Introduction
Despite ongoing advances in diagnostics, operative
technique, and treatment strategy for the decades, gastric cancer
remains one of the most common cancers in the world and a lethal
disease (1). Gastrectomy for
gastric cancer is essential for improving the survival rate, it may
cause persistent functional disorders such as reduced amount of
oral intake, insulin resistance, increased protein catabolism, and
metabolic changes, leading to weight loss and the development of
sarcopenia (2). It is reported that
preoperative sarcopenia has been associated with long-term
prognosis as well as short-term outcomes such as the development of
postoperative pneumonia, poor activities of daily living, longer
hospital stay, and the incidence of postoperative complications
(3-10),
which may restrict the following treatment options (11). Treatment option for patient with
recurrence of gastric cancer after gastrectomy is limited to
chemotherapy or best supportive care. It is known that sarcopenia
could influence on pharmacokinetics of chemotherapy which could be
associated with adverse effects of chemotherapy in several cancers
(4). However, little is known about
the effects of reduced skeletal muscle volume after gastrectomy on
prognosis and treatment strategy after the recurrence of gastric
cancer. In the present study, we investigated the effects of
reduced skeletal muscle volume after gastrectomy on the treatment
and prognosis in patients with recurrent gastric cancer.
Materials and methods
Patients
The study protocol was approved by the Institutional
Review Board of National Defense Medical College (Saitama, Japan).
Of the 553 patients who underwent radical gastrectomy for gastric
cancer at the National Defense Medical College between 2011 and
2016, 67 patients who had gastric cancer recurrence were included
in this study. We retrospectively evaluated the clinicopathological
findings, serum albumin levels, C-reactive protein (CRP), total
cholesterol, and neutrophil and lymphocyte counts at the time of
preoperative and recurrence of gastric cancer. In addition, the
neutrophil-lymphocyte ratio (NLR), the CRP-albumin ratio (CAR), the
controlling nutrition status (CONUT) score, the prognostic
nutritional index (PNI), and the modified Glasgow prognostic (mGPS)
score were calculated as markers of nutrition or inflammation.
The tumor pathological findings were recorded in
accordance with the third English edition of the Japanese
Classification of Gastric Carcinoma, edited by the Japanese Gastric
Cancer Association (12). All
patients were followed-up using an oncologically appropriate plan
on a per-patient basis. For patients with stage II or III disease,
postoperative adjuvant chemotherapy with S-1 (80 mg/m²/day) was
recommended for 1 year. In 45 cases of pathological stage II or III
gastric cancer patients, 26 cases (57.8%) received adjuvant
chemotherapy. There was no significant correlation between age and
receiving adjuvant chemotherapy.
Definition of sarcopenia
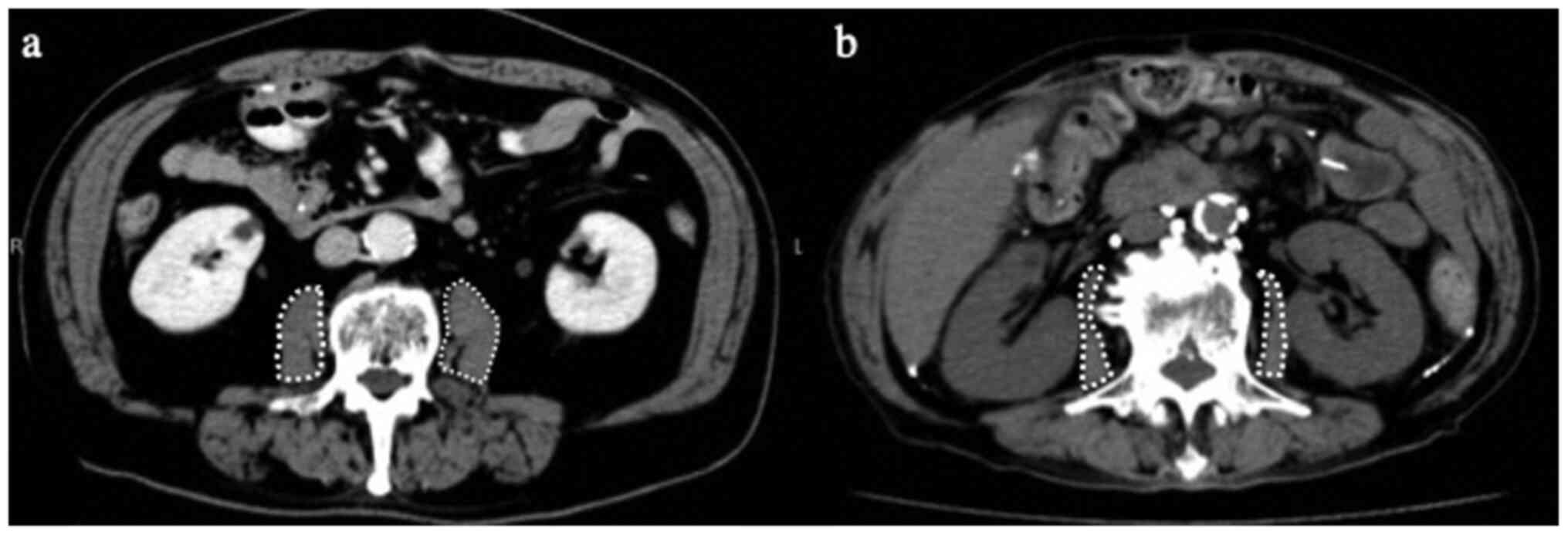
The psoas muscle index (PMI:
cm2/m2) was calculated from CT images
(Aquilion 64; Toshiba Medical Systems) and the psoas muscle
cross-sectional area at the third lumbar vertebra (L3) normalized
length preoperatively and at the recurrence of gastric cancer by a
physician who was blinded to the clinicopathological
characteristics of the patients (Fig.
1) (13,14). The reduction rate of PMI from the
preoperative value to that at the recurrence of gastric cancer was
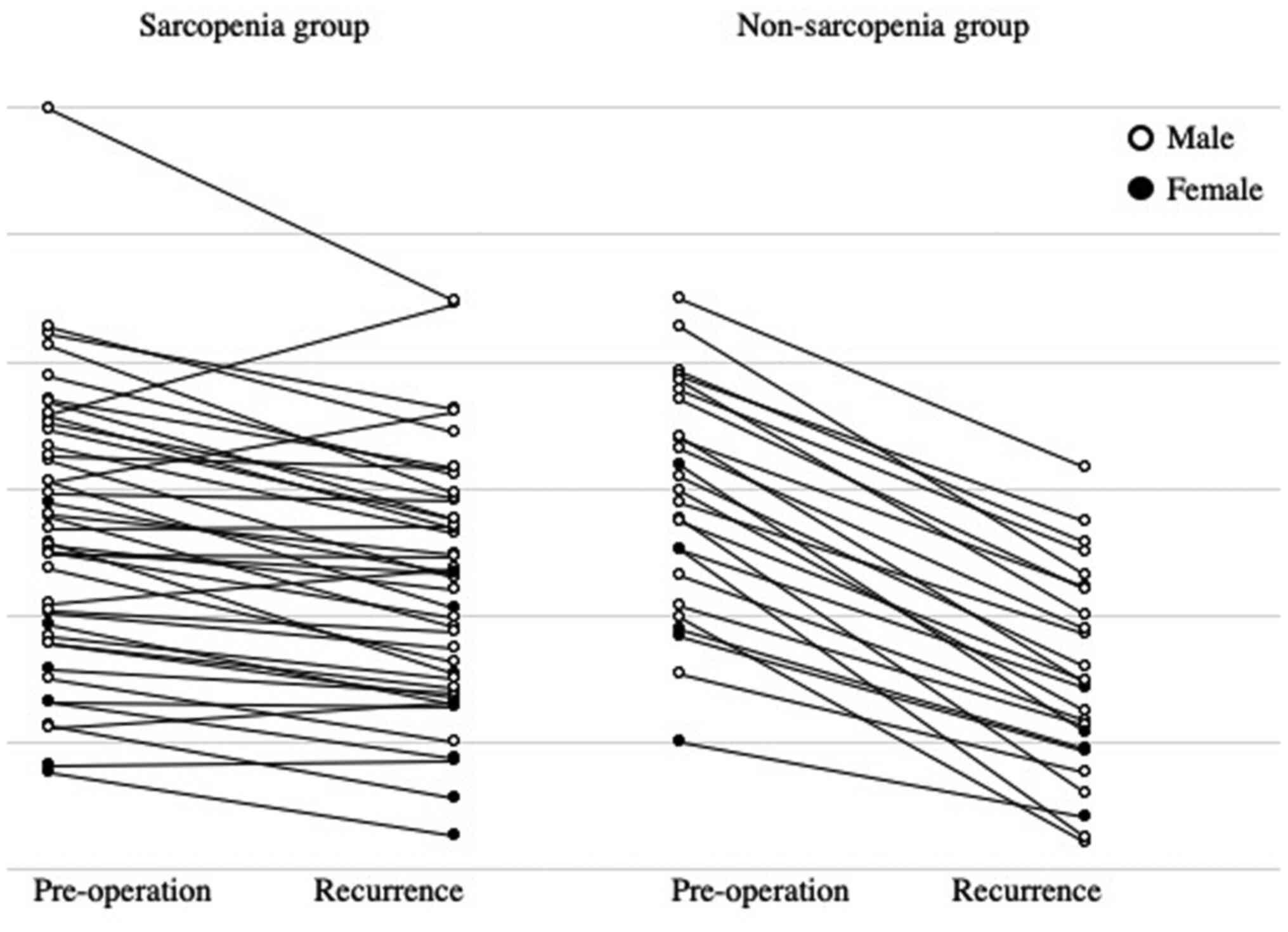
calculated. The patients were divided into two groups by the cutoff
value using area under the receiver operating characteristic curves
(ROC); the sarcopenia group (n=25 patients) had less than the
cutoff value of the reduction rate of PMI (male=0.766 and
female=0.704), and the non-sarcopenia group (n=42 patients) had
more than the cutoff value (Fig.
2). The median value of the total cohort was 0.793.
Statistical analyses
All statistical analyses were performed using the
JMP® Pro 14.0.0 software package (SAS Institute Inc.).
The Student's t-test and Pearson's Chi-square test were performed,
as appropriate. A receiver operating characteristics (ROC) curve
was constructed to estimate the optimal cutoff value of the
reduction of PMI. Survival rates were obtained by the Kaplan-Meier
method, and the statistical significance was determined by the
log-rank test.
Univariate and multivariate analyses were performed
using the Cox proportional hazards regression model. The data are
expressed as mean ± standard deviation. A P-value of <0.05 was
considered statistically significant.
Results
Patient characteristics
Patients' clinical factors at the recurrence of
gastric cancer and pathological factors diagnosed from resected
specimens are shown in Table I.
There were no significant differences in age, sex, Charlson
Comorbidity Index score, and surgical procedure including
reconstruction methods between the two groups. The sarcopenia group
had a higher body weight and body mass index (BMI) than did the
non-sarcopenia group. In addition, the reduction rates of body
weight and BMI due to the recurrence of gastric cancer in the
sarcopenia group were higher than those of the non-sarcopenia
group. There was no significant difference in the pathological
factors between the two groups except for tumor depth. The NLR,
CAR, CONUT score, PNI, and mGPS at the recurrence of gastric cancer
were not significantly different between the two groups (Table II). There was no significant
difference in the time between gastrectomy and recurrence, the
number of patients who received adjuvant chemotherapy and
chemotherapy after the recurrence, the number of discontinued
chemotherapies due to adverse effect, the number of chemotherapy
regimens after recurrence, the kind of basic chemotherapy after
recurrence, and the total courses of chemotherapies between the two
groups. The sarcopenia group had a significantly shorter OS from
recurrence than did the non-sarcopenia group (median survival time,
interquartile range: 118, 43.5-180.5 vs. 300, 133.8-636.3 days,
P<0.001).
 | Table IPatient's clinicopathological
factors. |
Table I
Patient's clinicopathological
factors.
| Characteristic | Sarcopenia (n=25,
37.3%) | Non-Sarcopenia (n=42,
62.7%) | Total (n=67) | P-value |
|---|
| Age | 72.1±7.8 | 70.0±8.6 | 70.8±8.3 | 0.393 |
| Sex | | | | 0.718 |
|
Male | 20 | 32 | 52 | |
|
Female | 5 | 10 | 15 | |
| Body weight (kg) | | | | |
|
Preoperatively | 55.4±8.9 | 59.5±9.8 | 57.9±9.6 | 0.082 |
|
At the time
of recurrence | 46.2±8.1 | 51.2±7.9 | 49.3±8.3 | 0.014a |
|
Recurrence/preoperatively | 0.8±0.1 | 0.9±0.1 | 0.9±0.1 |
0.422b |
| Body mass index
(kg/m2) | | | | |
|
Preoperatively | 21.3±2.9 | 22.6±3.2 | 22.1±3.1 | 0.071 |
|
At the time
of recurrence | 17.7±2.2 | 19.5±2.6 | 18.8±2.6 | 0.010a |
|
Recurrence/preoperatively | 0.8±0.1 | 0.9±0.1 | 0.9±0.1 | 0.338 |
| Psoas muscle
index | | | | |
|
Preoperatively | 3.9±0.9 | 3.7±1.1 | 3.8±1.0 | 0.223 |
|
At the time
of recurrence | 2.5±0.8 | 3.2±1.0 | 3.0±1.0 | 0.005a |
|
Recurrence/Preoperatively | 0.6±0.1 | 0.9±0.1 | 0.8±0.2 |
<0.001a |
| CCI score 2≤ | | | | 0.630 |
|
Yes | 6 | 8 | 14 | |
|
No | 19 | 34 | 53 | |
| Tumor location
U/M/L | | | | 0.579 |
|
U | 11 | 15 | 26 | |
|
M | 9 | 12 | 21 | |
|
L | 5 | 15 | 20 | |
| Histology
int/dif/other | | | | 0.210 |
|
Int | 14 | 18 | 32 | |
|
Dif | 8 | 22 | 30 | |
|
Other | 3 | 2 | 5 | |
| Tumor depth | | | | 0.418 |
|
pT1 | 4 (16.0%) | 3 (7.1%) | 7 (10.5%) | |
|
pT2 | 3 (12.0%) | 3 (7.1%) | 6 (9.0%) | |
|
pT3 | 7 (28.0%) | 19 (45.2%) | 26 (38.8%) | |
|
pT4 | 11 (44.0%) | 17 (40.5%) | 28 (41.8%) | |
| Lymph node
metastasis | | | | 0.978 |
|
pN0 | 5 (20.0%) | 8 (19.1%) | 13 (19.4%) | |
|
pN1 | 4 (16.0%) | 8 (19.1%) | 12 (17.9%) | |
|
pN2 | 6 (24.0%) | 11 (26.2%) | 17 (25.4%) | |
|
pN3 | 10 (40.0%) | 15 (35.7%) | 25 (37.3%) | |
| Pathological cancer
stage | | | | 0.267 |
|
pStageI | 3 (12.0%) | 3 (7.2%) | 6 (9.1%) | |
|
pStageII | 6 (24.0%) | 12 (28.6%) | 18 (27.3%) | |
|
pStageIII | 16 (64.0%) | 27 (64.3%) | 43 (63.6%) | |
| Lymphatic
invasion | | | | 0.613 |
|
Ly0 | 2 | 5 | 7 | |
|
Ly1 | 23 | 37 | 60 | |
| Venous
invasion | | | | 0.429 |
|
V0 | 4 | 4 | 8 | |
|
V1 | 21 | 38 | 59 | |
| DG/TG/other | | | | 0.161 |
|
DG | 6 | 19 | 25 | |
|
TG | 17 | 22 | 39 | |
|
Other | 2 | 1 | 3 | |
|
Open/laparoscopy | | | | 0.161 |
|
Open | 18 | 23 | 41 | |
|
Laparoscopy | 7 | 19 | 26 | |
|
Billroth-I/Roux-en-Y/other | | | | 0.130 |
|
Billroth-I | 6 | 15 | 21 | |
|
Roux-en-Y | 2 | 27 | 44 | |
|
Other | 17 | 0 | 2 | |
| Recurrence
pattern | | | | 0.455 |
|
Peritoneal | 9 (36.0%) | 9 (7.1%) | 18 (26.9%) | |
|
Hematogenous | 6 (24.0%) | 17 (7.1%) | 23 (34.3%) | |
|
Lymph
node | 6 (24.0%) | 11 (7.1%) | 17 (25.4%) | |
|
Local | 2 (8.0%) | 1 (7.1%) | 3 (4.5%) | |
|
Other | 2 (8.0%) | 4 (45.2%) | 6 (8.9%) | |
 | Table IIPatient's characteristics at the time
of recurrence of gastric cancer. |
Table II
Patient's characteristics at the time
of recurrence of gastric cancer.
| Characteristic | Sarcopenia (n=25,
37.3%) | Non-Sarcopenia
(n=42, 62.7%) | Total (n=67) | P-value |
|---|
| Neutrophil
lymphocyte ratio | | | | |
|
Preoperatively | 3.0±2.4 | 3.5±2.8 | 3.3±2.6 | 0.828 |
|
At the time
of recurrence | 4.2±3.1 | 4.6±4.7 | 4.5±4.1 | 0.589 |
| CAR | | | | |
|
Preoperatively | 0.3±0.4 | 0.1±0.2 | 0.2±0.3 | 0.213 |
|
At the time
of recurrence | 0.7±1.0 | 0.5±0.6 | 0.6±0.8 | 0.474 |
| CONUT score | | | | |
|
Preoperatively | 2.3±1.1 | 2.5±1.3 | 2.4±1.2 | 0.597 |
|
At the time
of recurrence | 4.8±2.8 | 3.0±1.7 | 3.9±2.5 | 0.062 |
| Prognostic
nutritional index | | | | |
|
Preoperatively | 37.2±9.5 | 35.9±13.3 | 36.4±11.7 | 0.746 |
|
At the time
of recurrence | 39.7±7.5 | 43.7±9.0 | 42.1±8.6 | 0.114 |
| Modified GPS
score | | | | |
|
Preoperatively | 0.4±0.7 | 0.5±0.7 | 0.5±0.7 | 0.927 |
|
At the time
of recurrence | 1.2±0.7 | 1.0±0.6 | 1.1±0.7 | 0.180 |
| Adjuvant
chemotherapy | | | | 0.874 |
|
Yes | 8 | 16 | 24 | |
|
No | 17 | 26 | 43 | |
| Discontinued
adjuvant chemotherapy due to adverse effect | 6 (75.0%) | 10 (62.5%) | 16 (66.7%) | 0.540 |
| Duration from
gastrectomy to recurrence (day) | 572.9±513.2 | 444.8±331.0 | 492.6±409.5 | 0.484 |
| Chemotherapy after
recurrence | | | | 0.729 |
|
Yes | 12 | 22 | 34 | |
|
No | 13 | 20 | 33 | |
| 1st line regimen
after recurrence | | | | 0.100 |
|
Pyrimidine
fluoride | 8 | 20 | 28 | |
|
Taxane | 2 | 2 | 4 | |
|
Other | 2 | 0 | 2 | |
| Number of
chemotherapy regimens after recurrence | 1.6±0.8 | 1.7±1.0 | 1.7±0.9 | 0.855 |
| Total courses of
chemotherapy after recurrence | 3.7±2.4 | 7.5±5.6 | 6.3±5.1 | 0.092 |
| Discontinued
chemotherapy due to adverse effect | 6 (50.0%) | 7 (31.8%) | 13 (38.2%) | 0.297 |
| Overall survival
from the recurrence of gastric cancer (day) | 169.0±52.8 | 492.9±83.4 | 372.0±58.8 | <0.001 |
Prognostic factors
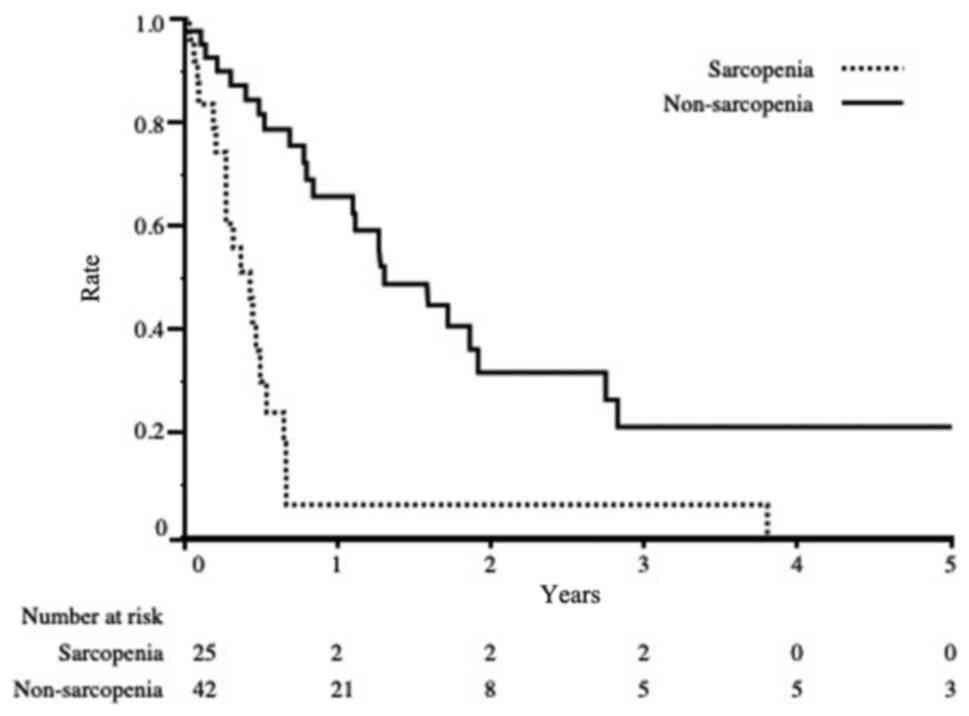
The survival rate from the time of recurrence in the
sarcopenia group was significantly worse than that in the
non-sarcopenia group (3-year OS 6.0% vs. 21.0%, P<0.001;
Fig. 3). Univariate and
multivariate analyses that might affect the survival rate from the
time of the recurrence of gastric cancer were shown in Table III. Univariate analysis
demonstrated that the total courses of chemotherapy after
recurrence <5 [hazard ratio (HR)=3.82], sarcopenia (HR=2.66),
NLR ≥3.0 (HR=2.63), and PNI ≤40 (HR=2.59) were significantly
associated with the prognosis after recurrence. The sarcopenia
group more frequently had peritoneal recurrence, which didn't
affect prognosis.
 | Table IIIPrognostic factor for the overall
survival from the time at recurrence. |
Table III
Prognostic factor for the overall
survival from the time at recurrence.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.69 | 0.91-3.22 | 0.096 | | | |
|
≥70 years
old | | | | | | |
| Body weight
reduction rate | 1.24 | 0.67-2.28 | 0.490 | | | |
|
≥Median | | | | | | |
| Body mass index
reduction rate | 1.21 | 0.65-2.23 | 0.530 | | | |
|
≥Median | | | | | | |
| CCI score | 0.90 | 0.37-1.92 | 0.802 | | | |
|
≥2 | | | | | | |
| Tumor depth | 0.71 | 0.35-1.66 | 0.404 | | | |
|
≥pT3 | | | | | | |
| Lymph node
metastasis | 1.65 | 0.88-3.24 | 0.117 | | | |
|
≥pN2 | | | | | | |
| Lymphatic
invasion | 0.99 | 0.45-2.62 | 0.984 | | | |
|
Ly1 | | | | | | |
| Venous
invasion | 0.95 | 0.43-2.52 | 0.908 | | | |
|
V1 | | | | | | |
| Neutro Lymph
ratio | 2.63 | 1.28-5.53 | 0.008a | 1.48 | 0.40-5.01 | 0.544 |
|
≥3.0 | | | | | | |
| CONUT score | 2.26 | 0.90-6.20 | 0.084 | | | |
|
≥4 | | | | | | |
| Prognostic
Nutritional Index | 2.59 | 1.23-5.50 | 0.012a | 1.25 | 0.33-5.01 | 0.746 |
|
≤40 | | | | | | |
| modified GPS
score | 1.91 | 0.90-4.70 | 0.097 | | | |
|
≥1 | | | | | | |
| Sarcopenia | 4.18 | 2.17-8.07 |
<0.001a | 5.04 | 1.28-22.61 | 0.021a |
|
Yes | | | | | | |
| Adjuvant
chemotherapy | 1.05 | 0.58-1.93 | 0.872 | | | |
|
No | | | | | | |
| Recurrence
site | 1.49 | 0.75-2.80 | 0.246 | | | |
|
Peritoneal | | | | | | |
| Chemotherapy after
recurrence | 1.32 | 0.71-2.43 | 0.376 | | | |
|
No | | | | | | |
| Chemotherapy
regimen after recurrence | 0.45 | 0.16-1.59 | 0.192 | | | |
|
Pyrimidine
fluoride | | | | | | |
| Number of regimens
after recurrence | 1.13 | 0.50-2.58 | 0.762 | | | |
|
<2 | | | | | | |
| Total courses of
chemotherapy after recurrence | 3.82 | 1.33-11.42 | 0.013a | 3.88 | 1.19-13.69 | 0.025a |
|
<5 | | | | | | |
Multivariate analysis revealed that sarcopenia at
the recurrence (HR=5.04) and the total courses of chemotherapy
after recurrence (HR=3.88) were independent unfavorable prognostic
factors.
Table IV shows
univariate and multivariate analysis for the OS from the time at
recurrence among the difference time of sarcopenia. Sarcopenia at
the recurrence and the reduction rate of PMI from surgery to the
recurrence were selected as the independent poor prognostic factors
via multivariate analysis, but preoperative sarcopenia was not.
 | Table IVUnivariate and multivariate analysis
for the overall survival from the time at recurrence among the
difference time of sarcopenia. |
Table IV
Univariate and multivariate analysis
for the overall survival from the time at recurrence among the
difference time of sarcopenia.
| | Univariate
analysis | Multivariate
analysis |
|---|
| | | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sarcopenia by
skeletal muscle mass reduction rate | Yes | 4.18 | 2.17-8.07 |
<0.001a | 3.05 | 1.44-6.48 | 0.004a |
| Preoperative
sarcopenia | Yes | 0.93 | 0.51-1.73 | 0.813 | 0.80 | 0.39-1.67 | 0.551 |
| Sarcopenia at the
time of recurrence | Yes | 3.58 | 1.78-7.42 |
<0.001a | 3.05 | 1.30-7.31 | 0.010a |
Discussion
In the present study, we demonstrated that the high
reduction rate of PMI from the preoperative value to that at the
recurrence of gastric cancer and the fewer courses of chemotherapy
performed after recurrence were independently associated with poor
prognosis after the recurrence.
Since Rosenberg has reported the concept of
sarcopenia in 1997, and many studies have evaluated the
associations between sarcopenia and clinical factors, such as poor
quality of life, aspiration pneumonia, osteoporosis, swallowing
function, and respiratory function (15). In patients with malignancies,
sarcopenia is more likely to be developed due to increased protein
catabolism, inflammatory reactions, metabolic abnormalities, and
poor oral intake and may be associated with cancer cachexia. Many
recent studies have shown that the frequency of serious
postoperative complications was high and the long-term prognosis
was poor in gastric cancer patients with preoperative sarcopenia
(5-7,9,10).
In addition, postoperative loss of the muscle mass affects the
continuation rate of postoperative adjuvant chemotherapy,
especially in the elderly, because of increased severe adverse
events (16,17). However, no study has evaluated the
relationship between prognosis after the recurrence and the
reduction of skeletal muscle mass after gastrectomy. This study
indicated that the reduction of PMI was a risk factor of poor OS
after the recurrence of gastric cancer, which is consistent with a
previous report that skeletal muscle loss during postoperative
adjuvant chemotherapy is associated with poor prognosis (18).
We also demonstrated that patients who failed to
continue chemotherapy more than five courses after the recurrence
of gastric cancer had a poor prognosis. There are several factors
affecting the continuity of chemotherapy after the recurrence,
i.e., adverse events, age, performance status, the amounts of oral
intakes, economic problem, and other social circumstances (19). Physicians can intervene the
continuity of chemotherapy by providing appropriate nutritional
management and preventing loss of skeletal muscle mass at the time
of recurrence, which may be associated with longer survival after
the recurrence of gastric cancer.
Preoperative exercises and nutritional support
programs were effective for increasing total caloric intake,
protein, and grip strength, maintaining skeletal muscle volume and
improving postoperative outcomes in patients with gastric cancer
(19,20). However, there were few reports on
the effects of postoperative nutritional supports on the
postoperative development of sarcopenia and outcome. In addition,
it has been reported that subtotal gastrectomy for the upper third
of gastric cancer had advantages over total gastrectomy in terms of
maintaining weight and nutritional status (20,21).
Thus, it will be essential to ensure thorough nutritional
management after surgery, as well as surgical procedures, for
maintaining skeletal muscle volume and nutritional status at the
recurrence of gastric cancer.
In the present study, we also evaluated the NLR,
CAR, PNI, CONUT, and mGPS at the recurrence of gastric cancer,
which were well known to be preoperative prognostic markers in
various malignancies (22-26).
We demonstrated that these markers were not associated with
prognosis when the values at the recurrence were used, indicating
the importance of preoperative value but not at the recurrence.
We compared the clinical importance of the
sarcopenia preoperatively, at the recurrence, and the reduction
rate of PMI from surgery to the recurrence. Sarcopenia at the
recurrence and the reduction rate of PMI from surgery to the
recurrence were selected as the independent poor prognostic factors
by multivariate analysis.
This study has several potential limitations. The
retrospective design of the study and relatively small number of
patients in this study may have resulted in bias. In addition, we
did not evaluate the relation of amounts of oral intake and the
exercise after gastrectomy to the occurrence of gastric cancer,
which made it difficult to determine whether skeletal muscle loss
was caused by eating disorder after gastrectomy or with the
progression of cancer.
In conclusion, fewer total courses of chemotherapy
after recurrence and sarcopenia were poor prognostic factors for
patients with gastric cancer recurrence. Our data suggested that
prospective interventional study to prevent the reduction of
skeletal muscle volume should be promising for improving survival
after the recurrence of gastric cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
KK, HT, HS, YIt, YIs, ST, YK and HU contributed to
the study conception and design. Material preparation and data
collection and analysis were performed by KK. The first draft of
the manuscript was written by KK and HT, and all authors commented
on previous versions of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures followed were in accordance with the
Helsinki Declaration of 1964 and later versions. The study protocol
was approved by the Institutional Review Board of the National
Defense Medical College, and written informed consent was obtained
from every patient.
Patient consent for publication
Informed consent for publication was obtained from
every patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cruz-Jentoft AJ, Baeyens JP, Bauer JM,
Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y,
Schneider SM, et al: Sarcopenia: European consensus on definition
and diagnosis: Report of the European working group on sarcopenia
in older people. Age Ageing. 39:412–423. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nagata K, Tsujimoto H, Nagata H, Harada M,
Ito N, Kanematsu K, Nomura S, Horiguchi H, Hiraki S, Hase K, et al:
Impact of reduced skeletal muscle volume on clinical outcome after
esophagectomy for esophageal cancer: A retrospective study.
Medicine (Baltimore). 97(e11450)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Antoun S, Borget I and Lanoy E: Impact of
sarcopenia on the prognosis and treatment toxicities in patients
diagnosed with cancer. Curr Opin Support Palliat Care. 7:383–389.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fukuda Y, Yamamoto K, Hirao M, Nishikawa
K, Nagatsuma Y, Nakayama T, Tanikawa S, Maeda S, Uemura M, Miyake
M, et al: Sarcopenia is associated with severe postoperative
complications in elderly gastric cancer patients undergoing
gastrectomy. Gastric Cancer. 19:986–993. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang DD, Chen XX, Chen XY, Wang SL, Shen
X, Chen XL, Yu Z and Zhuang CL: Sarcopenia predicts 1-year
mortality in elderly patients undergoing curative gastrectomy for
gastric cancer: A prospective study. J Cancer Res Clin Oncol.
142:2347–2356. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kawamura T, Makuuchi R, Tokunaga M,
Tanizawa Y, Bando E, Yasui H, Aoyama T, Inano T and Terashima M:
Long-term outcomes of gastric cancer patients with preoperative
sarcopenia. Ann Surg Oncol. 25:1625–1632. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ongaro E, Buoro V, Cinausero M,
Caccialanza R, Turri A, Fanotto V, Basile D, Vitale MG, Ermacora P,
Cardellino GG, et al: Sarcopenia in gastric cancer: When the loss
costs too much. Gastric Cancer. 20:563–572. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang SL, Zhuang CL, Huang DD, Pang WY, Lou
N, Chen FF, Zhou CJ, Shen X and Yu Z: Sarcopenia adversely impacts
postoperative clinical outcomes following gastrectomy in patients
with gastric cancer: A prospective study. Ann Surg Oncol.
23:556–564. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhuang CL, Huang DD, Pang WY, Zhou CJ,
Wang SL, Lou N, Ma LL, Yu Z and Shen X: Sarcopenia is an
independent predictor of severe postoperative complications and
long-term survival after radical gastrectomy for gastric cancer:
Analysis from a large-scale cohort. Medicine (Baltimore).
95(e3164)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gosney MA: Clinical assessment of elderly
people with cancer. Lancet Oncol. 6:790–797. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hamaguchi Y, Kaido T, Okumura S, Kobayashi
A, Hammad A, Tamai Y, Inagaki N and Uemoto S: Proposal for new
diagnostic criteria for low skeletal muscle mass based on computed
tomography imaging in Asian adults. Nutrition. 32:1200–1205.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mourtzakis M, Prado CM, Lieffers JR,
Reiman T, McCargar LJ and Baracos VE: A practical and precise
approach to quantification of body composition in cancer patients
using computed tomography images acquired during routine care. Appl
Physiol Nutr Metab. 33:997–1006. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Rosenberg IH: Sarcopenia: Origins and
clinical relevance. J Nutr. 127 (5 Suppl):990S–991S.
1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aoyama T, Kawabe T, Fujikawa H, Hayashi T,
Yamada T, Tsuchida K, Yukawa N, Oshima T, Rino Y, Masuda M, et al:
Loss of lean body mass as an independent risk factor for
continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann
Surg Oncol. 22:2560–2566. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shen Y, Hao Q, Zhou J and Dong B: The
impact of frailty and sarcopenia on postoperative outcomes in older
patients undergoing gastrectomy surgery: A systematic review and
meta-analysis. BMC Geriatr. 17(188)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sugiyama K, Narita Y, Mitani S, Honda K,
Masuishi T, Taniguchi H, Kadowaki S, Ura T, Ando M, Tajika M and
Muro K: Baseline sarcopenia and skeletal muscle loss during
chemotherapy affect survival outcomes in metastatic gastric cancer.
Anticancer Res. 38:5859–5866. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Japanese Gastric Cancer Association.
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yamamoto K, Nagatsuma Y, Fukuda Y, Hirao
M, Nishikawa K, Miyamoto A, Ikeda M, Nakamori S, Sekimoto M,
Fujitani K and Tsujinaka T: Effectiveness of a preoperative
exercise and nutritional support program for elderly sarcopenic
patients with gastric cancer. Gastric Cancer. 20:913–918.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kosuga T, Hiki N, Nunobe S, Noma H, Honda
M, Tanimura S, Sano T and Yamaguchi T: Feasibility and nutritional
impact of laparoscopy-assisted subtotal gastrectomy for early
gastric cancer in the upper stomach. Ann Surg Oncol. 21:2028–2035.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ishibashi Y, Tsujimoto H, Hiraki S, Kumano
I, Yaguchi Y, Horiguchi H, Nomura S, Ito N, Shinto E, Aosasa S, et
al: Prognostic value of preoperative systemic immunoinflammatory
measures in patients with esophageal cancer. Ann Surg Oncol.
25:3288–3299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ishibashi Y, Tsujimoto H, Yaguchi Y, Kishi
Y and Ueno H: Prognostic significance of systemic inflammatory
markers in esophageal cancer: Systematic review and meta-analysis.
Ann Gastroenterol Surg. 4:56–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ignacio de Ulíbarri J, González-Madroño A,
de Villar NG, González P, González B, Mancha A, Rodríguez F and
Fernández G: CONUT: A tool for controlling nutritional status.
First validation in a hospital population. Nutr Hosp. 20:38–45.
2005.PubMed/NCBI
|
|
26
|
Toyokawa T, Kubo N, Tamura T, Sakurai K,
Amano R, Tanaka H, Muguruma K, Yashiro M, Hirakawa K and Ohira M:
The pretreatment controlling nutritional status (CONUT) score is an
independent prognostic factor in patients with resectable thoracic
esophageal squamous cell carcinoma: Results from a retrospective
study. BMC Cancer. 16(722)2016.PubMed/NCBI View Article : Google Scholar
|