and it was found that the expression levels of GPX8, DIO2, GPX2,
SELENOI and SCLY were significantly increased, while those of
SELENOW, DIO1, SELENBP1, MSRB3 and GPX3 were significantly
decreased in colon adenocarcinoma. In oesophageal carcinoma, the
only gene with increased expression was SCLY, and the only gene
with decreased expression was GPX3. In liver hepatocellular
carcinoma, the genes with increased expression were GPX8, DIO2,
TXNRD1, GPX7, TRNAU1AP, SELENON and SELENOM, while the genes with
decreased expression were SEPSECS, SELENOP and DIO1. In stomach
adenocarcinoma, no genes had increased expression levels, and the
expression of SELENOM, SELENOW, SELENBP1, MSRB3 and GPX3 was
significantly decreased. In lung adenocarcinoma and lung squamous
cell carcinoma, the genes with increased expression were GPX8,
DIO2, TXNRD1, GPX7, GPX2 and SELENOI; the genes with decreased
expression were SELENOP, SELENBP1, MSRB3 and GPX3. SEPX1 and SEPHS2
had increased expression in lung adenocarcinoma, SEPHS1, SELENOV
and SELENOO were increased in lung squamous cell carcinoma, and
DIO3 was decreased in lung adenocarcinoma, Levels of GPX5, GPX1 and
DIO1 were decreased in lung squamous cell carcinoma. Expression
survival analysis (Fig. 1B) showed
that the GPX3, which had an increased level of expression in
stomach adenocarcinoma and lung squamous cell carcinoma, SELENOV in
oesophageal carcinoma, GPX8 and GPX4 in colon adenocarcinoma,
TXNRD1 and SEPHS1 in liver hepatocellular carcinoma and GPX8 in
lung adenocarcinoma were associated with poor survival. The genes
with decreased expression: SELENOP in liver hepatocellular
carcinoma and GPX3, and SELENOW, SELENOK, SELENBP1 and SECISBP2 in
lung adenocarcinoma had a poor prognosis. OS in lung and liver
cancer supported these findings, and are shown in Figs. S2 and S3. The expression of SELENOM was
significantly increased in liver hepatocellular carcinoma, while it
was significantly decreased in stomach adenocarcinoma. GPX3
expression was significantly decreased in lung adenocarcinoma, lung
squamous cell carcinoma and stomach adenocarcinoma. However, lower
expression of GPX3 in lung adenocarcinoma and the high expression
of GPX3 in lung squamous cell carcinoma and stomach adenocarcinoma
had a poor prognosis.
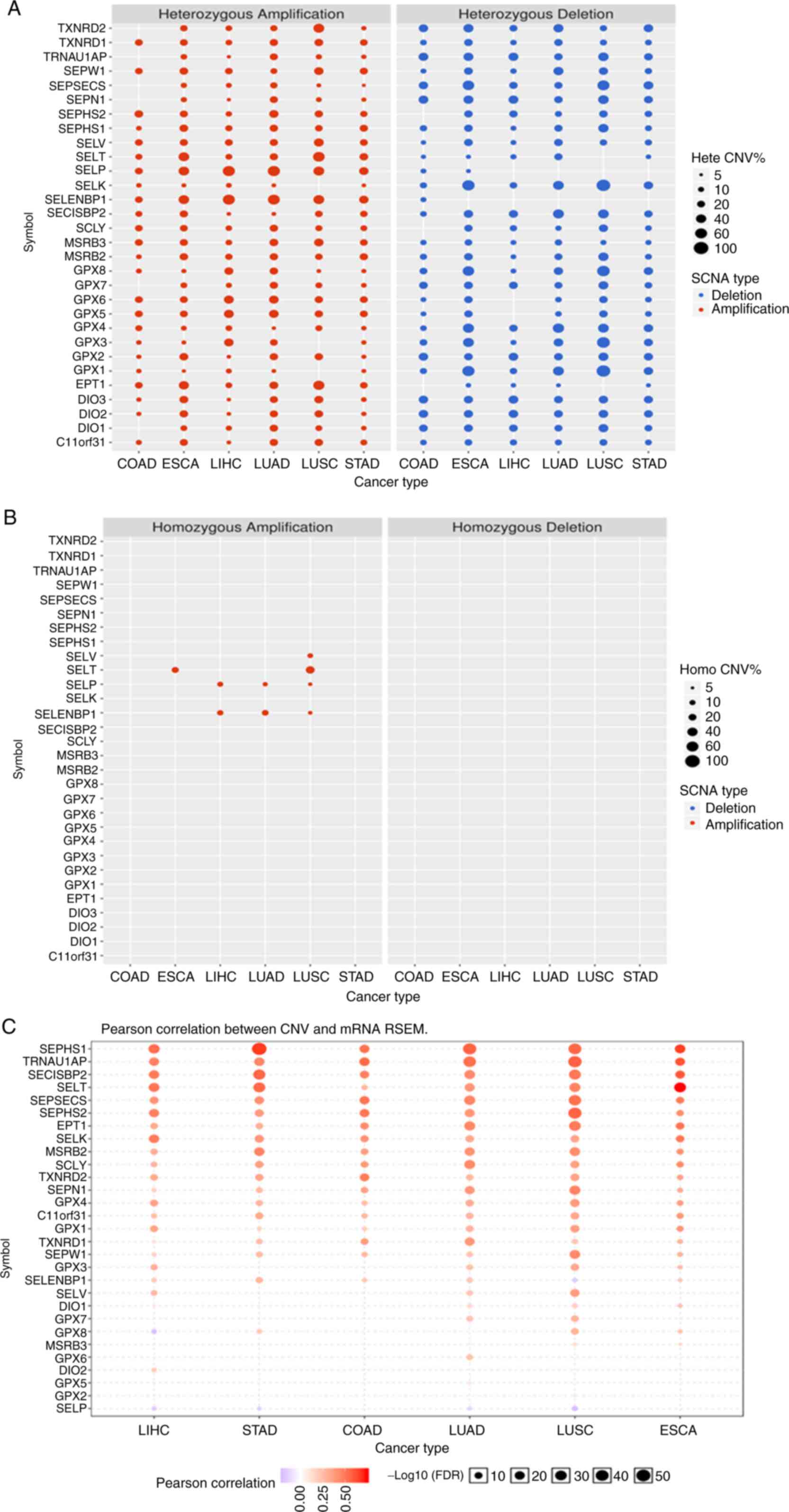
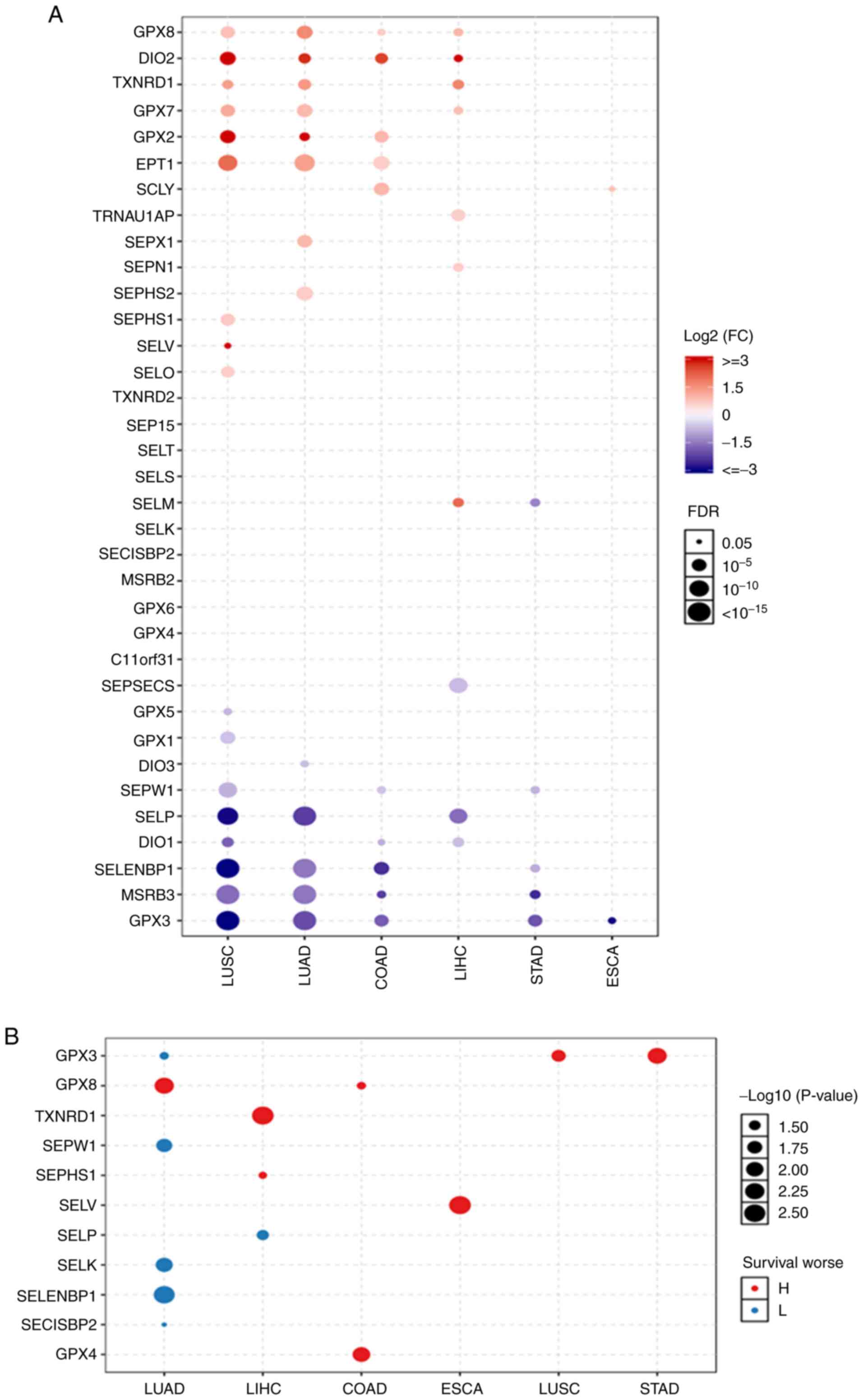 | Figure 1The differential selenoproteome
between (A) normal tissues and tumors, and (B) the expression
survival analysis in COAD, ESCA, LIHC, LUAD, LUSC and STAD. The
selenoproteome expression levels and survival analysis in the five
leading types of cancer death were performed using GSCALite, and
the results with significant differences were presented in the
figures. Blue and red represent lower expression and higher
expression, respectively, in (A) blue and red indicates the worse
of the low or high expression in the cancer types. The size dot
indicates the significance. COAD, colon adenocarcinoma; ESCA,
oesophageal carcinoma; LIHC, liver hepatocellular carcinoma; LUAD,
lung adenocarcinoma; LUSC, lung squamous cell carcinoma; STAD,
stomach adenocarcinoma. |
Single nucleotide variations in the
selenoproteome and survival
Fig. 2 show that the
SNV frequency of the selenoproteome was 69.05% (270 of 391
tumours). The top 10 mutated genes were SELENOP, DIO2, SECISBP2,
TXNRD1, DIO3, SELENOO, SELENBP1, GPX5, GPX6 and SEPHS1, and the
most frequent type of SNV was a missense mutation. The SNV
frequency of the selenoproteome was increased in oesophageal
carcinoma, liver hepatocellular carcinoma, colon adenocarcinoma,
stomach adenocarcinoma, lung squamous cell carcinoma and lung
adenocarcinoma. SNV survival analysis found no significant
difference between mutated and non-mutated genes.
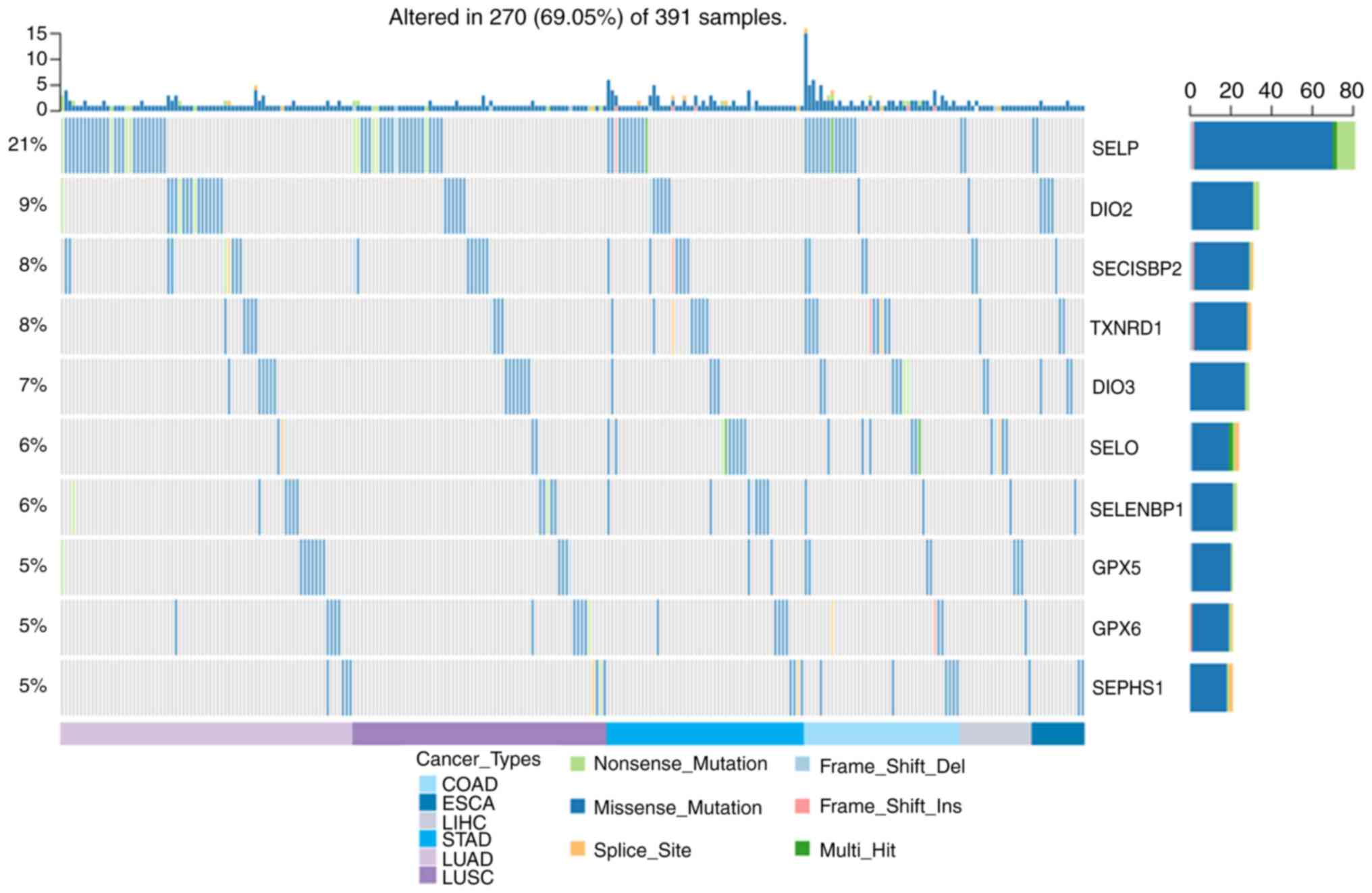 | Figure 2The SNV of selenoproteome in COAD,
ESCA, LIHC, LUAD, LUSC and STAD. The SNV of selenoproteome in the
five leading types of cancer associated mortality were performed
using GSCALite, and the top ten genes and the SNV type were
presented in the figure. COAD, colon adenocarcinoma; ESCA,
oesophageal carcinoma; LIHC, liver hepatocellular carcinoma; LUAD,
lung adenocarcinoma; LUSC, lung squamous cell carcinoma; STAD,
stomach adenocarcinoma. |
Copy number variation of the
selenoproteome
The results shown in Fig. 3A suggested that the CNV was very
different for each gene in each type of cancer. The main type of
CNV was heterozygous amplification and deletion, and only a few
genes, such as SELENOP and SELENBP1, had a low frequency of
heterozygous deletion. The genes with homozygous amplification were
SELENOT in oesophageal carcinoma and lung squamous cell carcinoma;
SELENOP and SELENBP1 in liver hepatocellular carcinoma, lung
squamous cell carcinoma and lung adenocarcinoma; and SELENOV in
lung squamous cell carcinoma. There were no homozygous deletions
(Fig. 3B). Pearson correlation
showed a strong correlation between CNV and SELENOT, SELENOV mRNA
RSEM in oesophageal carcinoma and lung squamous cell carcinoma, and
a poor correlation for SELENOP and SELENBP1 (Fig. 3C).
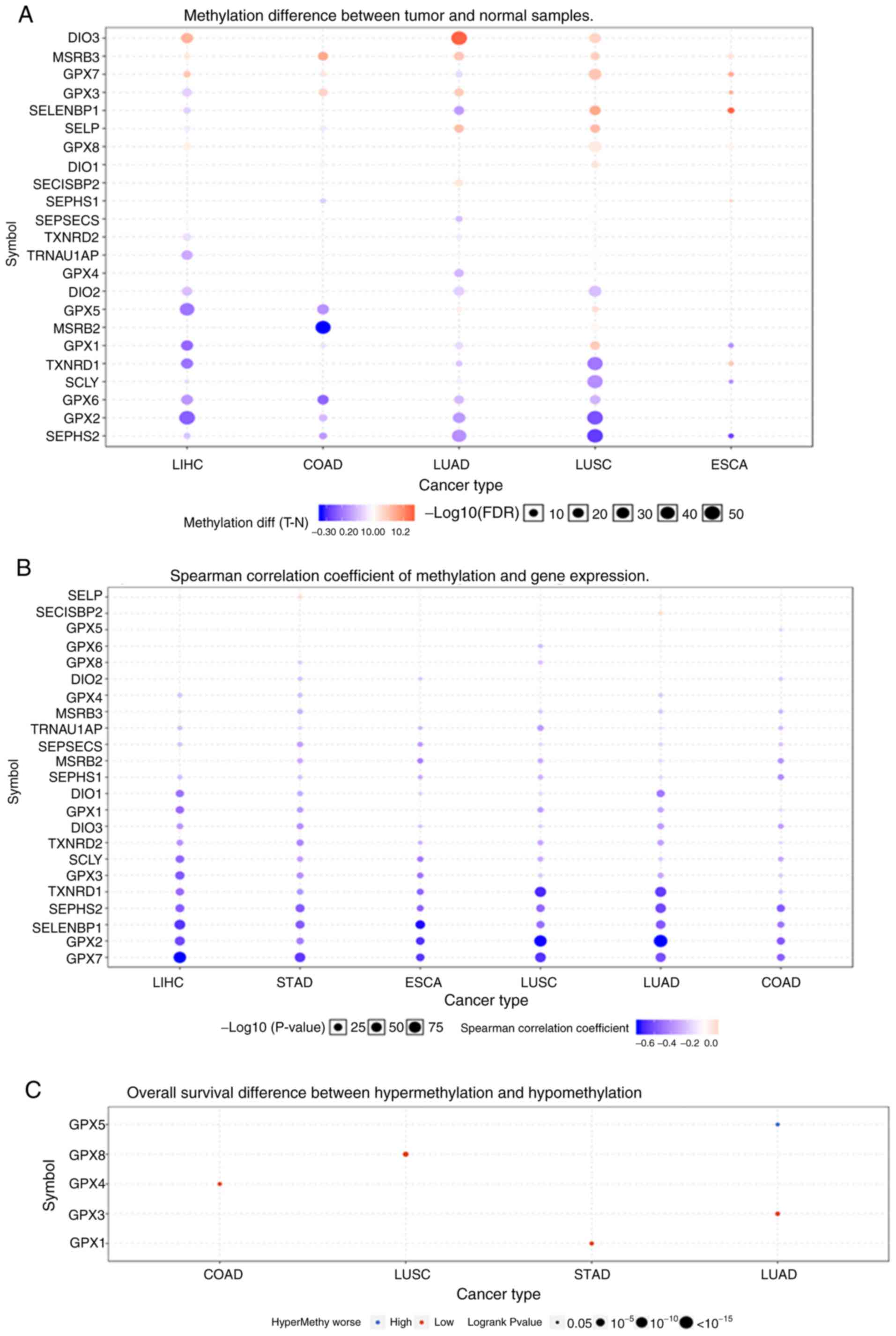
Methylation of the selenoproteome and
survival
Fig. 4A shows that
the methylation of the selenoproteome in different tumours was
highly heterogeneous. There were more hypermethylated than
hypomethylated genes in lung squamous cell carcinoma and
oesophageal carcinoma, and there were more hypomethylated than
hypermethylated genes in colon adenocarcinoma, liver hepatocellular
carcinoma and lung adenocarcinoma. There was no differential
methylation in stomach adenocarcinoma. The Spearman correlation
coefficient indicated that most of the genes were negatively
correlated, and only SELENOP in liver hepatocellular carcinoma and
stomach adenocarcinoma, and SECISBP2 in lung adenocarcinoma showed
a positive correlation between methylation and gene expression
(Fig. 4B). OS analysis suggested
that hypermethylation of GPX4 in colon adenocarcinoma, GPX8 in lung
squamous cell carcinoma, GPX1 in stomach adenocarcinoma and GPX3 in
lung adenocarcinoma had poor prognosis, and hypomethylation of GPX5
in lung adenocarcinoma had poor prognosis (Fig. 4C). GPX5 and GPX8, which were
associated with OS are not selenoproteins. The selenoproteins, GPX1
and GPX3 had lower expression levels in cancer tissues, and GPX4
showed no change.
Pathway activity
The pathway activity shown in Fig. 5A suggested that there were 24 genes
involved in tumour-related signalling pathways. For GPX3, the
expression level of which was decreased in cancer, the main
pathways were apoptosis inhibition, cell cycle inhibition, DNA
damage response inhibition, EMT activation, and RTK activation. For
TXNRD1, which was increased in some cancers, the main pathways were
apoptosis activation, cell cycle activation, and DNA damage
response activation. The relationship network between the 13
survival-related genes, SELENOM, which is increased in liver
hepatocellular carcinoma and decreased in stomach adenocarcinoma,
and pathways in the five leading types of cancer death, was
evaluated. The results shown in Fig.
5B revealed that GPX3 was not involved in the tumour-related
signalling pathways of oesophageal carcinoma, and TXNRD was not
involved in the tumour-related signalling pathways of colon
adenocarcinoma, stomach adenocarcinoma or liver hepatocellular
carcinoma. In colon adenocarcinoma, GPX4 was not involved in any of
the tumour-related signalling pathways, and GPX8 was involved in
the activation of EMT; in oesophageal carcinoma, neither reduced
GPX3 or the survival-related SELENOV genes were involved in
tumour-related signalling pathways; in liver hepatocellular
carcinoma, only one survival-related gene, SELENOP, was involved in
HormoneAR and RTK activation; for lung squamous cell carcinoma,
both GPX3 and GPX8 were involved in EMT activation; for lung
adenocarcinoma, survival-related genes were involved in RAS/MAPK,
RTK, EMT and PI3K/AKT activation, as well as apoptosis inhibition;
and in stomach adenocarcinoma, survival-related genes were involved
in PI3K/AKT activation and HormoneAR inhibition.
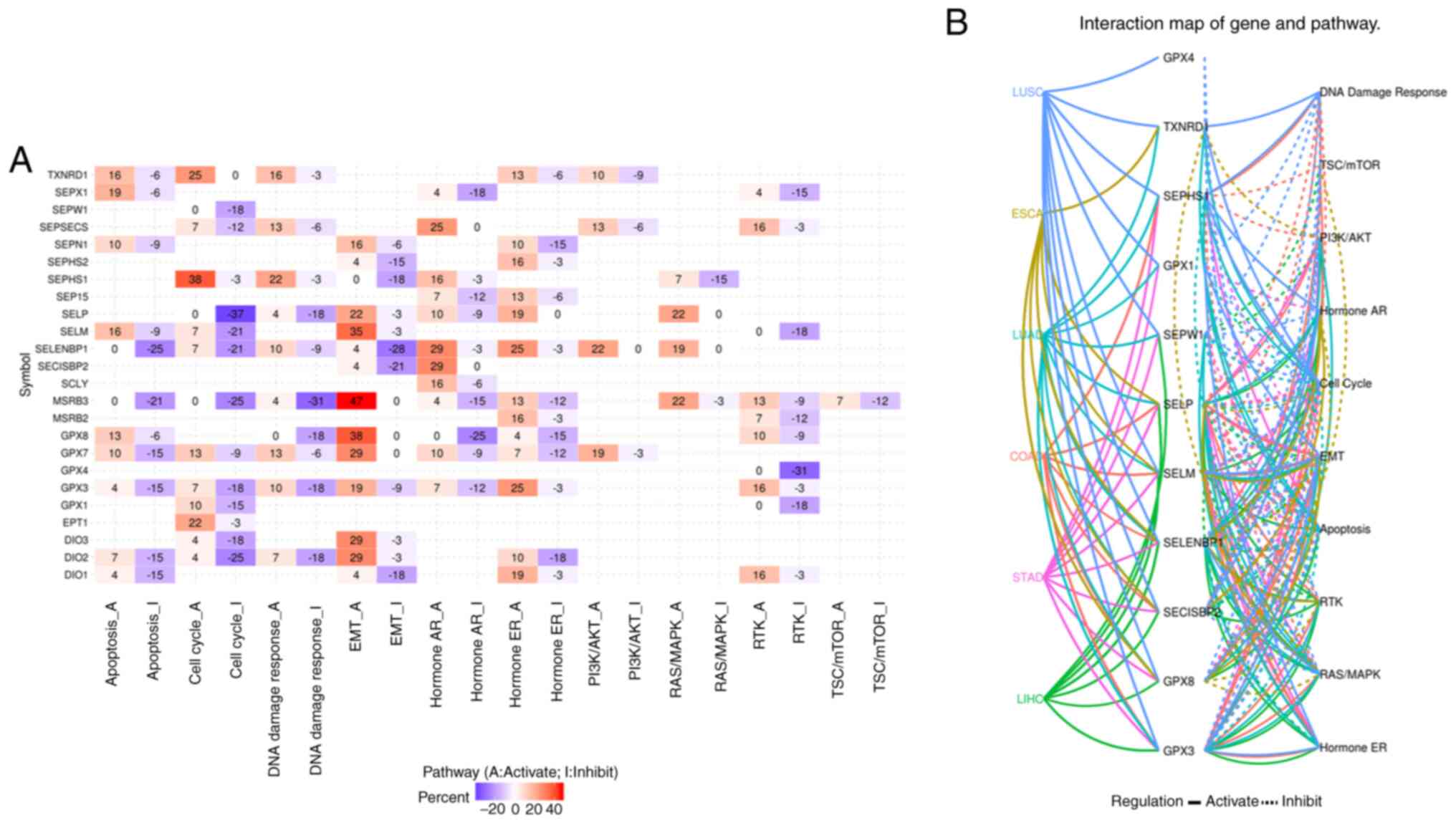 | Figure 5(A) The pathways involved in cancer
development, growth and progression of selenoproteome, and (B)
cancer pathway analysis of overall survival related selenoproteome
in COAD, ESCA, LIHC, LUAD, LUSC and STAD. The cancer pathway of
selenoproteome in the five leading types of cancer associated
mortality were performed using GSCALite. The luminance of red and
blue represents the degree of activation and inhibition of related
pathways, respectively, in (A). The solid line indicates that the
pathway is activated, the dotted line indicates that the pathway is
inhibited, and different colors represent different cancers in (B).
COAD, colon adenocarcinoma; ESCA, oesophageal carcinoma; LIHC,
liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC,
lung squamous cell carcinoma; STAD, stomach adenocarcinoma. |
Discussion
In the present study a global analysis of
differential expression, SNV, CNV, methylation and pathways in the
selenoproteomes of the five leading types of cancer death was
performed using GSCALite. The selenoproteomes were highly
heterogeneous, especially with respect to the effect on the OS of
cancer patients, although some genes performed the same role in
different tumours. To better understand the role of the
selenoproteome in the development, growth, and progression of
different tumours, we discuss the potential relationships between
the selenoproteome and cancer, one by one.
In this study, it was found that the expression
levels of GPX8, DIO2, GPX2, SELENOI and SCLY were significantly
increased, and those of SELENOW, DIO1, SELENBP1, MSRB3 and GPX3
were significantly decreased in colon adenocarcinoma. Previously,
TXNRD1, GPX1 and GPX4 have been found to be significantly increased
compared with corresponding normal tissues in 32 colon
adenocarcinoma patients from Germany (20), and the mRNA and protein levels of
TXNRD were increased several fold in 50% (5/10) of colon
adenocarcinoma patients (21). A
study in Japan showed that GPX1, GPX3 and SELENOP were decreased
and GPX2 was increased in colorectal cancer (CRC) (22). A study reviewed by Peters et
al found that the redox homeostasis related selenoproteins
GPX1-4, TXNRD1, SELENOF and SELENOP, and the WNT/β-catenin
signalling pathway associated selenoproteins DIO3, GPX2, TXNRD3 and
SELENOP may effect CRC risk and development (23). To the best of our knowledge, there
are many selenoproteins whose roles in CRC are unknown (23). These observations indicate that the
selenoproteomes which exhibited significant changes in this study
are a promising research target, which should have high priority.
Of particular interest are genes associated with survival, such as
GPX4 and GPX8. Although there was no observed relationship between
SNV and colorectal cancer/colon adenocarcinoma risk based on big
genomic data (24), the SNV of
GPX1, GPX2, GPX4, TXNRD1, TXNRD2, TXNRD3, SELENOF, SELENOP, SELENOS
and SELENOW have been well studied in CRC, and the SNV of SELENOP
and SELENOS had a strong effect on CRC risk (23-25).
For colon adenocarcinoma, the top eight potential risk
selenoprotein-related genes for SNV analysis were SELENOP, TXNRD1,
DIO3, SELENOO, SEPHS1, SCLY, TXNRD2 and SELENOI (Fig. S4). The results of methylation
analysis showed no difference in GPX4 methylation between tumour
and normal samples, but the hypermethylation of GPX4 in colon
adenocarcinoma was associated with poor survival. There was a
negative correlation between GPX4 methylation and expression.
Ferroptosis, which can be regulated by GPX4, has great prospects
for application in tumour immunotherapy (26) and tumour death (27), and might play important roles in
colon adenocarcinoma development, growth and progression due to the
potential correlation between higher GPX4 and lower survival.
Although GPX4 was not involved in the tumour-related signalling
pathways, we hypothesized that higher GPX4 expression inhibits
tumour cell ferroptosis, while GPX8 activates EMT, so patients with
higher GPX4 and GPX8 have poor survival.
In oesophageal carcinoma, levels of SCLY were
increased and those of GPX3 were decreased. The survival related
gene was SELENOV, which is associated with a low survival rate at
high levels, and there were no genes identified as being involved
in any relationship between methylation and OS. In a study of
oesophageal squamous cell carcinoma (ESCC) in China, it was found
that the mRNA and protein levels of GPX2 were significantly
increased compared with normal tissue, and lack of GPX2 expression
was associated with poor prognosis (28). The expression of SELENBP1 was
significantly decreased in oesophageal adenocarcinoma (EA) in the
USA (29). It was suggested that
there was a positive correlation between plasma SELENOP and EA
risk, and there were no genetic variants related to EA risk
(30). There was no relationship
observed between selenium and Barrett's oesophagus in a study by
Steevens et al (31). In
this study, GPX3 and SELENOV did not appear to be involved in
tumour-related signalling pathways. The potential risk of SCLY,
which is involved in energy metabolism and therefore essential for
tumour development (32), needs
more research.
In liver hepatocellular carcinoma, the present study
found that the levels of GPX8, DIO2, TXNRD1, GPX7, TRNAU1AP,
SELENON and SELENOM were increased; and those of SEPSECS, SELENOP
and DIO1 were decreased. SELENOP mRNA was consistently
significantly decreased in hepatocellular carcinoma (HCC) in a
study conducted in Xi'an, China (33), and TXNRD1 expression level was also
increased in data from the GEO and TCGA databases (34). TXNRD1 mRNA was significantly
increased in HCC patients from Hong Kong (35), Zhengzhou (34), Chongqing (36,37),
and Guangzhou (38), China. High
levels of TXNRD1 were associated with poor prognosis (34-38),
and Auranofin, a TXNRD1 inhibitor, was shown to inhibit tumour
growth in a mouse model, and in HCC cells (35-37).
SELENOP also plays an important role in HCC development (39), and low levels of SELENOP were
associated with poor prognosis (39-41).
In this study, it was also demonstrated that higher levels of
TXNRD1 and SEPHS1, as well as lower levels of SELENOP, were
associated with poor prognosis. It was shown that SELENOP and
SELENBP1 had homozygous mutations, and there were no genes involved
in a relationship between methylation and OS. The expression levels
of TXNRD1, SELENOP and SELENBP1 were associated with
hypomethylation. Previously, it was suggested that SELENBP1 is
downregulated in HCC (42) and is
correlated with tumour prognosis (43). SELENOM, which is upregulated in HCC
cells, may also be involved in HCC development (44), through apoptosis, the cell cycle,
and EMT pathways. These results indicate that changes in the
selenoproteome in liver hepatocellular carcinoma should be the
subject of more attention. In particular, high expression of SEPHS1
can reduce redox damage and promote cell proliferation (45), leading to a poor prognosis for the
patients.
For lung cancer, the question of whether mutations
in the GPX1 gene are associated with lung cancer risk is difficult
to answer, due to differences in factors such as age, population,
and lifestyle (46). In the present
study, GPX1 was significantly decreased in lung squamous cell
carcinoma, but was unchanged in lung adenocarcinoma. Lower levels
of SELENOP were associated with higher lung cancer risk among Black
patients in the south-eastern USA (47), and SELENOP was also decreased in
Polish patients (48). SELENOP was
consistently decreased in both lung squamous cell carcinoma and
lung adenocarcinoma in this study. Lower levels of GPX3, SELENOW,
SELENOK, SELENBP1 and SECISBP2 in lung adenocarcinoma were
associated with poor survival, while high levels of GPX3 in lung
squamous cell carcinoma were associated with poor survival,
possibly related to EMT activation. Recently, it was shown that
GPX3 can inhibit the proliferation of lung cancer cells (49), and GPX3 levels, which can be
regulated by methylation, were lower in lung cancer patients
(50,51). In this study, hypermethylation of
GPX3 can inhibit GPX3 expression in lung adenocarcinoma, while
there was no difference in methylation between lung squamous cell
carcinoma and normal tissue. OS analysis showed that GPX3
hypermethylation and GPX5 hypomethylation in lung adenocarcinoma,
and GPX8 hypermethylation in lung squamous cell carcinoma were
associated with poor survival. More studies are needed to uncover
the role of GPX3, including its expression levels and methylation
status, in the development of lung squamous cell carcinoma and lung
adenocarcinoma. It has also been demonstrated that low levels of
SELENBP1 were associated with poor survival in lung adenocarcinoma
(52), SELENOK may regulate the
growth and migration of lung cancer through calcium ions (53), SELENOW may have antioxidant activity
in lung cancer (54), and SECISBP2
appears to be associated with the prognosis of the whole cancer
population (55). We think the role
of SELENOK in lung cancer is worth further study, due to its
important role in the immune system and cancer (56).
For stomach adenocarcinoma, unlike the other four
types of cancer, there were no significantly upregulated
selenoprotein genes. The downregulated genes were SELENOM, SELENOW,
SELENBP1, MSRB3 and GPX3. High levels of GPX3 and GPX1
hypermethylation were associated with poor survival. It has been
suggested that hypermethylation of GPX1 and GPX3 resulted in
decreased mRNA expression of GPX1 and GPX3 in gastric cancer cells,
and downregulation of GPX1 expression due to hypermethylation was
related to poor survival in cancer patients from Korea.
Downregulation of GPX3 has been associated with advanced gastric
cancer and lymph node metastasis, but no correlation with survival
was found (57). Another study
demonstrated that downregulation of GPX3 due to hypermethylation
was related to lymph node metastasis, while the overexpression of
GPX3 in gastric cancer cells did not inhibit cell growth but did
inhibit cell migration (58). High
levels of GPX3 expression associated with poor survival in stomach
adenocarcinoma may act through PI3K/AKT activation. Recently, it
was shown that SELENOM and SELENOW were significantly decreased in
gastric cancer in Harbin, China (59). Downregulation of SELENBP1 and
associated poor survival were also observed in stomach
adenocarcinoma in Wuhan, China (60). In this study, decreased levels of
SELENBP1 and GPX3 were correlated with hypermethylation of the two
genes, but the effects upon the levels of SELENOM and SELENOW were
unclear. Although levels of SELENOP were lower in stomach
adenocarcinoma, and were associated with the degree of stomach
adenocarcinoma differentiation (61), the roles of SELENOM and SELENOW in
stomach adenocarcinoma development needs further research.
In conclusion, we conducted a preliminary analysis
of the differences in expression of the selenoproteome in five
cancers, together with a comprehensive analysis of the effects of
SNV, CNV, methylation and cancer pathways on gene expression. The
effects of gene expression and methylation of the selenoproteome on
survival were investigated. In the context of previous research, we
concluded that the roles of GPX4 in colon adenocarcinoma, SCLY and
SELENOV in oesophageal carcinoma, SEPHS1 in liver hepatocellular
carcinoma, SELENOK in lung cancer, and SELENOM and SELENOW in
stomach adenocarcinoma need further research. This study provides a
valuable basis for the comprehensive understanding of the role of
the selenoproteome in the development of cancer, and may lead to
the identification of new biomarkers or potential therapeutic
targets for cancer.
Supplementary Material
Expression levels of selenoproteome in
normal tissues of colon, esophagus, liver, lung and stomach. The
brightness in red represents the relative expression levels.
The overall survival analysis of GPX3,
GPX8, SELENOW, SELENOK, SELENBP1 and SECISBP2 in lung cancer by the
Kaplan Meier plotter.
The overall survival analysis of
SELENOP, TXNRD1 and SEPHS1 in liver cancer by the Kaplan Meier
plotter.
The SNV analysis of selenoproteome in
colon adenocarcinoma by GSCALite. COAD, colon adenocarcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 21561006 and
21867007); and the Science and Technology Foundation of Guizhou
Province [grant nos. (2019)1258, LH-(2016)7372 and
J-(2014)2028].
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ, JD and ZZ performed the experiments; YJ and JD
contributed to the data analysis and presentation. YJ and ZZ
designed the experiment and wrote the manuscript. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Feng RM, Zong YN, Cao SM and Xu RH:
Current cancer situation in China: Good or bad news from the 2018
Global Cancer Statistics? Cancer Commun (Lond).
39(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen W, Zheng R, Zhang S, Zeng H, Xia C,
Zuo T, Yang Z, Zou X and He J: Cancer incidence and mortality in
China, 2013. Cancer Lett. 401:63–71. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gatzka MV: Targeted tumor therapy
remixed-an update on the use of small-molecule drugs in combination
therapies. Cancers (Basel). 10(155)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Avery JC and Hoffmann PR: Selenium,
selenoproteins, and immunity. Nutrients. 10(1203)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang Z, Rose AH and Hoffmann PR: The role
of selenium in inflammation and immunity: From molecular mechanisms
to therapeutic opportunities. Antioxid Redox Sign. 16:705–743.
2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fairweather-Tait SJ, Bao Y, Broadley MR,
Collings R, Ford D, Hesketh JE and Hurst R: Selenium in human
health and disease. Antioxid Redox Sign. 14:1337–1383.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rayman MP: Selenium and human health.
Lancet. 379:1256–1268. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Short SP and Williams CS: Selenoproteins
in tumorigenesis and cancer progression. Adv Cancer Res. 136:49–83.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Méplan C: Association of single nucleotide
polymorphisms in selenoprotein genes with cancer risk. Methods Mol
Biol. 1661:313–324. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hatfield DL, Carlson BA, Tsuji PA, Tobe R
and Gladyshev VN: Selenium and cancer. In: Molecular, Genetic, and
Nutritional Aspects of Major and Trace Minerals. Collins J (ed).
Academic Press, New York, NY, pp463-473, 2017.
|
|
14
|
Meyer HA, Endermann T, Stephan C, Stoedter
M, Behrends T, Wolff I, Jung K and Schomburg L: Selenoprotein P
status correlates to cancer-specific mortality in renal cancer
patients. PLoS One. 7(e46644)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lincoln DT, Ali EME, Tonissen KF and
Clarke FM: The thioredoxin-thioredoxin reductase system:
Over-expression in human cancer. Anticancer Res. 23:2425–2433.
2003.PubMed/NCBI
|
|
16
|
Jabłońska E and Reszka E: Selenium and
epigenetics in cancer: Focus on DNA methylation. Adv Cancer Res.
136:193–234. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu CJ, Hu FF, Xia MX, Han L, Zhang Q and
Guo AY: GSCALite: A web server for gene set cancer analysis.
Bioinformatics. 34:3771–3772. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8(e82241)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Menyhárt O, Nagy Á and Győrffy B:
Determining consistent prognostic biomarkers of overall survival
and vascular invasion in hepatocellular carcinoma. R Soc Open Sci.
5(181006)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yagublu V, Arthur JR, Babayeva SN, Nicol
F, Post S and Keese M: Expression of selenium-containing proteins
in human colon carcinoma tissue. Anticancer Res. 31:2693–2698.
2011.PubMed/NCBI
|
|
21
|
Berggren M, Gallegos A, Gasdaska JR,
Gasdaska PY, Warneke J and Powis G: Thioredoxin and thioredoxin
reductase gene expression in human tumors and cell lines, and the
effects of serum stimulation and hypoxia. Anticancer Res.
16:3459–3466. 1996.PubMed/NCBI
|
|
22
|
Murawaki Y, Tsuchiya H, Kanbe T, Harada K,
Yashima K, Nozaka K, Tanida O, Kohno M, Mukoyama T, Nishimuki E, et
al: Aberrant expression of selenoproteins in the progression of
colorectal cancer. Cancer Lett. 259:218–230. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Peters KM, Carlson BA, Gladyshev VN and
Tsuji PA: Selenoproteins in colon cancer. Free Radic Biol Med.
127:14–25. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Méplan C and Hesketh J: The influence of
selenium and selenoprotein gene variants on colorectal cancer risk.
Mutagenesis. 27:177–186. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Méplan C, Hughes DJ, Pardini B, Naccarati
A, Soucek P, Vodickova L, Hlavatá I, Vrána D, Vodicka P and Hesketh
JE: Genetic variants in selenoprotein genes increase risk of
colorectal cancer. Carcinogenesis. 31:1074–1079. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang W, Green M, Choi JE, Gijón M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al: CD8(+) T
cells regulate tumour ferroptosis during cancer immunotherapy.
Nature. 569:270–274. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hassannia B, Vandenabeele P and Berghe TV:
Targeting ferroptosis to iron out cancer. Cancer Cell. 35:830–849.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lei Z, Tian D, Zhang C, Zhao S and Su M:
Clinicopathological and prognostic significance of GPX2 protein
expression in esophageal squamous cell carcinoma. BMC Cancer.
16(410)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Silvers AL, Lin L, Bass AJ, Chen G, Wang
Z, Thomas DG, Lin J, Giordano TJ, Orringer MB, Beer DG and Chang
AC: Decreased selenium-binding protein 1 in esophageal
adenocarcinoma results from posttranscriptional and epigenetic
regulation and affects chemosensitivity. Clin Cancer Res.
16:2009–2021. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Takata Y, Kristal AR, Santella RM, King
IB, Duggan DJ, Lampe JW, Rayman MP, Blount PL, Reid BJ, Vaughan TL
and Peters U: Selenium, selenoenzymes, oxidative stress and risk of
neoplastic progression from Barrett's esophagus: Results from
biomarkers and genetic variants. PLoS One. 7(e38612)2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Steevens J, Schouten LJ, Driessen ALC,
Huysentruyt CJ, Keulemans YC, Goldbohm RA and van den Brandt PA:
Toenail selenium status and the risk of Barrett's esophagus: The
Netherlands Cohort Study. Cancer Causes Control. 21:2259–2268.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Seale LA: Selenocysteine β-Lyase:
Biochemistry, regulation and physiological role of the
selenocysteine decomposition enzyme. Antioxidants (Basel).
8(357)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li CL, Nan KJ, Tian T, Sui CG and Liu YF:
Selenoprotein P mRNA expression in human hepatic tissues. World J
Gastroenterol. 13:2363–2368. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zheng Y, Liu Y, Zhao S, Zheng Z, Shen C,
An L and Yuan Y: Large-scale analysis reveals a novel risk score to
predict overall survival in hepatocellular carcinoma. Cancer Manag
Res. 10:6079–6096. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee D, Xu IMJ, Chiu DKC, Leibold J, Tse
AP, Bao MH, Yuen VW, Chan CY, Lai RK, Chin DW, et al: Induction of
oxidative stress through inhibition of thioredoxin reductase 1 is
an effective therapeutic approach for hepatocellular carcinoma.
Hepatology. 69:1768–1786. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tuo L, Xiang J, Pan X, Gao Q, Zhang G,
Yang Y, Liang L, Xia J, Wang K and Tang N: PCK1 downregulation
promotes TXNRD1 expression and hepatoma cell growth via the
Nrf2/Keap1 pathway. Front Oncol. 8(611)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gao Q, Zhang G, Zheng Y, Yang Y, Chen C,
Xia J, Liang L, Lei C, Hu Y, Cai X, et al: SLC27A5 deficiency
activates NRF2/TXNRD1 pathway by increased lipid peroxidation in
HCC. Cell Death Differ. 27:1086–1104. 2020.PubMed/NCBI
|
|
38
|
Fu B, Meng W, Zeng X, Zhao H, Liu W and
Zhang T: TXNRD1 is an unfavorable prognostic factor for patients
with hepatocellular carcinoma. Biomed Res Int.
2017(4698167)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shang N, Wang X, Shu Q, Wang H and Zhao L:
The functions of selenium and selenoproteins relating to the liver
diseases. J Nanosci Nanotechnol. 19:1875–1888. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tarek M, Louka ML, Khairy E, Ali-Labib R,
Zakaria Zaky D and Montasser IF: Role of microRNA-7 and
selenoprotein P in hepatocellular carcinoma. Tumor Biol.
39(1010428317698372)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hughes DJ, Duarte-Salles T, Hybsier S,
Trichopoulou A, Stepien M, Aleksandrova K, Overvad K, Tjønneland A,
Olsen A, Affret A, et al: Prediagnostic selenium status and
hepatobiliary cancer risk in the European Prospective Investigation
into Cancer and Nutrition cohort. Am J Clin Nutr. 104:406–414.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
DI Stasio M, Volpe MG, Colonna G, Nazzaro
M, Polimeno M, Scala S, Castello G and Costantini S: A possible
predictive marker of progression for hepatocellular carcinoma.
Oncol Lett. 2:1247–1251. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jia Y, Dai J, Zhang LL and Xia H:
Biological functions of selenium-binding protein 1 and its
relationship with diseases. Prog Biochem Biophys. 46:128–137.
2019.
|
|
44
|
Guariniello S, Colonna G, Raucci R,
Costantini M, Di Bernardo G, Bergantino F, Castello G and
Costantini S: Structure-function relationship and evolutionary
history of the human selenoprotein M (SelM) found over-expressed in
hepatocellular carcinoma. Biochim Biophys Acta. 1844:447–456.
2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Na J, Jung J, Bang J, Lu Q, Carlson BA,
Guo X, Gladyshev VN, Kim J, Hatfield DL and Lee BJ: Selenophosphate
synthetase 1 and its role in redox homeostasis, defense and
proliferation. Free Radic Biol Med. 127:190–197. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jaworska K, Gupta S, Durda K, Muszyńska M,
Sukiennicki G, Jaworowska E, Grodzki T, Sulikowski M, Waloszczyk P,
Wójcik J, et al: A low selenium level is associated with lung and
laryngeal cancers. PLoS One. 8(e59051)2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Epplein M, Burk RF, Cai Q, Hargreaves MK
and Blot WJ: A prospective study of plasma Selenoprotein P and lung
cancer risk among low-income adults. Cancer Epidemiol Biomarkers
Prev. 23:1238–1244. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Gresner P, Gromadzinska J, Jablonska E,
Kaczmarski J and Wasowicz W: Expression of selenoprotein-coding
genes SEPP1, SEP15 and hGPX1 in non-small cell lung cancer. Lung
Cancer. 65:34–40. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
An BC, Choi YD, Oh IJ, Kim JH, Park JI and
Lee SW: GPx3-mediated redox signaling arrests the cell cycle and
acts as a tumor suppressor in lung cancer cell lines. PLoS One.
13(e0204170)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Oh IJ, Kim HE, Song SY, Na KJ, Kim KS, Kim
YC and Lee SW: Diagnostic value of serum glutathione peroxidase 3
levels in patients with lung cancer. Thorac Cancer. 5:425–430.
2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Choi JY, An BC, Jung IJ, Kim JH and Lee
SW: MiR-921 directly downregulates GPx3 in A549 lung cancer cells.
Gene. 700:163–167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen G, Wang H, Miller CT, Thomas DG,
Gharib TG, Misek DE, Giordano TJ, Orringer MB, Hanash SM and Beer
DG: Reduced selenium-binding protein 1 expression is associated
with poor outcome in lung adenocarcinomas. J Pathol. 202:321–329.
2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Marciel MP, Khadka VS, Deng Y, Kilicaslan
P, Pham A, Bertino P, Lee K, Chen S, Glibetic N, Hoffmann FW, et
al: Selenoprotein K deficiency inhibits melanoma by reducing
calcium flux required for tumor growth and metastasis. Oncotarget.
9:13407–3422. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Jeong D, Kim TS, Chung YW, Lee BJ and Kim
IY: Selenoprotein W is a glutathione-dependent antioxidant in vivo.
FEBS Lett. 517:225–228. 2002.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Xu X, Huang L, Chan CH, Yu T, Miao R and
Liu C: Assessing the clinical utility of genomic expression data
across human cancers. Oncotarget. 7:45926–45936. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Marciel MP and Hoffmann PR: Molecular
mechanisms by which Selenoprotein K regulates immunity and cancer.
Biol Trace Elem Res. 192:60–68. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Min SY, Kim HS, Jung EJ, Do Jee C and Kim
WH: Prognostic significance of glutathione peroxidase 1 (GPX1)
down-regulation and correlation with aberrant promoter methylation
in human gastric cancer. Anticancer Res. 32:3169–3175.
2012.PubMed/NCBI
|
|
58
|
Peng DF, Hu TL, Schneider BG, Chen Z, Xu
ZK and El-Rifai W: Silencing of glutathione peroxidase 3 through
DNA hypermethylation is associated with lymph node metastasis in
gastric carcinomas. PLoS One. 7(e46214)2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lan X, Xing J, Gao H, Li S, Quan L, Jiang
Y, Ding S and Xue Y: Decreased expression of selenoproteins as a
poor prognosticator of gastric cancer in humans. Biol Trace Elem
Res. 178:22–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhang J, Dong W and Lin J: Reduced
selenium-binding protein 1 is associated with poor survival rate in
gastric carcinoma. Med Oncol. 28:481–487. 2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang Q, Gong L, Dong R, Qiao Q, He XL, Chu
YK, Du XL, Yang Y, Zang L, Nan J, et al: Tissue microarray
assessment of selenoprotein P expression in gastric adenocarcinoma.
J Int Med Res. 37:169–174. 2009.PubMed/NCBI View Article : Google Scholar
|