Introduction
Intraductal papillary-mucinous neoplasm (IPMN) of
the pancreas is a cystic tumor arising from the cells lining the
pancreatic ducts. IPMN is divided into main-duct, branch-duct and
mxixed-type lesions, depending on the site of origin (1). The disease spectrum ranges from
low-grade dysplasia to invasive intraductal papillary-mucinous
carcinoma (IPMC) (2). Moreover,
IPMN may occasionally be associated with pancreatic carcinoma at a
site distant from IPMN (1).
Histologically, IPMNs are subdivided into intestinal,
pancreatobiliary, gastric and oncocytic types based on the
expression of immunohistochemical markers, such as mucin core
protein (MUC)1, MUC2 and CDX2(3).
Generally, the prognosis following surgical resection of IPMN with
an associated invasive carcinoma has been reported to be superior
to that of pancreatic ductal adenocarcinoma (PDAC) (4). However, the prognosis of the
pancreatobiliary type IPMN is worse compared with that of the other
subtypes (5). We herein report a
case of a pancreatobiliary type IPMC with formation of a portal
vein tumor thrombus and multifocal liver metastasis.
Case report
A 78-year-old man visited a regional hospital with
complaints of fever and vomiting. An abdominal plain computed
tomography (CT) scan at the hospital revealed dilatation of the
bile ducts and the presence of cystic lesions in the pancreatic
head and liver, and the patient was referred to Saiseikai Chuwa
Hospital (Sakurai, Japan) for further examination and
treatment.
The patient had a history of hypertension and benign
prostatic hyperplasia and received regular treatment with
nifedipine and tamsulosin hydrochloride. There was no history of
liver dysfunction or a specific family history. The patient
reported no allergies, cigarette smoking, or alcohol
consumption.
When the patient first visited our hospital in March
2018, his vital signs were stable, with a body temperature of
36.3˚C, heart rate of 56 bpm, blood pressure of 120/66 mmHg,
respiratory rate of 16 breaths/min and SpO2 of 96% in
room air. The conjunctivas were slightly anemic. There were no
murmurs or rales on chest auscultation, and the abdomen was soft
and flat with no tenderness on palpation. The laboratory tests
(Table I) revealed mild normocytic
anemia, reduced levels of albumin and increased levels of hepatic
and biliary enzymes, including aspartate aminotransferase, alanine
aminotransferase, alkaline phosphatase, γ-glutamyl transpeptidase
and pancreatic amylase. The levels of tumor markers, including
carbohydrate antigen (CA)19-9, DUPAN-2, carcinoembryonic antigen
and α-fetoprotein were measured, and the CA19-9 level was found to
be increased. To evaluate the pancreatic and liver masses, an
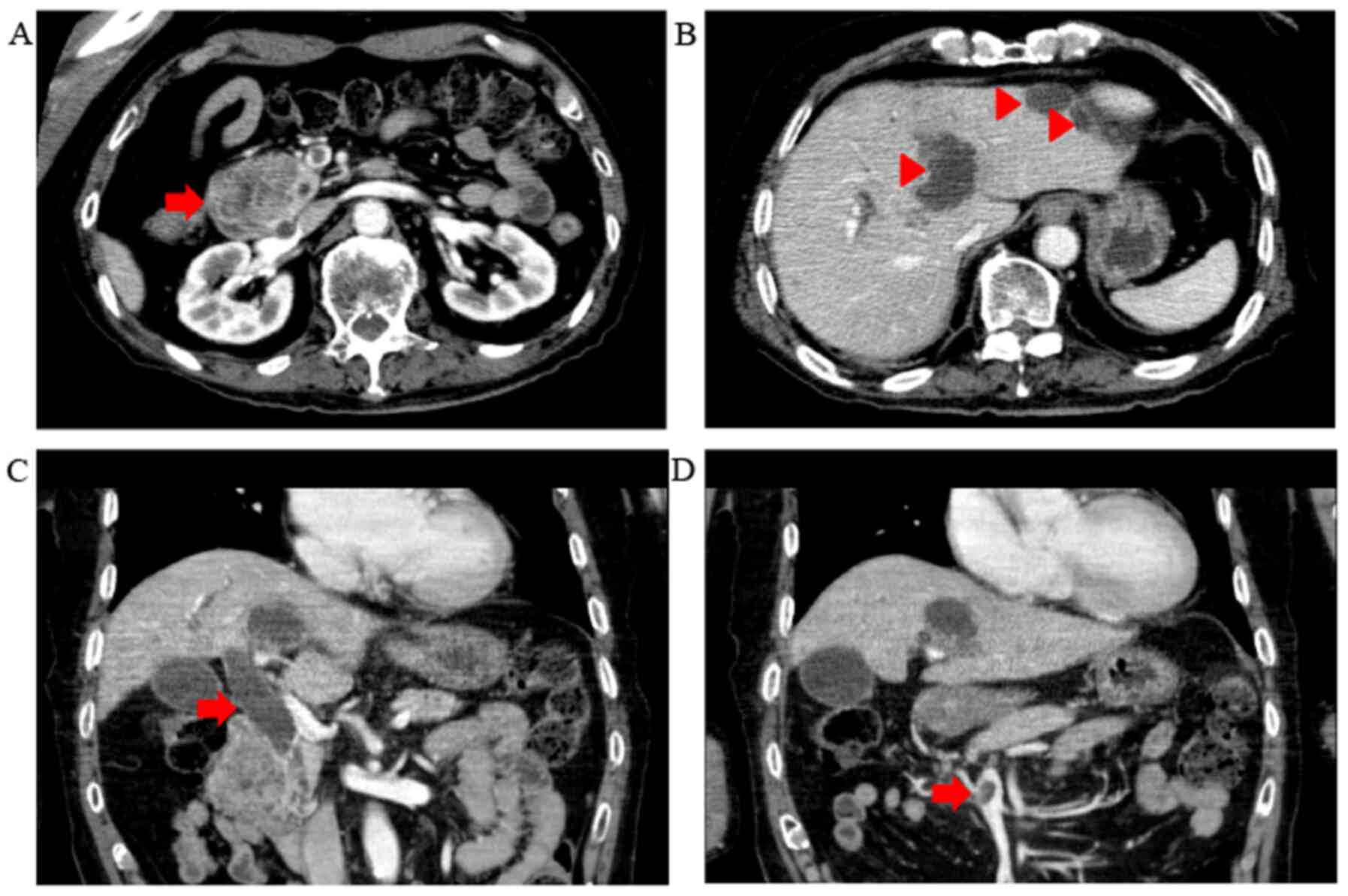
abdominal contrast-enhanced CT scan was performed (Fig. 1). A polycystic low-density area was
identified in the pancreatic head, with a width of 46 mm, and the
wall of this polycystic area was partially well-enhanced. The
common bile duct was obstructed due to the polycystic tumor in the
pancreatic head and was dilated to 20 mm. Multiple polycystic
low-density areas were identified in the liver, with a morphology
similar to that of the cystic lesion in the pancreatic head.
Moreover, a 10-mm filling defect was observed in the superior
mesenteric vein (SMV). The magnetic resonance
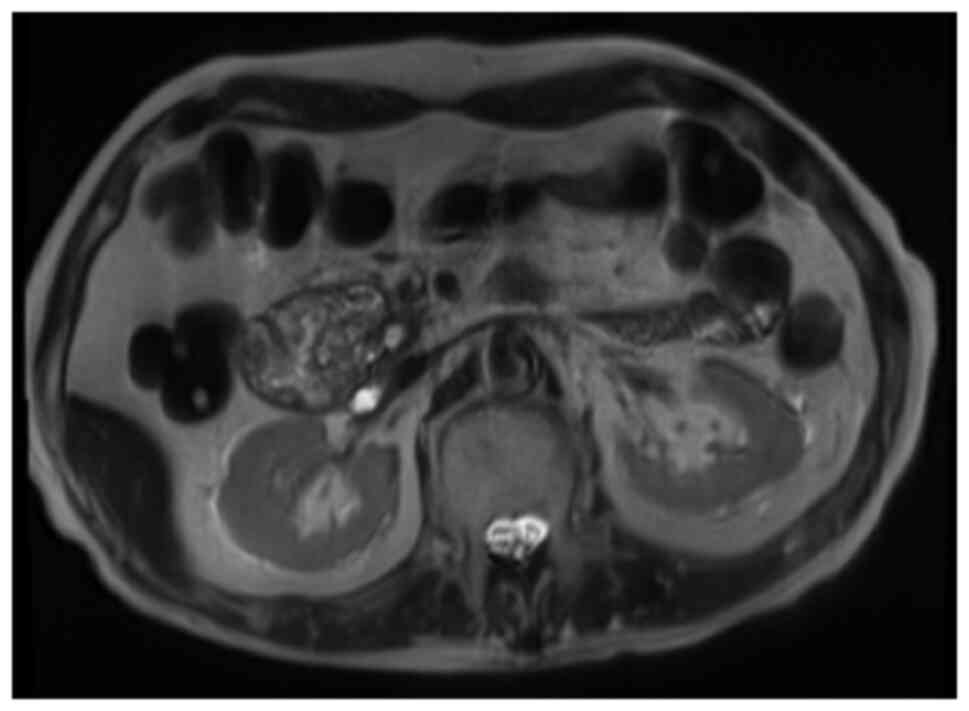
cholangiopancreatography signals from a T2-weighted image revealed
that the pancreatic head tumor exhibited low intensity, with a
small high-intensity area (Fig. 2).
Similarly, on the CT scan, the common bile duct was obstructed by
the polycystic tumor in the pancreatic head and was dilated to a
width of 20 mm. In order to drain the bile duct and perform
diagnostic brush cytology, endoscopic retrograde
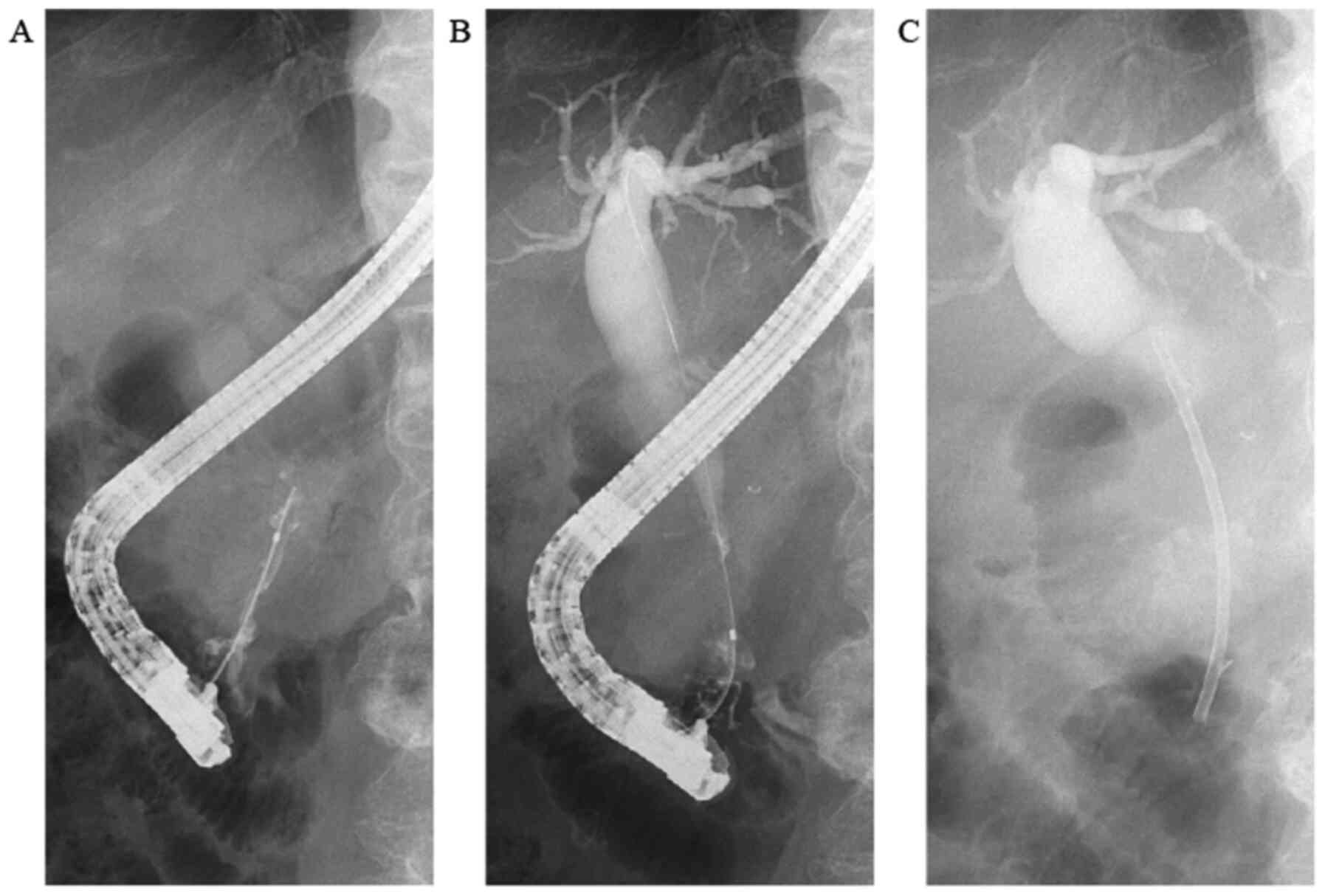
cholangiopancreatography (ERCP) was performed (Fig. 3). The papilla of Vater was normal.
First, contrast medium was injected into the pancreatic duct. The
main pancreatic duct was dilated to a width of 4 mm. Pooling of
mucus in the pancreatic duct was suspected due to the shallow
filling defects. Second, contrast medium was injected into the bile
duct. The proximal bile duct was markedly dilated, whereas the
distal bile duct was narrowed and was 20 mm in length. Brush
cytology of the narrowed bile duct was conducted, and endoscopic
biliary stenting (EBS) was performed using a plastic stent in order
to drain the bile duct. The brush cytology findings revealed the
presence of atypical ductal cells.
 | Table ILaboratory data at the patient's first
visit. |
Table I
Laboratory data at the patient's first
visit.
| Laboratory parameters
(normal range) | Value | Unit |
|---|
| Hematology | | |
|
White blood
cell count (3,300-8,600) | 4,600 | /µl |
|
Red blood
cell count (435-555x104) |
351x104 | /µl |
|
Hematocrit
(40.7-50.1) | 32.1 | % |
|
Hemoglobin
(13.7-16.8) | 10.6 | g/dl |
|
Mean
corpuscular volume (83.6-98.2) | 91.5 | fl |
|
Platelet
count (15.8-34.8x104) |
16.1x104 | /µl |
| Coagulation | | |
|
Prothrombin
time-INR (0.85-1.15) | 1.03 | INR |
|
Activated
partial thromboplastin time (25-40) | 31.9 | sec |
|
Fibrinogen
(150-400) | 491 | mg/dl |
|
Fibrin
degradation products (0-10) | 3.4 | µg/ml |
|
D-dimer
(0-1) | 1.0 | µg/ml |
| Chemistry | | |
|
Albumin
(4.1-5.1) | 3.2 | g/dl |
|
Aspartate
transaminase (13-30) | 41 | IU/l |
|
Alanine
transaminase (10-42) | 66 | IU/l |
|
Lactate
dehydrogenase (124-222) | 86 | IU/l |
|
Alkaline
phosphatase (106-322) | 788 | IU/l |
|
γ-Glutamyl
transpeptidase (13-64) | 160 | IU/l |
|
Amylase
(44-132) | 357 | IU/l |
|
P-amylase
(16-52) | 282 | IU/l |
|
Total
bilirubin (0.4-1.5) | 0.5 | mg/dl |
|
Blood urea
nitrogen (8-20) | 16.5 | mg/dl |
|
Creatinine
(0.65-1.07) | 0.75 | mg/dl |
|
Na
(138-145) | 134 | mEq/l |
|
K
(3.6-4.8) | 4.2 | mEq/l |
|
C-reactive
protein (0-0.29) | 5.165 | mg/dl |
|
Procalcitonin
(0-0.4) | 0.39 | ng/ml |
|
Brain
natriuretic peptide (0-18.4) | 19.6 | pg/ml |
|
Hemoglobin
A1c (4.7-6.2) | 8.7 | % |
| Tumor markers | | |
|
Carbohydrate
antigen 19-9 (0-37) | 84.6 | U/ml |
|
DUPAN-2
(0-150) | 46 | U/ml |
|
Carcinoembryonic
antigen (0-5) | 3.5 | ng/ml |
|
α-Fetoprotein
(0-15) | 2.0 | ng/ml |
|
Protein
induced by vitamin K absence-II (0-39) | 25.45 | mAU/ml |
The comprehensive diagnosis was advanced IPMC with
invasion of the bile duct and liver metastasis. The clinical stage
was stage IV (T3N0M1) according to the Union for International
Cancer Control TNM classification (6). As regards treatment, systemic
chemotherapy was recommended; however, the patient and his family
opted for best supportive care due to his declining performance
status (performance status score: 4). Thus, EBS was performed to
drain the bile duct on the 2nd day of hospitalization
and the plastic stent was exchanged for a metallic stent on the
8th day to prolong stent patency. Subsequently, the
patient developed cholangitis and aspiration pneumonia, and he
eventually succumbed to IPMC 90 days after the initial
admission.
After obtaining informed consent from the patient's
daughter, an autopsy was performed to examine the tumor.
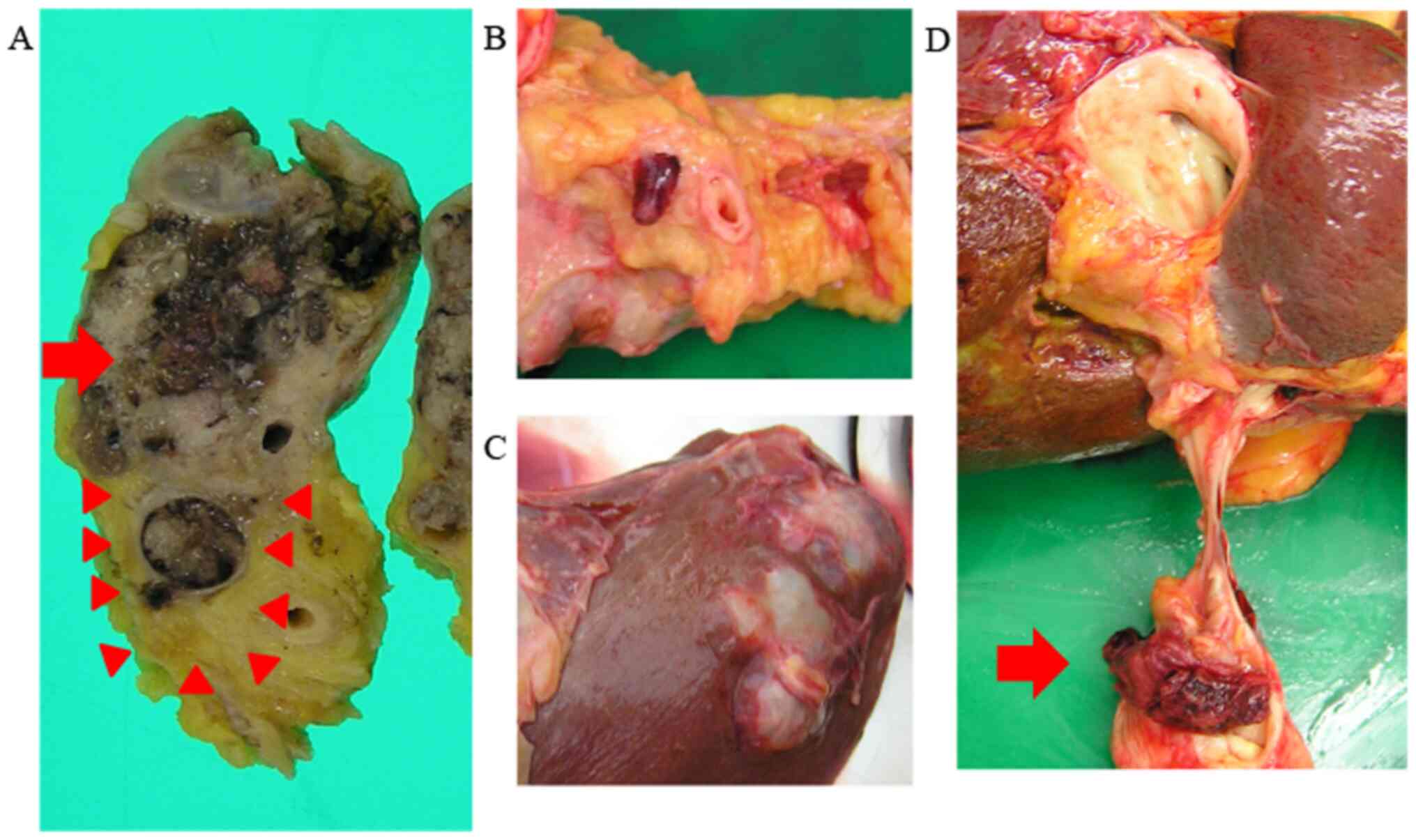
Macroscopically, the polycystic tumor in the pancreatic head was
sized 5x4.5 cm and the cysts contained mucus. Moreover, the tumor
had invaded the portal vein and formed an elongated tumor thrombus,
extending from the SMV to the hilar portal vein. Polycystic tumor
lesions were also identified in the liver, and the structure of
these metastatic lesions was identical to that of the primary
pancreatic head tumor (Fig. 4).
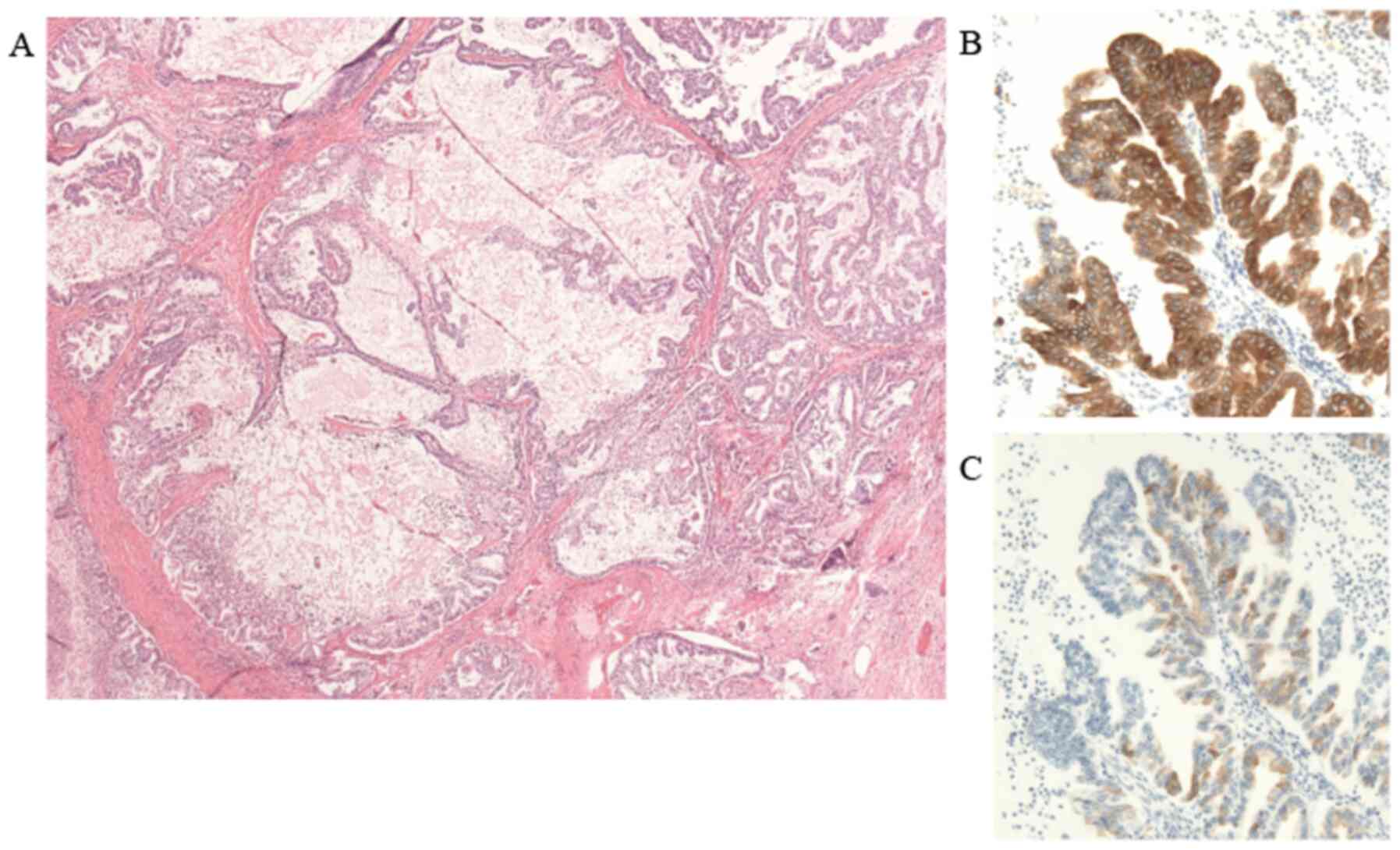
Microscopically, on hematoxylin and eosin staining,
the pancreatic head tumor exhibited a papilliform structure
consisting of small tumor cells and the tumor cysts were filled
with mucus. On immunostaining, MUC1 and MUC5AC were positive
(Fig. 5), but MUC2 was negative
(data not shown). Therefore, the histological diagnosis was
pancreatobiliary type IPMC, and the morphological diagnosis, which
was based on the dilation of both the main pancreatic duct as well
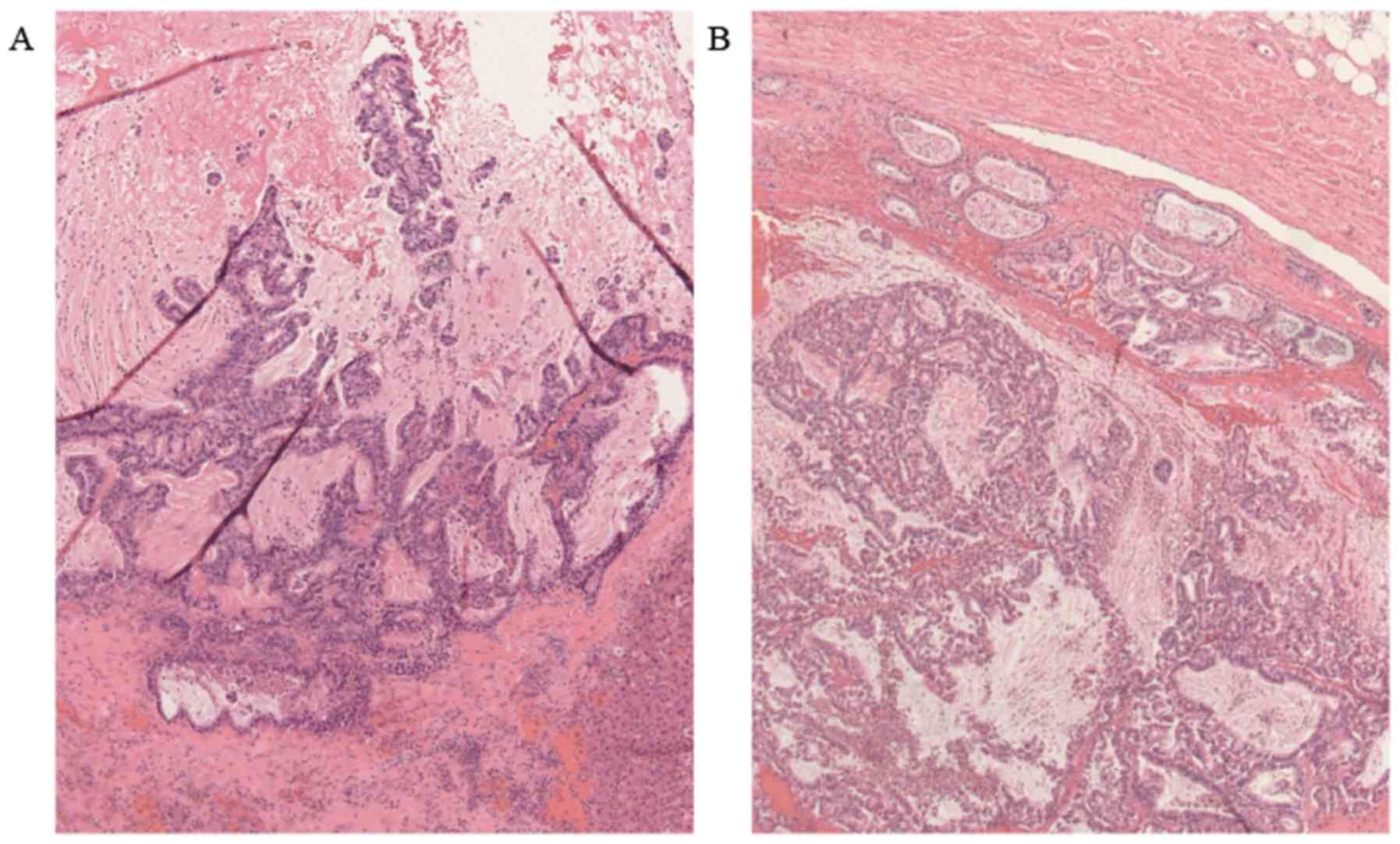
as its branches, was mixed-type IPMC. The polycystic liver tumors
and SMV tumor thrombus had the same morphology as the pancreatic
tumors on pathological examination, with pooling of mucus in the
cysts of the liver metastatic lesions (Fig. 6). The pancreatic head tumor had not
metastasized to the regional lymph nodes, but rather directly
metastasized to the liver via the portal vein, retaining the IPMC
structure and mucus production.
Discussion
There were two clinical issues with the present
case: First, the pancreatic tumor directly invaded the portal vein
and caused tumor thrombosis. Second, the pancreatic tumor
metastasized to the liver via the portal vein, retained the IPMC
structure and produced mucus. Currently, invasive IPMN is
considered to have a better prognosis compared with conventional
PDAC (7,8). Ueda et al (9) reported the case of a female patient
with IPMN with metastases to the lymph nodes, liver and lung, who
achieved long-term survival (5 years and 2 months), as she
responded to chemotherapy following pancreaticoduodenectomy.
However, in the present case, the patient's survival duration after
diagnosis was very short due to the widespread local invasion,
distant metastases and lack of anticancer treatment, such as
systemic chemotherapy or radiotherapy.
IPMN and other cystic pancreatic tumors may be
accurately and timely diagnosed due to the advances in imaging
techniques, including MRI and ERCP (10). On occasion, these tumors are
incidentally found on abdominal CT in asymptomatic patients. Other
patients may present with variable symptoms, such as abdominal
pain, obstructive jaundice and vomiting, as in the present case.
The progression of obstructive jaundice with IPMN has been
previously reported, as mucus produced from IPMN may obstruct the
common bile duct (11). However, in
the present case, the common bile duct was directly invaded and
obstructed by the tumor.
Serum CA19-9 level is a well-known predictor of PDAC
and poor survival. Elevated serum CA19-9 levels were found to be
associated with mixed invasive IPMC, which is consistent with
several previous reports (12,13),
and this finding may indicate that invasive IPMC shares similar
characteristics with PDAC. Hirono et al (14) evaluated the diagnostic value of the
factors associated with invasive IPMC identified in their study,
and branch-duct IPMN with elevated serum CA19-9 levels was
associated with invasive IPMC, with a sensitivity of 86.4%,
specificity of 96.6%, positive predictive value of 86.4%, negative
predictive value of 96.6% and accuracy of 94.5%.
IPMNs are classified into four subtypes (intestinal,
pancreatobiliary, gastric and oncocytic) based on their
histomorphology and mucin phenotype (15). The subtype of the present case was
pancreatobiliary, as determined based on the positive expression of
MUC1 and MUC5AC and negative expression of MUC2. The
pancreatobiliary type is the most malignant among the four subtypes
(5). Moreover, the main-duct or
mixed-type morphological classifications of IPMN are associated
with a higher risk of evolving into invasive IPMC compared with the
branch-duct type (16). Mixed-type
IPMN includes various histological types. Masuda et al
(17) previously reported that
mixed IPMN with positive MUC2 expression had a significantly higher
prevalence of high-grade dysplasia and invasive IPMC compared with
MUC2-negative IPMN. However, the tumor in the present case was
classified as invasive IPMC, despite the negative MUC2 expression.
It was previously reported that the mixed type of IPMN rarely
includes the pancreatobiliary type (17). The pancreatobiliary type is
MUC2-negative, as are the gastric and oncocytic types. Thus, this
contradiction regarding whether mixed-type IPMN is invasive may be
attributed to selection bias in the report.
It was reported that 19-45% of IPMNs are accompanied
by invasive carcinomas (8).
However, the presence of portal vein invasion was significantly
lower among patients with invasive IPMC compared with those with
PDAC (8). Invasive IPMCs with
portal vein invasion and tumor thrombosis, such as in the present
case, have rarely been reported (16,18).
Moreover, our patient had a long tumor thrombus and liver
metastases. When an abdominal contrast-enhanced CT was first
performed, the tumor thrombus was only 10 mm. However, when the
autopsy was performed after 90 days, the tumor thrombus had grown
and extended from the SMV to the hilar portal vein. The rapid
growth of the portal thrombosis indicated a highly malignant tumor.
In addition, invasion into the vasculature is a significant
predictor of poor outcome (19).
Moreover, in the present case, the structure of the polycystic
liver tumors was identical to that of the pancreatic tumors on
pathological examination. The SMV tumor thrombosis metastasized to
the liver, maintaining its polycystic structure and mucus
production. As the pancreatic head tumor had not metastasized to
the regional lymph nodes, it was inferred that part of the SMV
tumor thrombus, with a polycystic structure and mucus content, was
detached and reached the liver via the portal vein.
While the patient and his family opted for best
supportive care in the present case, several therapies for invasive
IPMC have been reported. Pancreatectomy with lymph node dissection
are recommended for invasive IPMC and PDAC, if the tumor is
resectable (20), whereas the
efficacy of postoperative adjuvant therapy for invasive IPMC
remains controversial. McMillan et al (21) reported that postoperative adjuvant
therapy may improve the survival of patients with advanced-stage
invasive IPMC or lymph node metastases. However, another study
reported that postoperative adjuvant therapy does not affect the
survival of patients with invasive IPMC (22). Moreover, it is unclear whether
systemic chemotherapy improves the survival of patients with
unresectable invasive IPMN. Chemoradiotherapy has been demonstrated
to prolong the survival of patients with PDAC (23). Regarding chemotherapy for PDAC,
gemcitabine or S-1 (tegafur, gimeracil, oteracil potassium) as
systemic chemotherapy for unresectable invasive IPMC is clinically
considered (24).
To date, there have been reports of IPMC with portal
invasion or liver metastasis (25,26).
However, to the best of our knowledge, this is the first report of
a IPMC metastasizing to the liver while maintaining its IPMC
structure.
As regards the limitations of the present study, the
expression of proliferating cell nuclear antigen, tumor protein
p53, vascular endothelial growth factor and the KRAS mutation
status were not determined due to health insurance-related
restrictions. From an academic perspective, however,
immunohistochemical examination must be performed to evaluate the
status of carcinogenesis.
In conclusion, we herein present our experience with
an autopsy case of IPMC accompanied by a long tumor thrombus in the
portal vein and multifocal liver metastasis that maintained the
structure of the primary pancreatic head tumor.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NM wrote the report. NM and AD were the attending
physicians for the patient. NM, AD, HU, SA, KM and KY conducted the
ERCP. MT made the pathological diagnosis. AD supervised the report.
All the authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The family of the patient consented to the
publication of the case details and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tanaka M, Fernández-Del Castillo C,
Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M
and Wolfgang CL: Revision of international consensus Fukuoka
guidelines for the management of IPMN of the pancreas.
Pancreatology. 17:738–753. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Adsay V, Mino-Kenudson M, Furukawa T,
Basturk O, Zamboni G, Marchegiani G, Bassi C, Salvia R, Malleo G,
Paiella S, et al: Pathologic evaluation and reporting of
intraductal papillary mucinous neoplasms of the pancreas and other
tumoral intraepithelial neoplasms of pancreatobiliary tract:
Recommendations of verona consensus meeting. Ann Surg. 263:162–177.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Adsay NV, Merati K, Basturk O,
Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH and
Klimstra DS: Pathologically and biologically distinct types of
epithelium in intraductal papillary mucinous neoplasms: Delineation
of an ‘intestinal’ pathway of carcinogenesis in the pancreas. Am J
Surg Pathol. 28:839–848. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aronsson L, Andersson R and Ansari D:
Intraductal papillary mucinous neoplasm of the
pancreas-epidemiology, risk factors, diagnosis, and management.
Scand J Gastroenterol. 52:803–815. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim J, Jang KT, Mo Park S, Lim SW, Kim JH,
Lee KH, Lee JK, Heo JS, Choi SH, Choi DW, et al: Prognostic
relevance of pathologic subtypes and minimal invasion in
intraductal papillary mucinous neoplasms of the pancreas. Tumour
Biol. 32:535–542. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
James D: Brierley and Marry K:
Gospodarowicz, Christian Wittekind. TMN Classification of Malignant
Tumors, 8th ed. New York, Wiley-Blackwell, 2016.
|
|
7
|
Sohn TA, Yeo CJ, Cameron JL, Hruban RH,
Fukushima N, Campbell KA and Lillemoe KD: Intraductal papillary
mucinous neoplasms of the pancreas an updated experience. Ann Surg.
239:788–797; discussion 797-9. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Murakami Y, Uemura K, Sudo T, Hayashidani
Y, Hashimoto Y, Nakashima A and Sueda T: Invasive intraductal
papillary-mucinous neoplasm of the pancreas: Comparison with
pancreatic ductal adenocarcinoma. J Surg Oncol. 100:13–18.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ueda N, Kaida D, Tomita Y, Ohnishi T,
Funaki H, Fujita H, Kinami S, Nakano Y and Kosaka T: A patient with
lnvasive carcinoma derived from IPMN who achieved long-term
survival despite lymph node metastasis. Gan To Kagaku Ryoho.
41:2202–2204. 2014.PubMed/NCBI(In Japanese).
|
|
10
|
Aşkan G, Bağci P, Memiş B and Baştürk O:
Intraductal neoplasms of the pancreas: An update. Turk Patoloji
Derg. 33:87–102. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Patel A, Lambiase L, Decarli A and Fazel
A: Management of the mucin filled bile duct. A complication of
intraductal papillary mucinous tumor of the pancreas. JOP.
6:255–259. 2005.PubMed/NCBI
|
|
12
|
Fritz S, Hackert T, Hinz U, Hartwig W,
Büchler MW and Werner J: Role of serum carbohydrate antigen 19-9
and carcinoembryonic antigen in distinguishing between benign and
invasive intraductal papillary mucinous neoplasm of the pancreas.
Br J Surg. 98:104–110. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Kim JR, Jang JY, Kang MJ, Park T, Lee SY,
Jung W, Chang J, Shin Y, Han Y and Kim SW: Clinical implication of
serum carcinoembryonic antigen and carbohydrate antigen 19-9 for
the prediction of malignancy in intraductal papillary mucinous
neoplasm of pancreas. J Hepatobiliary Pancreat Sci. 22:699–707.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Hirono S, Kawai M, Okada KI, Miyazawa M,
Shimizu A, Kitahata Y, Ueno M, Yanagisawa A and Yamaue H: Factors
associated with invasive intraductal papillary mucinous carcinoma
of the pancreas. JAMA Surg. 152(e165054)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Furukawa T: Subtyping of IPMN. Methods Mol
Biol. 1882:1–8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Castellano-Megías VM, Andrés CI,
López-Alonso G and Colina-Ruizdelgado F: Pathological features and
diagnosis of intraductal papillary mucinous neoplasm of the
pancreas. World J Gastrointest Oncol. 6:311–324. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Masuda A, Arisaka Y, Hara S, Matsumoto I,
Takenaka M, Sakai A, Shiomi H, Matsuki N, Sugimoto M, Fujita T, et
al: MUC2 expression and prevalence of high-grade dysplasia and
invasive carcinoma in mixed-type intraductal papillary mucinous
neoplasm of the pancreas. Pancreatology. 13:583–588.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tomimaru Y, Ishikawa O, Ohigashi H, Eguchi
H, Yamada T, Sasaki Y, Kishi K, Takachi K, Noura S, Miyashiro I, et
al: Intraductal papillary-mucinous carcinoma of the pancreas with
tumor thrombus in the portal vein: A report of two cases.
Hepatogastroenterology. 54:1585–1588. 2007.PubMed/NCBI
|
|
19
|
D'Angelica M, Brennan MF, Suriawinata AA,
Klimstra D and Conlon KC: Intraductal papillary mucinous neoplasms
of the pancreas: An analysis of clinicopathologic features and
outcome. Ann Surg. 239:400–408. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hirono S and Yamaue H: Surgical strategy
for intraductal papillary mucinous neoplasms of the pancreas. Surg
today. 50:50–55. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McMillan MT, Lewis RS, Drebin JA,
Teitelbaum UR, Lee MK, Roses RE, Fraker DL and Vollmer CM: The
efficacy of adjuvant therapy for pancreatic invasive intraductal
papillary mucinous neoplasm (IPMN). Cancer. 122:521–533.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hirono S, Shimizu Y, Ohtsuka T, Kin T,
Hara K, Kanno A, Koshita S, Hanada K, Kitano M, Inoue H, et al:
Recurrence patterns after surgical resection of intraductal
papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter,
retrospective study of 1074 IPMN patients by the Japan Pancreas
Society. J Gastroentrol. 55:86–99. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ochiai T, Igari K, Furuyama T, Ito H,
Mitsunori Y, Aihara A, Kumagai Y, Iida M, Odajima H, Tanaka S, et
al: Favorable response after Gemcitabine-Radiotherapy for invasive
pancreatic intraductal papillary mucinous neoplasm: A case report.
Int Surg. 98:340–345. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kameyama S, Motonari H, Ishimine T and Isa
T: Successful treatment with conversion surgery following
chemoradiotherapy for unresectable invasive intraductal papillary
mucinous neoplasm. Clin J Gastroenterol. 13:579–584.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Marsoner K, Haybaeck J, Csengeri D, Waha
JE, Schagerl J, Langeder R, Mischinger HJ and Kornprat P:
Pancreatic resection for intraductal papillary mucinous neoplasm- a
thirteen-year single center experience. BMC Cancer.
16(844)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Matsuda Y, Hagio M, Naito Z and Ishiwata
T: Clinicopathological features of 30 autopsy cases of pancreatic
carcinoma. J Nippon Med Sch. 79:459–467. 2012.PubMed/NCBI View Article : Google Scholar
|