Introduction
The American Cancer Society estimates that ~10% of
women will have a detectable breast mass in their lifetime
(1). A phyllodes tumor (PT) of the
breast is a rare fibroepithelial neoplasm, accounting for 0.3-1.0%
of all breast tumors (2). PTs may
occur at any age but are commonly observed in women aged between 40
and 50 years (3). PTs can recur
locally and rarely metastasize, and only a few become malignant
(~10%) (4,5). Whether benign or malignant, certain
PTs grow rapidly, even to a diameter >10 cm (6). Because the clinical outcomes of all
PTs range from local recurrence in 6.3-32.0% of patients to
metastasis in 1.7-40.0% of patients, the National Comprehensive
Cancer Network guidelines recommend complete surgical resection of
PTs, particularly malignant PTs, to obtain a negative margin ≥1 cm
(7). However, the scope of surgery
[breast-conserving surgery (BCS) vs. mastectomy] and role of
adjuvant radiation therapy have been controversial (8). The initial surgical removal of a giant
PT is often challenging (6).
Numerous studies have shown that preoperative tumor reduction
improves the success rate of breast cancer surgery and results in
good prognosis (6,9). The present study reports an unusual
case of a giant malignant PT of the breast (MPTB) with a rich blood
supply. Preoperative interventional embolization without arterial
infusion chemotherapy and and protective embolization on
extrahepatic branches, such as the internal mammary artery, were
performed. Following arterial embolization, the heart rate
gradually decreased to a normal level, tumor surface necrosis
quickly appeared and the patient exhibited no fever, chest pain or
other symptoms; surgery successfully treated the pain and tumor
necrosis. The patient was transferred to chemoradiotherapy from
local radiotherapy. No recurrence was found at the 14-month
follow-up. Written informed consent was obtained from the
patient.
Case report
A previously healthy 41-year-old woman without
underlying systemic disease detected an olive-sized lump in her
left breast 6 months before hospital admission. The lump grew
rapidly, with itching and peeling breast skin observed after ~1
month; less than half a month prior to admission, a skin ulceration
appeared on the left breast. The patient had no family history of
breast cancer or childbearing. Upon admission to The First
Affiliated Hospital of Kunming Medical University, the tumor
measured >20 cm in diameter and was larger than the right breast
and the ulceration measured 2 cm in diameter; the engorgement of
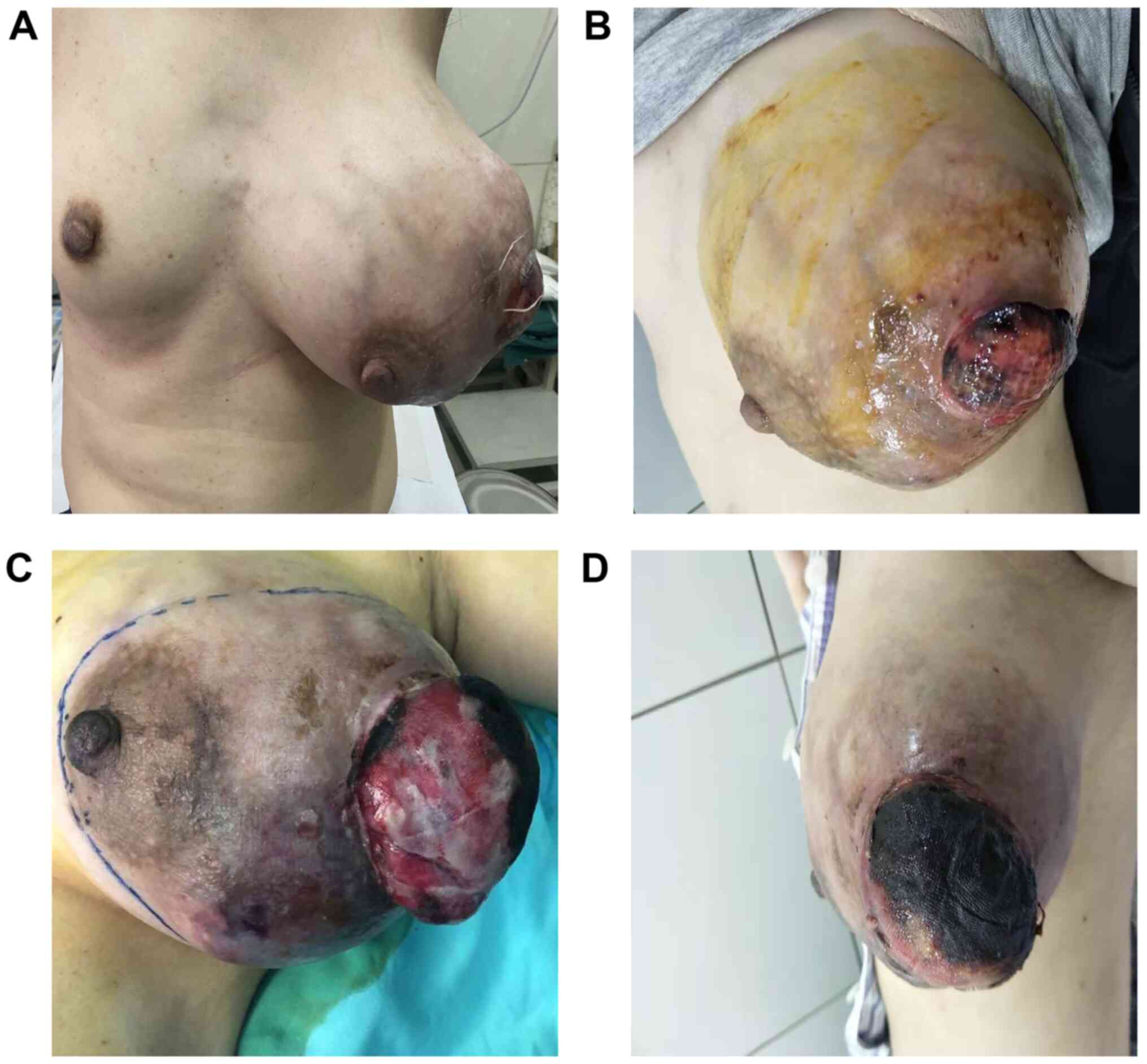
multiple veins was observed in the subcutaneous tissue (Fig. 1A and B). Palpation revealed that the tumor was
slightly hard and elastic, and certain areas were fluctuant.
Because of hypermetabolism and chronic blood loss from the tumor,
the patient’s heart rate increased to 128 beats per minute.
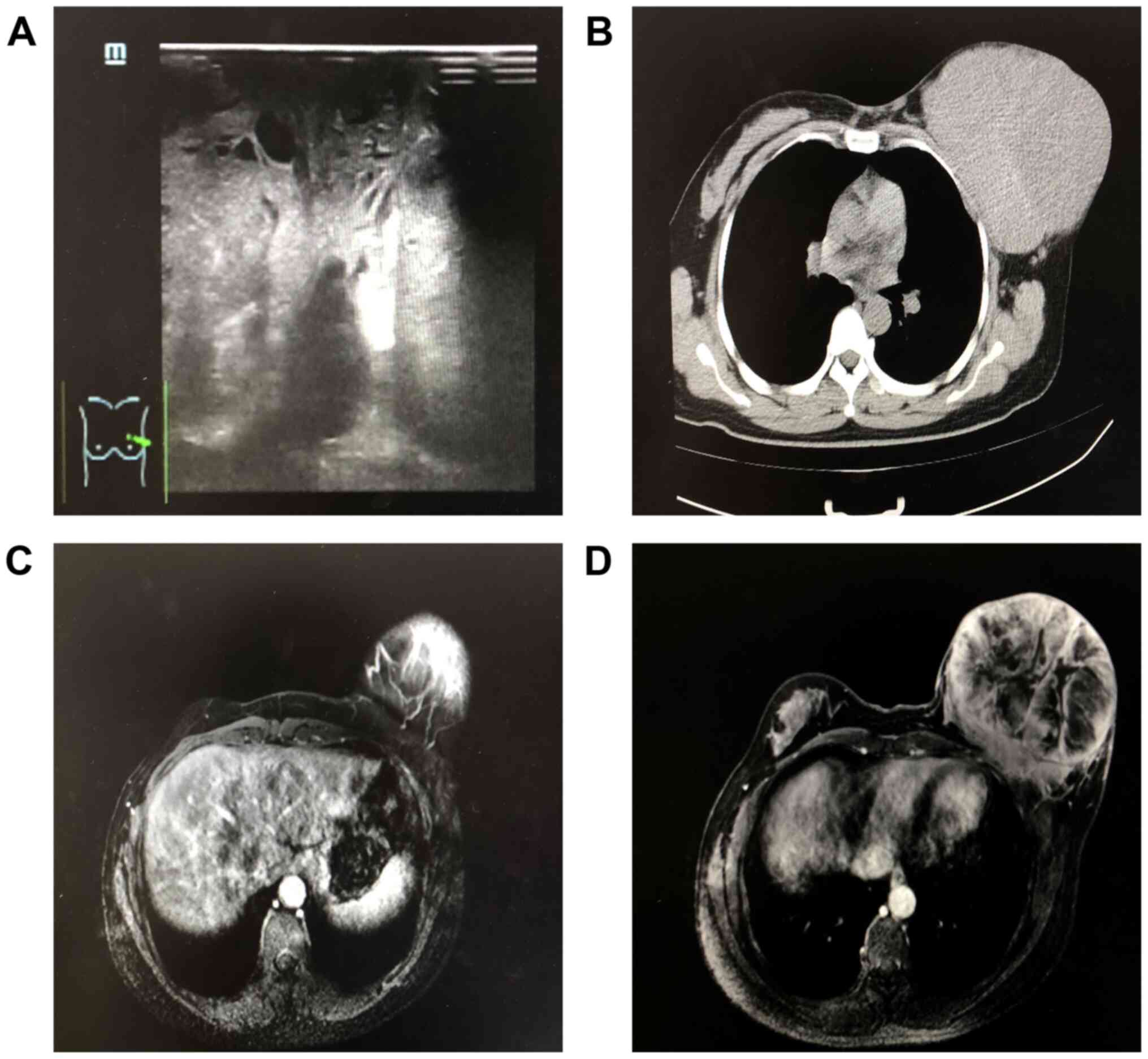
Color Doppler ultrasound revealed a very large
heterogeneous echo in the left mammary gland but could not
accurately measure the size of the lesion, which was ~192.6x117.2
mm. The boundary was obscure and the form was irregular, with a
rich blood supply inside. Multiple lymph nodes were enlarged and
rounded and hypoechoic nodules were observed in the left axillary
region (Fig. 2A). Computed
tomography (CT) showed a giant tumor occupying the left breast and
suspected invasion of the major pectoralis muscle, which indicated
that the tumor was potentially malignant (Fig. 2B). MRI showed a giant circular tumor
with a clear boundary occupying the whole breast and a partially
patchy unenhanced zone in the center measuring ~13x13x14 cm (upper
and lower diameter x left and right diameter x before and after
diameter). MRI also revealed possible invasion of the major
pectoralis muscle and several enlarged lymph nodes in the left
axillary region, the largest of which measured ~1.6x0.8 cm. No
abnormality was observed in the entire right breast tissue
(Fig. 2C and D). Preoperative core needle biopsy (CNB)
revealed that the biopsy specimen of the left breast was malignant
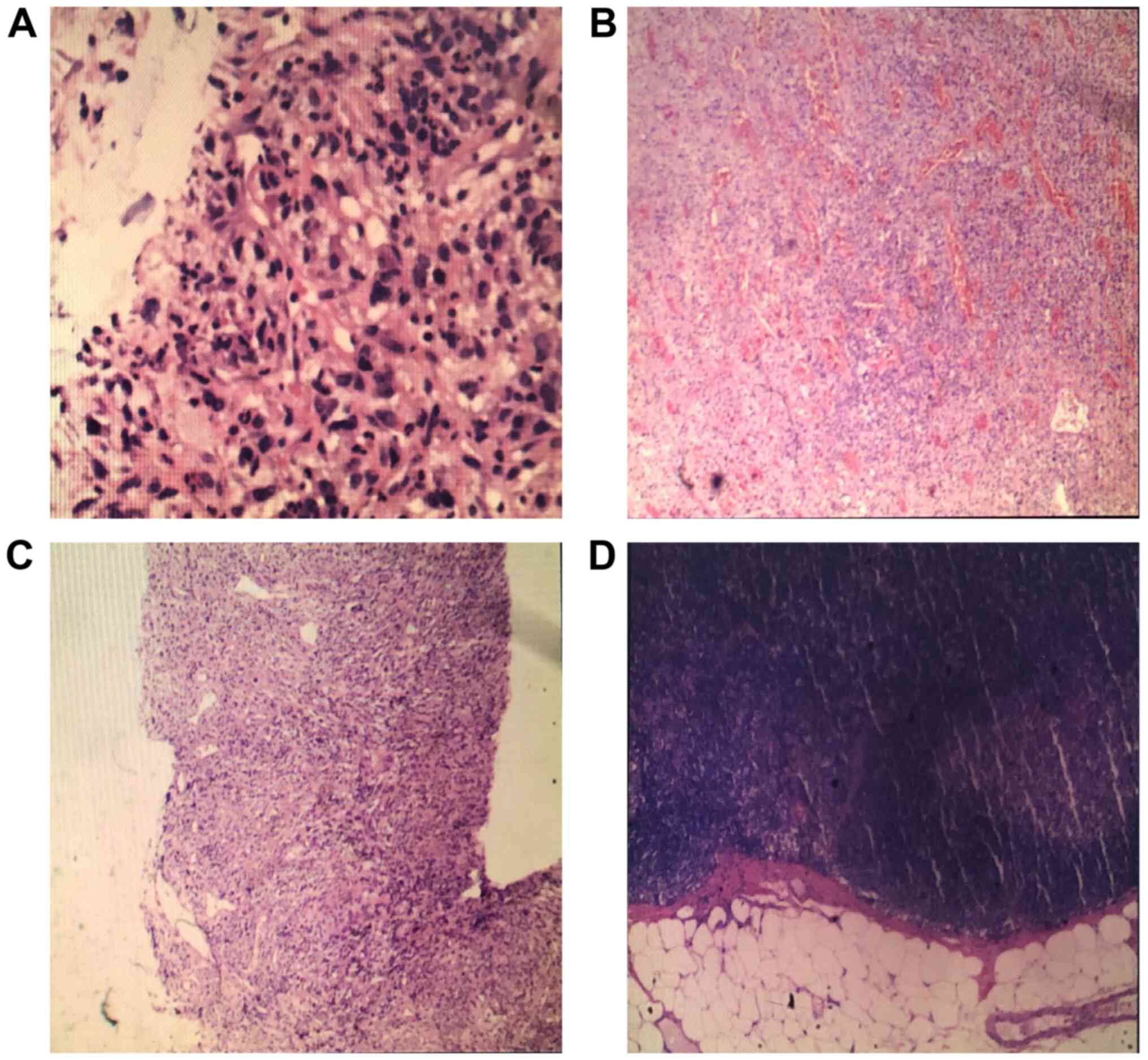
myxofibrosarcoma. The immunohistochemical report was as follows:
SMA (+), Des (-), KI-67 (+)30%, CK5/6 (-/+), P63 (-), CK (-) and
VIM (+) (Fig. 3A). Additionally,
the patient received comprehensive CT and MRI examination; the
results showed that the brain, lung, liver, bones and other organs
exhibited no signs of metastasis.
The tumor measured 24 cm in diameter; the fungating
wound was 5 cm above the breast surface and measured 5 cm in
diameter on the day before surgery. Because of the rich blood
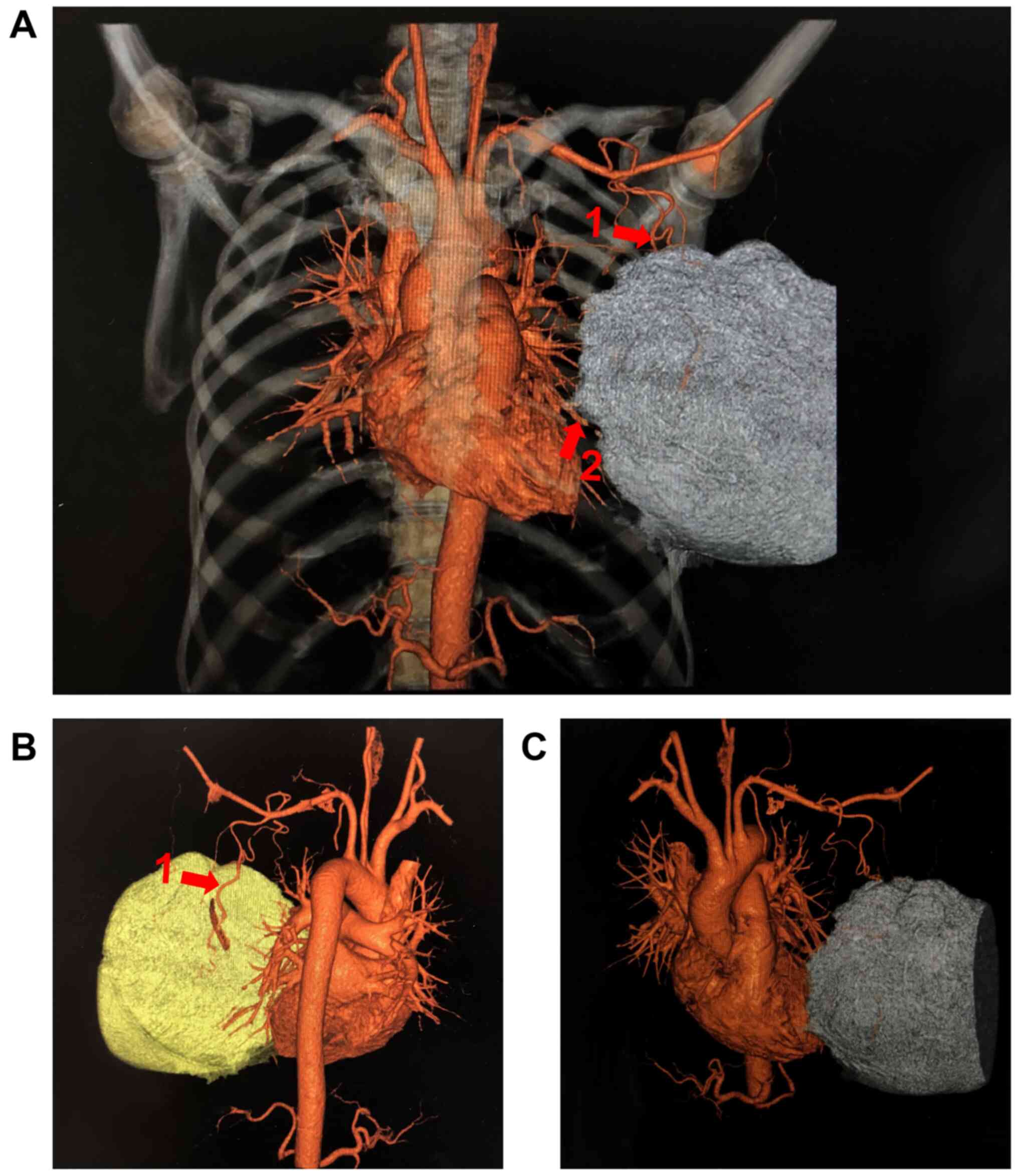
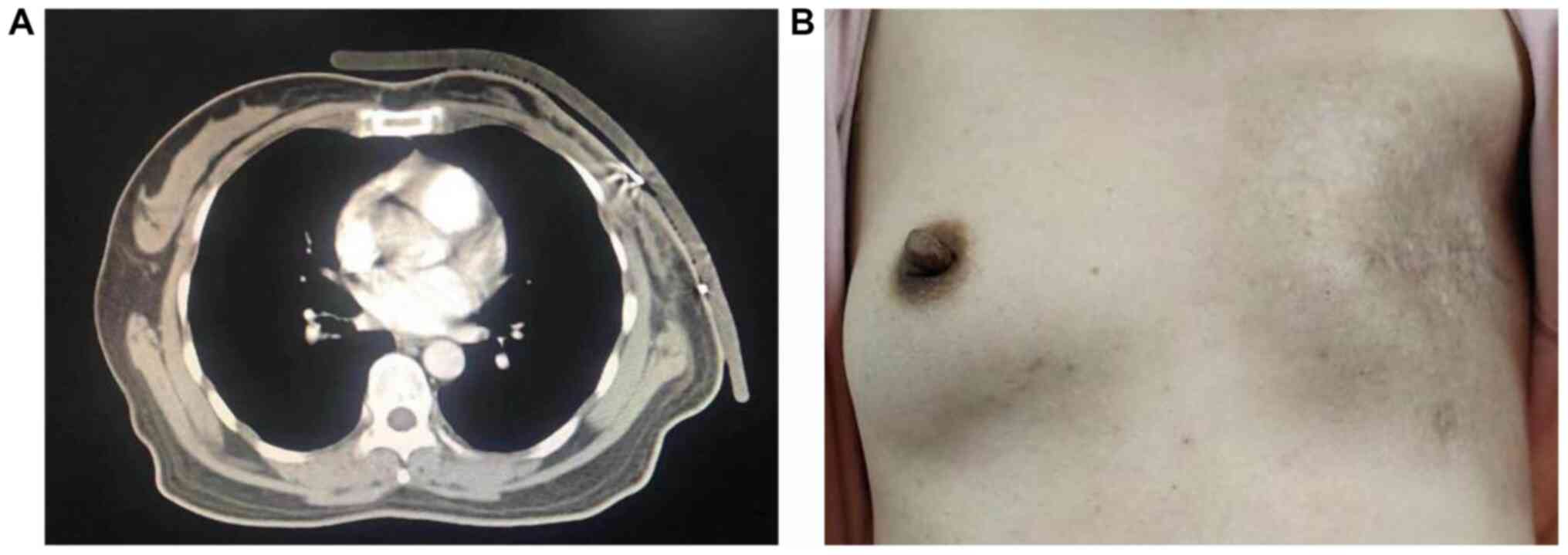
vessel supply revealed by MRI, the patient underwent CT angiography
of the breast tumor, which revealed that the perforating branch of
the left internal thoracic artery and branch of the left subclavian
artery were the dominant feeding arteries transporting blood to the
tumor in the left breast. These vessels were disordered and tangled
(Fig. 4). During the hospital
examination, the tumor grew rapidly with bleeding. In order to slow
tumor growth, decrease intraoperative blood loss and improve the
success rate of the operation, preoperative dominant blood supply
internal thoracic artery interventional embolization was performed,
and surgery was delayed.
The patient underwent percutaneous arterial
embolization, performed by an interventional physician, resulting
in tumor necrosis and contraction by limiting the tumor blood
supply. The process was performed as follows: After administering
local anesthesia, a 5F arterial sheath was inserted into the right
femoral artery using the Seldinger puncture technique. An elbowed
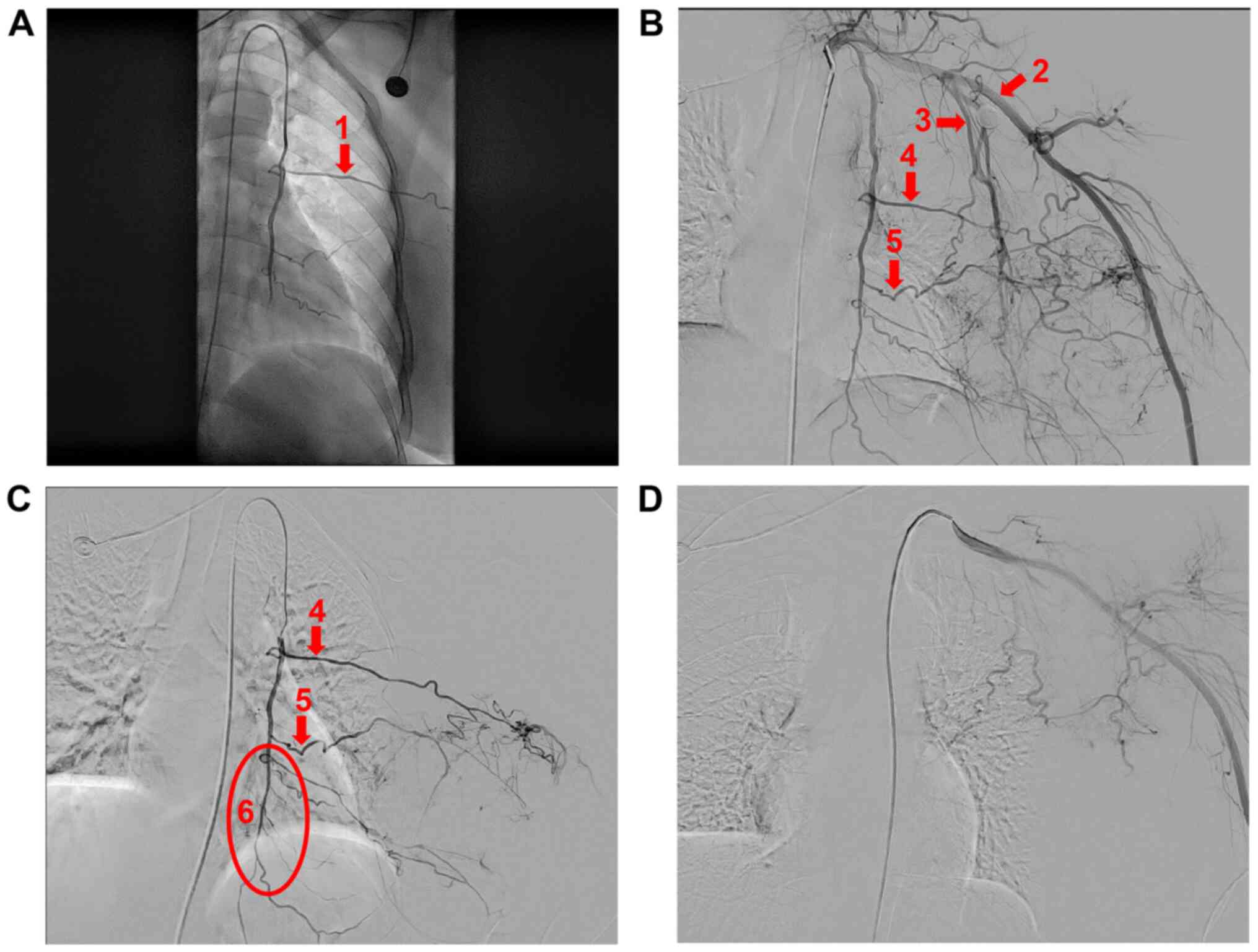
catheter was used to image the left subclavian artery; abnormal
staining was observed in the tumor, superselective to the internal
and external thoracic arteries (Fig.
5A and B). Protective
embolization was performed distal to the internal thoracic artery
using a gelatin sponge (Fig. 5C),
and the 5F arterial sheath was replaced with a 2.6F micropipe
superselective to the dominant blood-supplying arteries guided by a
0.018-inch micro godet. Next, gelatin sponge granules (710-1,000
µm) were injected; imaging revealed that most of the staining in
the tumor had disappeared (Fig.
5D). Following artery embolization, fever and black necrotic
tissue at the tumor site were observed. Fever resolved within 24 h,
but the area of necrosis on the tumor site expanded. No other
serious complications were observed and the tumor stopped growing
following artery embolization (Fig.
1C and D).
Three days after artery embolization, the patient
underwent surgery. Although axillary metastases of PTs are rare,
mastectomy and level I axillary lymph node excision due to swelling
were performed for safety after discussion. The tumor measured
17x16x11 cm. The bleeding volume during the operation was <80 ml
and the elasticity of several vessels was lost. The whole process
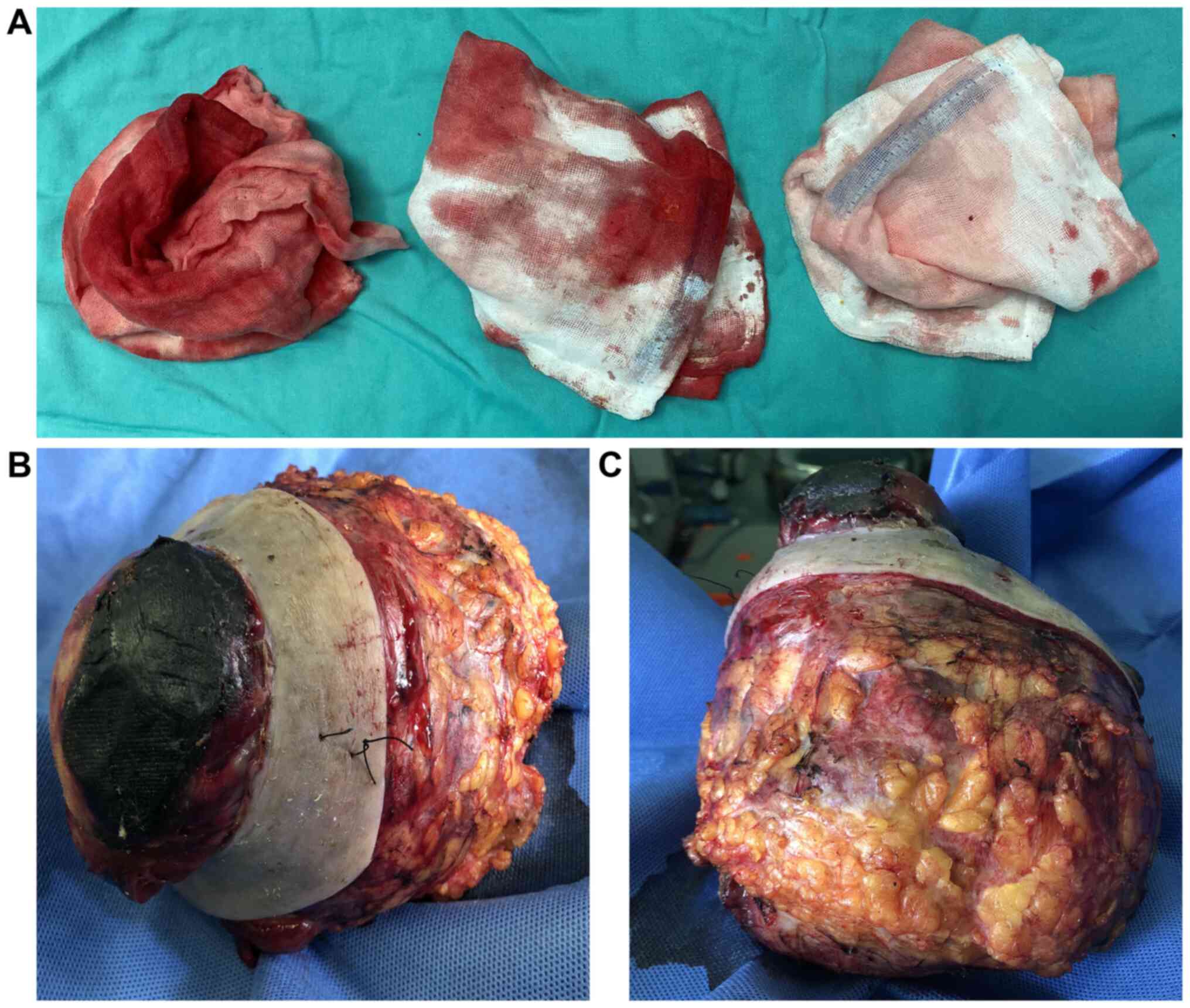
lasted 1.5 h (Fig. 6).
The postoperative biopsy specimen was diagnosed as a
malignant tumor, specifically myxofibrosarcoma (Fig. 3B and C). The tumor measured 17x16x11 cm, and the
following parameters were noted: Nerve invasion (-), vascular
invasion (-), invisible edge of the breast (-), invisible edge of
the base (-), invisible edge of the skin (-), nipple (-) and skin
(-). The immunohistochemical report was as follows: SMA (+), Des
(-), KI-67 (+)30%, CK5/6 (+), P63 (-), CK (-), and VIM (+). A total
of 11 lymph nodes were found in the left axillary gland, with no
metastatic tumors (Fig. 3D).
The surgery successfully treated the pain and tumor
necrosis (Fig. 7A).
Postoperatively, the patient recovered well and the incision healed
desirably. After surgery, it was recommended that the patient
undergo circulating tumor cell (CTC) detection and whole-genome
sequencing (WGS); CTC analysis was negative. Since the tumor
invaded the muscle layer of the chest wall, it was hypothesized
that the relevant microscopic lesion may still be present.
Therefore, we recommend that patients receive concurrent
chemoradiotherapy to prevent recurrence following surgery. The
patient was treated with local radiotherapy 18 times (3 Gy/time,
total dose 54 Gy) and with apatinib (250 mg/day) on the first and
fourth day and oral ticgio (40 mg/day). The patient refused further
chemotherapy and did not accept a recommendation for antiangiogenic
drugs. No recurrence was found at the 14-month follow-up (Fig. 7B).
Discussion
PTs are rare but complex fibroepithelial lesions of
the breast that are classified as benign, borderline or malignant
(5). PTs and malignant PTs present
significant challenges in diagnosis, classification, predictive
behavior and clinical treatment (10). From a diagnostic and management
perspective, correctly identifying malignant PTs is critical. They
should be surgically eradicated and effectively treated at the time
of diagnosis because the risks of metastasis and death from such
tumors, although relatively small, are well-established (5).
PTs have non-specific clinical and radiological
manifestations that can easily be confused with those of other
similar breast masses (11).
Clinical and radiological diagnoses are difficult, requiring joint
confirmation from multiple assessments. In the present case, color
Doppler ultrasound showed feature information that could assist in
diagnosis. CT showed whether lung metastasis occurred and
infiltration was assessed by MRI. However, these methods cannot
help to make a definitive preoperative diagnosis. CNB is a widely
used, highly sensitive method to obtain a preoperative diagnosis of
breast cancer (12), although the
tissue obtained in CNB does not represent the whole tumor. Here,
these results and clinical appearance were combined, and the
diagnosis was confirmed.
Surgical excision is the preferred procedure because
it can obtain a negative margin in the case of a final diagnosis of
a malignant PT (5,11). Although mastectomy is the only
treatment method for large tumors, the extent of surgery (BCS vs.
mastectomy) for small tumors remains controversial (8). Though axillary lymph node metastasis
is rare, some cases of giant PT-mixed breast cancer have been
reported. In the present case, the tumor grew rapidly and was very
large. The patient had metabolic consumption and risks were
associated with surgery.
Interventional embolization was developed to manage
vascular areas of bleeding, including in the brain, lung and liver
(13) and is an effective and safe
choice (14). Vascular occlusion by
embolization has also been used to control severe or recurrent
bleeding in fungal breast cancer (15). In the case of sternal erosion of
breast cancer, embolization can effectively control bleeding and
save lives (16). Interventional
embolization is used to decrease the preoperative breast tumor
volume and resection is performed 23 days after embolization.
Embolization effectively decreases intraoperative bleeding and
improves surgical results (16). In
the present case, the left breast tumor was primarily supplied by
the left internal thoracic artery branch and left internal thoracic
artery perforator. The blood supply vessels were thickened, the
blood supply was rich and the tumor grew rapidly with bleeding.
Therefore, catheter embolization was performed before surgery to
slow tumor growth and decrease intraoperative bleeding. Three days
after embolization, resection was successfully performed. No clear
guidance exists to determine the operation time after embolization,
and the operation should be performed carefully according to the
tumor growth rate, bleeding and ulceration for each patient.
Complications of interventional embolization are rare (14), and no complications were observed in
the present case. However, potential complications associated with
this type of operation, such as infection after tumor necrosis,
distal embolization and cerebral infarction (9), should be carefully monitored during
the process.
The precise role of adjuvant radiotherapy remains
debatable, but it has been personalized for certain patients with
malignant PT. Although radiotherapy has no significant effect on
survival rate, it can decrease local recurrence (5). When patients do not receive radiation
and chemotherapy, they may obtain passive results. For example, Kuo
et al (6) described patients
who received preoperative embolization chemotherapy and successful
tumor suppression. However, chemotherapy is not an option for
patients two months after recurrence (6). In the present case, since the tumor
invaded the muscle layer of the chest wall, it was recommended that
the patient receive chemotherapy and local radiotherapy to prevent
recurrence; the 14-month follow-up after chemoradiotherapy was
good. This result suggests that, in the case of an MPTB,
particularly an MPTB with chest wall invasion, chemoradiotherapy is
necessary.
WGS and CTC detection lead to high-resolution
analysis of cancers and reliably guided personalized therapy
(17); the patient accepted CTC
detection but rejected WGS. The CTC detection results were
negative.
Patients with large lesions are inclined to undergo
mastectomy. In cases of giant malignant PTs, preoperative
intervention artery embolization can inhibit tumor growth, decrease
bleeding during an operation, increase the operation speed and
shorten the period of postoperative convalescence. Preoperative
intervention artery embolization was effective and safe for the
present patient, and postoperative local radiotherapy may be
necessary for a good prognosis.
Acknowledgements
Not applicable.
Funding
The present report was supported by the National Natural Science
Foundation of China (grant no. 81660438).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FG, AJ and WC conceived and designed the study. YT,
LC, SZ, RS, WZ and LL performed data acquisition and analysis. YT,
LL and FG drafted the manuscript. FG and LL reviewed the
manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Approval was obtained from the ethics committee of
Kunming Medical University. The procedures performed in this study
adhered to the tenets of the Declaration of Helsinki. Written
informed consent was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chang J, Denham L, Dong EK, Malek K and
Lum SS: Trends in the diagnosis of phyllodes tumors and
fibroadenomas before and after release of WHO classification
standards. Ann Surg Oncol. 25:3088–3095. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Krings G, Bean GR and Chen YY:
Fibroepithelial lesions: The WHO spectrum. Semin Diagn Pathol.
34:438–452. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tan PH, Ellis I, Allison K, Brogi E, Fox
SB, Lakhani S, Lazar AJ, Morris EA, Sahin A, Salgado R, et al: The
2019 WHO classification of tumours of the breast. Histopathology.
77:181–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tan BY, Acs G, Apple SK, Badve S,
Bleiweiss IJ, Brogi E, Calvo JP, Dabbs DJ, Ellis IO, Eusebi V, et
al: Phyllodes tumours of the breast: A consensus review.
Histopathology. 68:5–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kuo CY, Lin SH, Lee KD, Cheng SJ, Chu JS
and Tu SH: Transcatheter arterial chemoembolization improves the
resectability of malignant breast phyllodes tumor with angiosarcoma
component: A case report. BMC Surg. 19(100)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Breast cancer, version 4.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:310–320. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mitus J, Reinfuss M, Mitus JW, Jakubowicz
J, Blecharz P, Wysocki WW and Skotnicki P: Malignant phyllodes
tumor of the breast: Treatment and prognosis. Breast J. 20:639–644.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hashimoto K, Mimura H, Arai Y, Doi M,
Kojima Y, Tsugawa K and Nakajima Y: Successful preoperative
chemoembolization in the treatment of a giant malignant phyllodes
tumor. Cardiovasc Intervent Radiol. 39:1070–1075. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Moten AS and Goldberg AJ: Malignant
phyllodes tumors of the breast: Association between race, clinical
features, and outcomes. J Surg Res. 239:278–283. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Atalay C, Kınaş V and Çelebioğlu S:
Analysis of patients with phyllodes tumor of the breast. Ulus
Cerrahi Derg. 30:129–132. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dillon MF, Quinn CM, McDermott EW,
O’Doherty A, O’Higgins N and Hill ADK: Needle core biopsy in the
diagnosis of phyllodes neoplasm. Surgery. 140:779–784.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moriarty JM, Xing MZ and Loh CT: Particle
embolization to control life-threatening hemorrhage from a
fungating locally advanced breast carcinoma: A case report. J Med
Case Rep. 6(186)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Corvino F, Giurazza F, Cangiano G,
Cavaglià E, Amodio F, Magistris GD, Corvino A and Niola R: Safety
and effectiveness of transcatheter embolization in the treatment of
internal mammary artery injuries. Radiol Med. 123:369–377.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aksoy Ş, Akçe B, Kiliçkesmez Ö, Gürsü RU,
Çakır MS, Nazlı MA and Aren A: Transcatheter arterial embolization
for controlling severe bleeding from recurrent locally-advanced
breast cancer. J Breast Health. 12:137–140. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Leung JWT, Gotway MB and Sickles EA:
Preoperative embolization of vascular phyllodes tumor of the
breast. AJR Am J Roentgenol. 184 (Suppl 3):S115–S117.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gulbahce N, Magbanua MJM, Chin R, Agarwal
MR, Luo XH, Liu J, Hayden DM, Mao Q, Ciotlos S, Li ZY, et al:
Quantitative whole genome sequencing of circulating tumor cells
enables personalized combination therapy of metastatic cancer.
Cancer Res. 77:4530–4541. 2017.PubMed/NCBI View Article : Google Scholar
|