Introduction
Immune checkpoint inhibitors (ICIs), either alone or
in combination, increase survival of patients with several types of
malignant tumor and are currently being studied in the context of
neoadjuvant and adjuvant care for multiple diseases. However, these
monoclonal antibodies, targeting programmed cell death (PD)-1, PD-1
ligand (PDL-1) and cytotoxic T-lymphocyte antigen (CTLA)-4, are
associated with a unique spectrum of adverse consequences that can
affect virtually every organ in the body. These include autoimmune
inflammation in the digestive tract, lung, skin, endocrine glands
and peripheral and central nervous systems (1,2). ICIs
have been shown to increase survival of patients with metastatic
non-small cell lung cancer (NSCLC) without activating genetic
alteration in EGFR, ALK or reactive oxygen species and are now
standard of care for these patients (3,4).
ICI-related pneumonitis (ICI-P) affects 3-5% of
patients treated with checkpoint inhibitors (5,6). It
appears to be more prevalent in patients with NSCLC than in those
with other types of cancer (6,7).
Similarly, it is more common in patients treated with PD-1/PDL-1
inhibitors than in those treated with CTLA-4 inhibitors alone
(6). The frequency of ICI-P is
higher when anti-PD-1 and anti-CTLA-4 are administered
concomitantly (6,7). Despite the low fatality rate of ~1%,
pneumonitis is one of the leading causes of ICI-associated
morbidity (8).
Treatment of ICI-P depends on its severity: The
majority of patients experience only mild to moderate pneumonitis
and improve with withdrawal of immunotherapy and/or a course of
corticosteroids (9,10). However, certain patients worsen
during treatment of pneumonitis and require additional
immunosuppressive therapy, such as infliximab, mycophenolate
mofetil (9,10) or immunomodulatory agents, such as
intravenous immunoglobulin (IVIG) (11). The clinical course may be further
complicated by opportunistic infection, secondary to prolonged
treatment with steroids and immunosuppressants (12,13).
The clinical course of cytomegalovirus (CMV)
pneumonia is typically indolent but fulminant disease may be
observed in immunocompromised patients and carries a mortality risk
mortality >30% (14). CMV
pneumonia has been infrequently described in patients receiving
cancer immunotherapy. Here, we describe a patient that developed
fatal CMV pulmonary infection following treatment for pneumonitis
induced by the anti PD-1 antibody pembrolizumab.
Case report
A 63-year-old man with a medical history of chronic
obstructive pulmonary disease, hyperlipidemia and diabetes mellitus
was diagnosed with metastatic squamous cell lung carcinoma in May
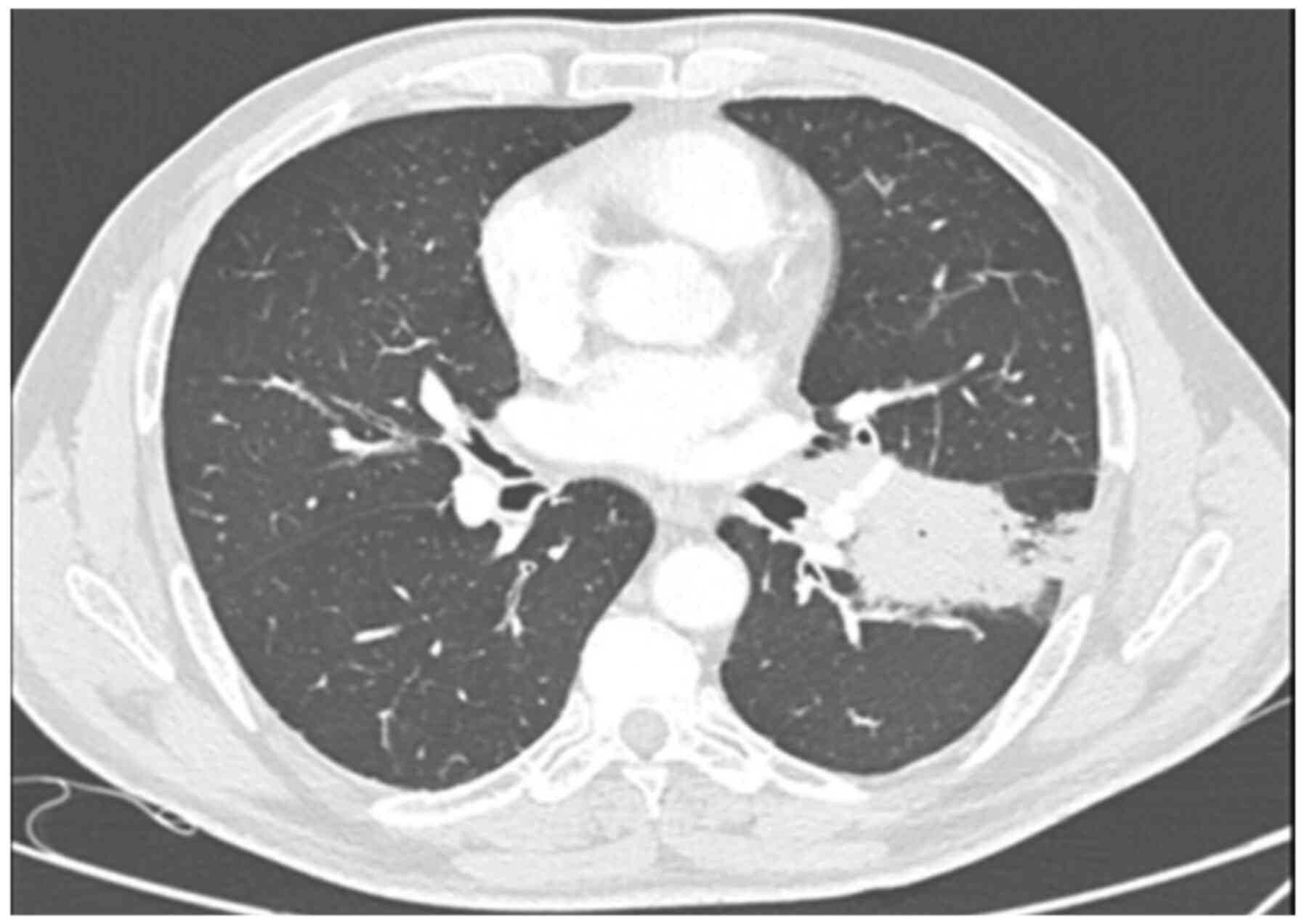
2017 (Fig. 1). Tumor cells stained
positively for PDL-1 with a count >50%, and the patient started
treatment with pembrolizumab in June 2017.
In February 2019, a PET-CT scan showed no
pathological uptake and the patient was in complete remission. The
only side effect noted at that time was hypothyroidism, which was
treated with levothyroxine.
In June 2019, the patient was admitted to Galilee
Medical Center (Nahariya, Israel) due to respiratory distress and
pulmonary infiltrates. He was treated with prednisone (1 mg/kg) and
levofloxacin and discharged following improvement with a
recommendation to taper the dose of prednisone. While receiving 10
mg prednisone, he experienced worsening dyspnea and was
re-admitted.
On admission, the patient was tachypneic, but other
vital signs were within the normal range. Blood oxygen saturation
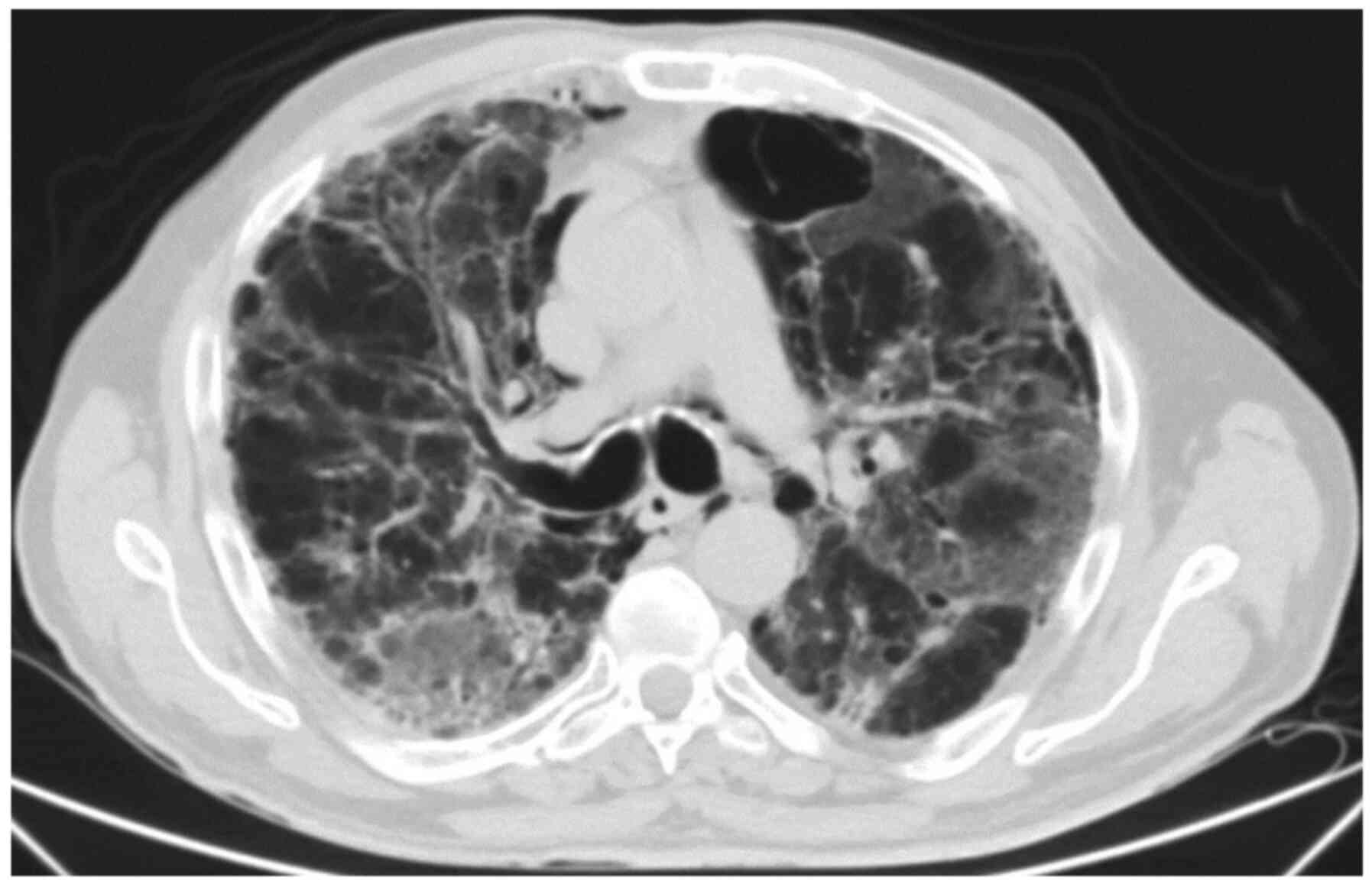
was 82% on ambient air. CT angiography demonstrated bilateral
reticular opacities (Fig. 2).
The clinical course and imaging studies were
consistent with grade 4 autoimmune pneumonitis secondary to ICIs in
a patient with chronic obstructive pulmonary disease. The patient
was treated with Prednisone 2 at a dose of 2 mg/kg, mycophenolate
mofetil, empiric broad-spectrum antibiotics and
trimethoprim-sulfamethoxazole. Despite these measures, his
condition worsened and the patient was intubated and mechanically
ventilated due to respiratory failure.
The patient was admitted to the intensive care unit
and IVIG was added to his treatment regimen. While intubated, he
underwent bronchoscopy and lavage, which was analyzed for potential
infectious agents. Following PCR analysis, CMV DNA was detected in
the lavage fluid and CMV IgM was detected in the serum. Both tests
supported the diagnosis of CMV pneumonia. Additionally, workup for
Pneumocystis carinii (PCP), Ziehl-Neelsen staining and
bacterial and fungal cultures were negative, and anti-viral
treatment with Ganciclovir was added to his treatment regimen.
The patient was weaned off mechanical ventilation
after two weeks and admitted to the Department of Oncology with
high flow oxygen support and continued antiviral therapy,
prednisone (slowly tapered to 0.5 mg/kg) and prophylactic
trimethoprim-sulfamethoxazole. However, despite these measures, his
condition continued to deteriorate. After discussion with the
patient and his family, the decision was made to avoid repeat
intubation. The patient died 3 weeks later.
Discussion
We describe a patient that suffered from ICI-P
complicated by CMV pneumonia, diagnosed by serology and a positive
PCR test using pulmonary lavage. An autopsy with pathology review
of lung tissue would constitute a definitive diagnostic test to
support CMV pneumonia diagnosis, however the family declined this
procedure. The clinical course, consisting of an initial
improvement with steroids and subsequent deterioration and
unresponsiveness to aggressive immune suppression, together with
positive serological and PCR tests, support the diagnosis of CMV
pneumonia in this patient. To the best of our knowledge, this is
the first published report of CMV pneumonia complicating ICI-P.
Although the majority of patients with pneumonitis
secondary to ICIs recover with corticosteroid treatment, fatalities
may occur. It is important to recognize factors that may complicate
the clinical course in these patients so they can be diagnosed and
promptly treated. The clinical and radiological findings suggestive
of CMV pneumonia are non-specific, overlapping with those of
immune-mediated pneumonitis (15-17).
Thus, a high degree of clinical suspicion is needed for early
diagnosis. The present patient was treated with steroids for
several weeks before diagnosis of CMV pneumonia, and doses were
tapered, as aforementioned, suggesting that this complication can
occur even after a relatively short course of steroids.
The range of clinical diseases due to CMV in
immunocompromised patients is broad and includes hepatitis,
retinitis, encephalitis, esophagitis and colitis (18). Our case demonstrates that workup for
CMV infection should be administered in patients failing to improve
with steroids for immune-mediated toxicity.
We report a case of fatal CMV pneumonia complicating
ICI-P. A high degree of clinical suspicion and prompt evaluation
for CMV infection is recommended in cases that do not improve with
steroids for treatment of immunotherapy-associated adverse effects
and in patients that relapse following initial improvement.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AS was responsible for conception of the work, data
acquisition and analysis, revising and finalizing the manuscript.
OB was responsible for data acquisition and analysis, drafting and
revising the manuscript. AO was responsible for acquisition and
analysis.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
De Velasco G, Je Y, Bossé D, Awad MM, Ott
PA, Moreira RB, Schutz F, Bellmunt V, Sonpavde GP, Hodi FS and
Choueiri TK: Comprehensive meta-analysis of key immune-related
adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer
patients. Cancer Immunol Res. 5:312–318. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yuan M, Huang LL, Chen JH, Wu J and Xu Q:
The emerging treatment landscape of targeted therapy in
non-small-cell lung cancer. Signal Transduct Target Ther.
4(61)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang H, Guo X, Zhou J, Li Y, Duan L, Si X
and Zhang L, Liu X, Wang M, Shi J and Zhang L: Clinical diagnosis
and treatment of immune checkpoint inhibitor-associated
pneumonitis. Thorac Cancer. 11:191–197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cadranel J, Canellas A, Matton L, Darrason
M, Parrot A, Jean-Marc N, Lavolé A, Anne-Marie R and Fallet V:
Pulmonary complication for immune checkpoint inhibitors in patient
with nonsmall cell lung cancer. Eur Respir Revc.
28(190058)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ma K, Lu Y, Jiang S, Tang J, Li X and
Zhang Y: The relative risk and incidence of immune checkpoint
inhibitors related pneumonitis in patients with advanced cancer: A
meta-analysis. Front Pharmacol. 9(1430)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-pd-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Naidoo J, Page DB, Li BT, Connell LC,
Schindler K, Lacouture ME, Postow MA and Wolchok JD: Toxicities of
the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Annals
of Oncology. Ann Oncol. 26:2375–2391. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Thompson JA, et al: Management of immune-related adverse events
in patients treated with immune checkpoint inhibitor therapy:
American society of clinical oncology clinical practice guideline.
J Clin Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Petri CR, Patell R, Batalini F, Rangachari
D and Hallowell RW: Severe pulmonary toxicity from immune
checkpoint inhibitor treated successfully with intravenous
immunoglobulin: Case report and review of the literature. Respir
Med Case Rep. 27(100834)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stuck AE, Minder CE and Frey FJ: Risk of
infectious complications in patients taking glucocorticosteroids.
Rev Infect Dis. 11:954–963. 1989.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fishman JA: Opportunistic
infections-coming to the limits of immunosuppression. Cold Spring
Harb Perspect Med. 3(a015669)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Konoplev S, Champlin RE, Giralt S, Ueno
NT, Khouri I, Raad I and Whimbey E: Cytomegalovirus pneumonia in
adult autologous blood and marrow transplant recipients. Bone
Marrow Transplantationc. 27:877–881. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Travi G and Pergam SA: Cytomegalovirus
pneumonia in hematopoietic stem cell recipients. J Intensive Care
Med. 29:200–212. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Franquet T, Lee KS and Müller NL:
Thin-section CT findings in 32 immunocompromised patients with
Cytomegalovirus pneumonia who do not have AIDS. Am J Roentgenol.
181:1059–1063. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kang EY, Patz EF Jr and Müller NL:
Cytomegalovirus pneumonia in transplant patients: CT findings. J
Comput Assist Tomogr. 20:295–299. 1996.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ljungman P, Boeckh M, Hirsch HH, Josephson
F, Lundgren J, Nichols G, Pikis A, Razonable RR, Miller V and
Griffiths PD: Definitions of cytomegalovirus infection and disease
in transplant patients for use in clinical trials. Clin Infect Dis.
64:87–91. 2017.PubMed/NCBI View Article : Google Scholar
|