Introduction
Ovarian cancer presents with a diverse range of
findings. Ovarian metastasis (OM), in particular, is not uncommon.
Such tumors mainly originate from the colon, stomach, endometrium,
appendix or breast (1,2). Approximately 15% of ovarian tumors are
metastatic (3). Bilateral small,
solid and highly vascularized ovarian masses are suggestive of OM
from breast cancer (4-8).
Tumors may metastasize to the ovaries through various routes,
including direct, hematogenous and lymphatic spread, as well as
transcoelomic dissemination (9).
Breast cancer is the most common malignant tumor and
one of the main causes of cancer-related mortality among women
worldwide (10). Under 10% of
patients with breast cancer exhibit evidence of distant metastasis
at the time of initial diagnosis (11). OM from breast cancer is frequently
asymptomatic until the tumor has grown to a considerable size. In
cases of malignant tumors of unknown histology, immunohistochemical
staining is performed as part of a thorough histological
examination. First, the histological type of the tumor is grossly
categorized as carcinoma, sarcoma, lymphoma, malignant melanoma or
germ cell tumor. There are indicative immunohistochemical markers
for each type, including cytokeratin (CK) for carcinoma, vimentin
and various differentiation markers for sarcoma, CD45 for lymphoma,
S100 protein and melanosome-associated antigen (also known as clone
HMB45) for malignant melanoma, and CD117/c-kit and Sal-like protein
4 for germ cell tumors. Once a tumor has been diagnosed as
carcinoma, particularly metastatic carcinoma of unknown primary
origin, immunohistochemistry is performed using a combination of
antibodies against CK7, CK20 and tissue-specific antigens, such as
thyroid transcription factor-1 (TTF1) for thyroid or lung cancer
and caudal-type homeobox 2 (CDX2) for colorectal cancer. In the
present case, these ancillary pathological procedures were also
employed to reach a conclusive diagnosis (12). OM from breast cancer may
occasionally mimic primary ovarian cancer. In recent years, our
knowledge on hereditary breast cancer caused by genetic factors has
increased. We herein report a case of OM from breast cancer
mimicking primary ovarian cancer, in which the patient was
diagnosed with synchronous bilateral breast cancer, indicating the
possible involvement of genetic factors, such as breast cancer type
1 susceptibility protein (BRCA)1 and BRCA2.
Case report
The patient was a 49-year-old Japanese woman,
gravida 1, para 1, who visited a local clinic complaining of
abdominal distention lasting for 10 days. A left-sided ovarian
tumor was identified on ultrasonography, and the patient was
referred to Tokyo Women's Medical University Hospital for further
examination and treatment in October 2019. Her medical history was
unremarkable, and she had no family history of hereditary breast
and ovarian cancer (HBOC). The patient had undergone menopause at
the age of 48 years. Physical examination did not reveal any
significant findings. Pelvic ultrasound examination revealed a
large amount of ascitic fluid and a left-sided ovarian tumor.
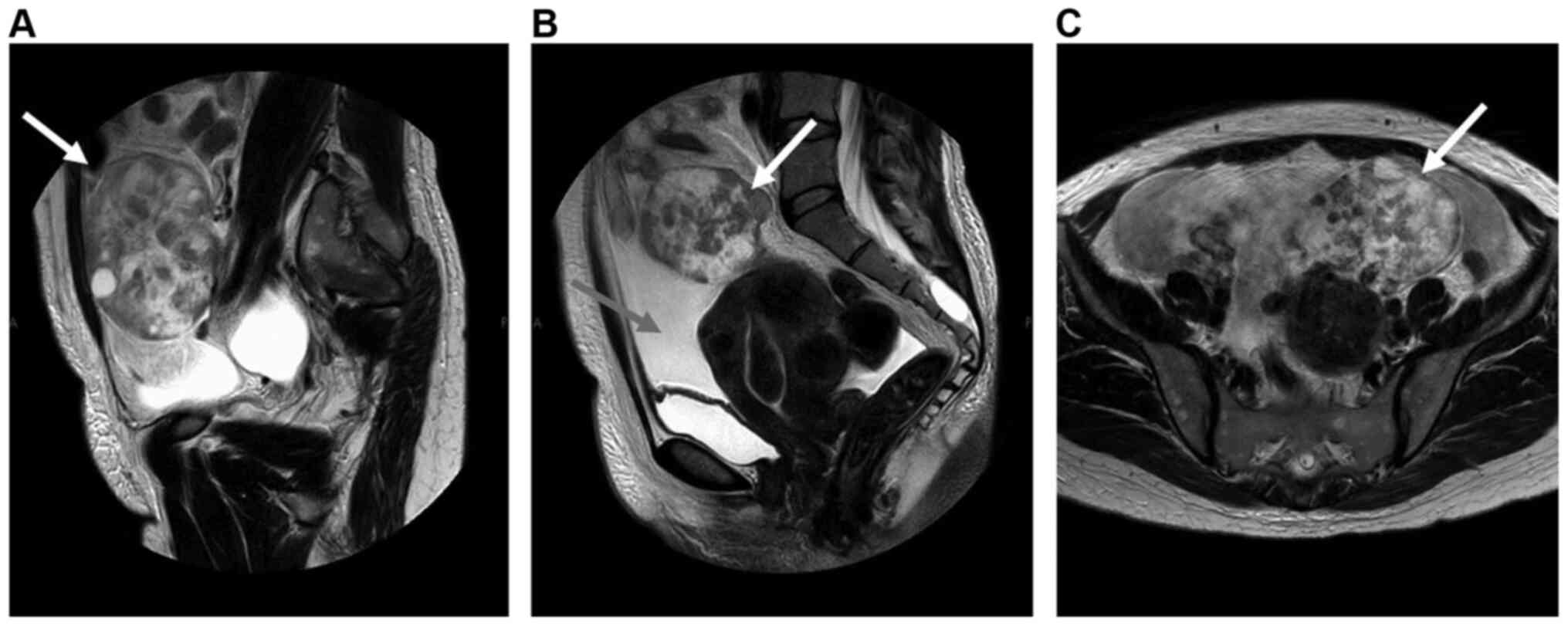
Pelvic magnetic resonance imaging examination revealed a left-sided
ovarian tumor, measuring 11 cm in largest diameter, and a massive
amount of ascitic fluid (Fig. 1).
The patient's laboratory findings included elevated serum levels of
cancer antigen (CA)125 (692 U/ml; normal range: ≤35 U/ml) and
CA15-3 (93.9 U/ml; normal range: ≤25 U/ml). The patient was
clinically diagnosed with ovarian carcinoma. Total abdominal
hysterectomy, bilateral salpingo-oophorectomy and omentectomy were
performed. Granulosa cell tumor, neuroendocrine carcinoma and
metastatic ovarian cancer were considered in the differential
diagnosis based on the morphological findings of the intraoperative
frozen section biopsy. Further postoperative histopathological
examination of the ovarian tumor raised the suspicion of metastasis
from lobular carcinoma of the breast. A breast and endocrine
surgeon at our hospital was consulted, and detailed examination
revealed invasive lobular carcinoma (ILC) of the left breast
(diameter: 5 cm), invasive ductal carcinoma (IDC) in the right
breast (diameter: 1 cm) and suspected multiple metastases to the
lumbar vertebrae. Genetic counseling and testing of BRCA1
and BRCA2 were performed, which did not reveal any germline
mutations. The patient was started on 125-mg palbociclib tablets
(once daily for 21 days followed by a break of 7 days), 2.5-mg
letrozole tablets (once daily) and 120 mg denosumab (once monthly).
The patient has remained well, with stable disease. She has been to
the hospital every 4 weeks for treatment and hematological
examinations. Imaging examinations were also performed every 3-6
months, and the patient has remained stable (last follow-up, March
2021).
Pathological findings of the ovarian
tumor Intraoperative pathological findings
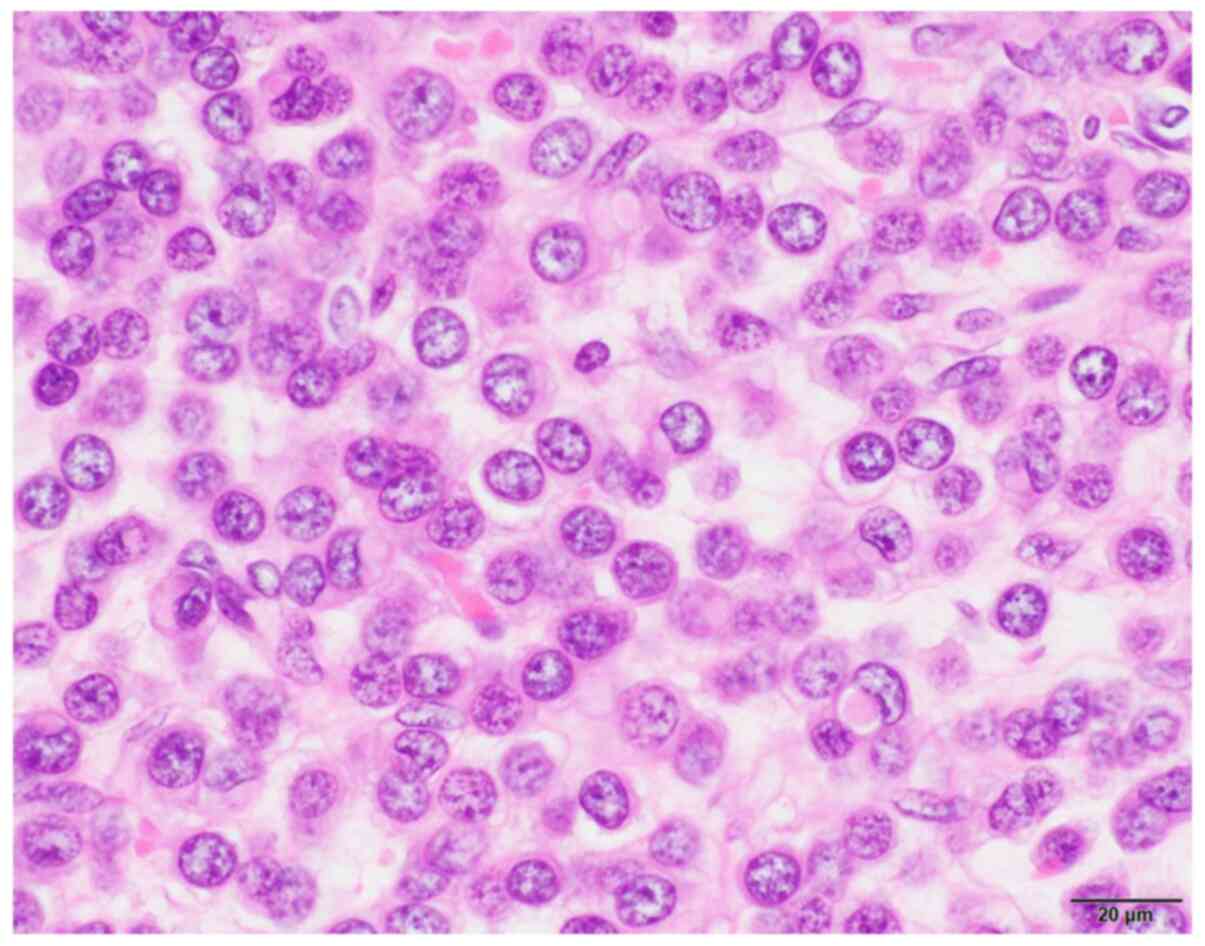
During the intraoperative pathological examination,
the histological type of the tumor could not be determined. The
tumor was composed of tightly packed, uniform, small round cells
(Fig. 2) and was tentatively
reported as a ‘small round cell tumor’. Sex cord tumor,
neuroendocrine carcinoma and metastatic carcinoma were considered
as diagnostic candidates.
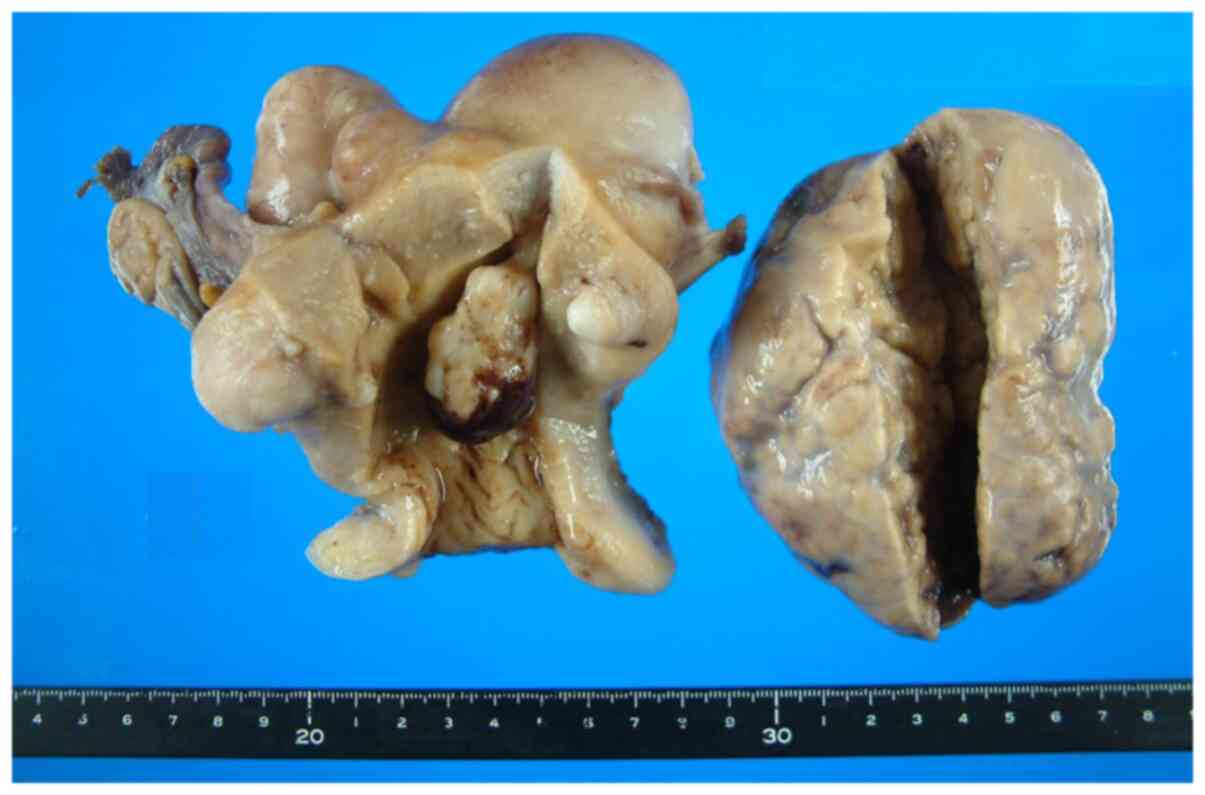
Gross findings
The left ovary was enlarged, solid, and measured
105x85x45 mm (Fig. 3). Its surface
was smooth and tense. Its cut surface was lobulated, homogenously
whitish in color, and firm in consistency. The right ovary was
atrophic and small. The uterus and oviducts were unremarkable.
Histopathological findings of the
ovarian tumor
The resected specimens were immediately fixed with
10% formalin, embedded in paraffin, cut into 4-µm sections and
subjected to histopathological examination. On hematoxylin and
eosin staining, the ovarian tumor was composed of small, uniformly
round tumor cells without intercellular connections, with a high
nuclear:cytoplasmic ratio. Occasionally, the nuclei of the tumor
cells were eccentrically situated within intracytoplasmic mucus.
Due to the possibility of the ovarian tumor being a metastatic
carcinoma rather than a primary ovarian tumor, ancillary
immunohistochemical studies were performed to identify the primary
site. Among the potential primary sites, the stomach (poorly
differentiated adenocarcinoma) and breast (ILC) were considered to
be the most likely, based on the characteristics of the disease and
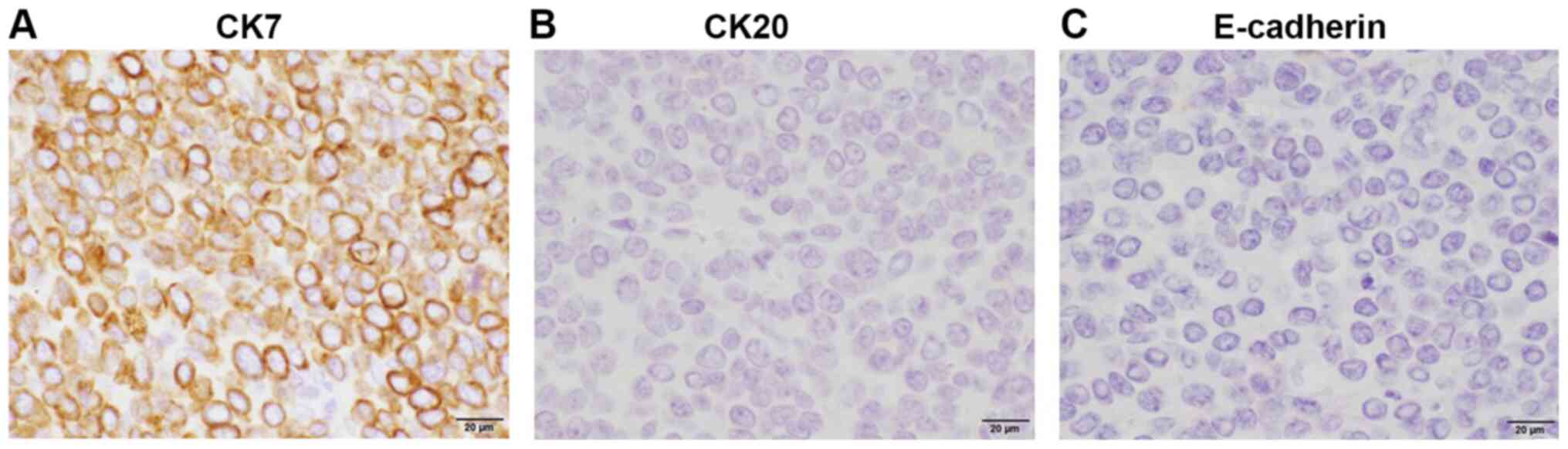
the morphology of the tumor. The tumor cells were positive for CK
(clones AE1/AE3 and CAM5.2) and CK7, but not CK20. Additional
immunostaining revealed positivity for the estrogen receptor (ER)
and negative results for TTF1 and CDX2. Accordingly, the breast was
considered as the likely primary site. To evaluate the possibility
of lobular carcinoma, E-cadherin expression was examined in the
tumor, which produced a negative result (Fig. 4). Based on these findings, detailed
breast examinations we performed, and bilateral breast carcinomas
were detected. One was diagnosed as IDC and the other was diagnosed
as ILC, the latter being the primary tumor of the OM.
Pathology of the breast tumors
Accordingly, detailed examinations and core-needle
biopsies of the bilateral breast tumors were performed. The
histological findings are shown in Fig.
5. Histologically, the left breast tumor was an ILC, which was
composed of small tumor cells (Fig.
5A) and was immunohistochemically negative for E-cadherin
(Fig. 5B). The ovarian tumor was
composed of similar small uniform tumor cells (Fig. 5G), which were also negative for
E-cadherin (Fig. 5H). By contrast,
the right breast tumor was an IDC, exhibiting formation of
neoplastic ducts (Fig. 5M) and
positivity for E-cadherin (Fig.
5N). The molecular subtype of the breast ILC was classified as
luminal A [positive for ER (Fig.
5C) and progesterone receptor (PgR; Fig. 5D), but negative for human epidermal
growth factor receptor 2 (HER2; Fig.
5E)], which was the same staining pattern as that of the
metastatic lesion (Fig. 5I,
J and K, respectively). Ki-67-positive cells were
scarce (Fig. 5L). The IDC in the
right breast was basal-like [negative for the ER, PgR and HER2
(Fig. 5O, P and Q,
respectively)], with a Ki-67 labeling index of ~5% (Fig. 5R).
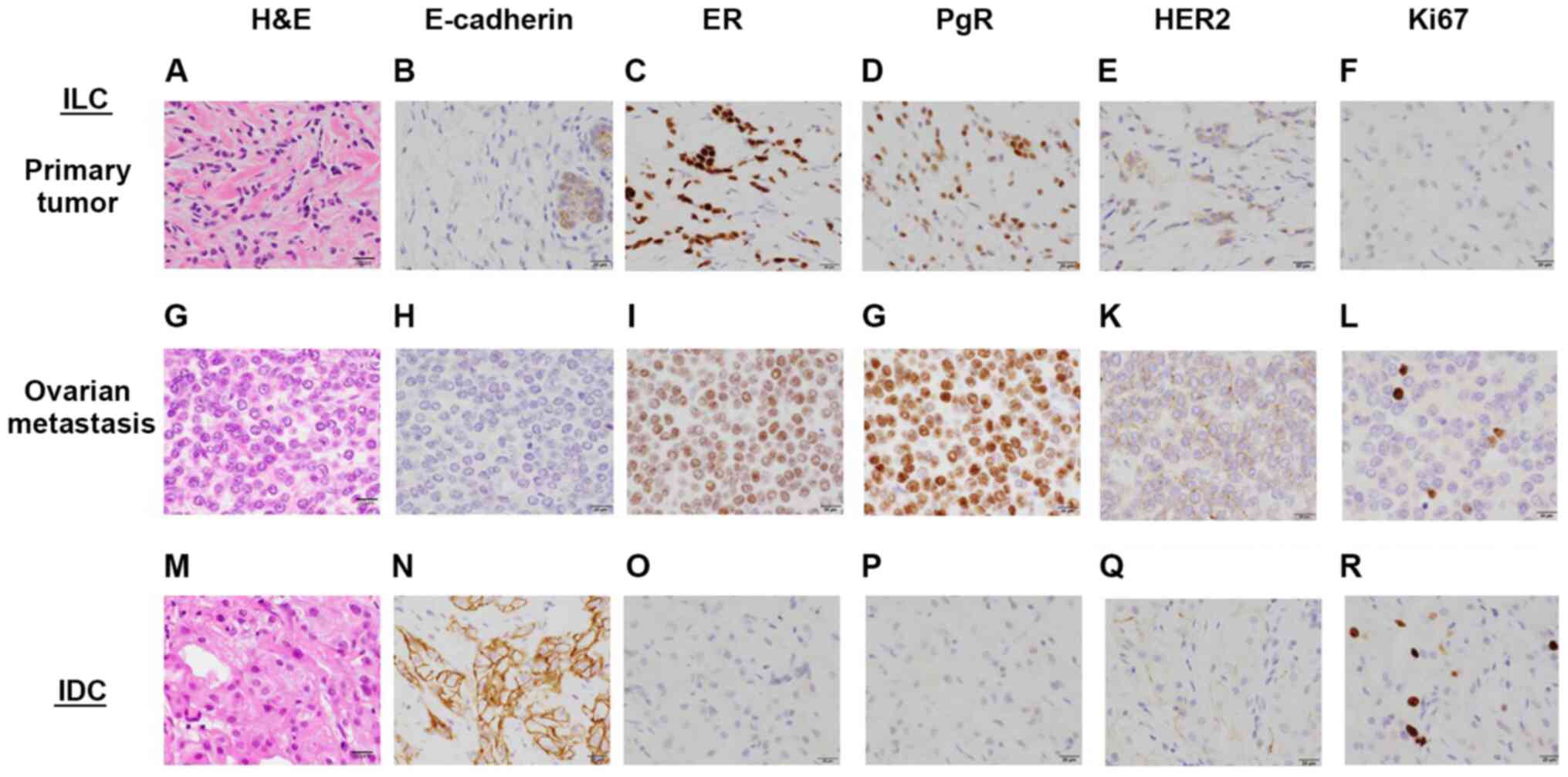 | Figure 5Pathological examination of the breast
and ovarian cancers. The primary tumor was found to be ILC of the
left breast based on (A) HE, (B) E-cadherin, (C) ER staining, (D)
PgR, (E) HER2 and (F) Ki-67 staining. (G-L) Respective staining
results for the ovarian metastasis. (M-R) Respective staining
results for the IDC of the right breast. Scale bars: 20 µm;
magnification, x40. ILC, invasive lobular carcinoma; IDC, invasive
ductal carcinoma, HE, hematoxylin and eosin; ER, estrogen receptor;
PgR, progesterone receptor; HER2, human epidermal growth factor
receptor. |
Discussion
The ovary is a common target of cancer metastasis,
and OM exhibits characteristic clinical presentations, such as
Krukenberg tumors (13) or
pseudo-Meigs syndrome (14-16).
However, it has been reported that the primary tumor could not be
identified in 15% of OM cases (17). The incidence of OM in patients with
breast cancer ranges between 13 and 47% (these percentages are
mainly based on autopsies or prophylactic or therapeutic
oophorectomies) (18-20).
OM from breast cancer is mainly found after surgery at the primary
site. The mean duration of the period between the diagnosis of the
primary tumor and the diagnosis of OM is ~2 years (21). OM from breast cancer is generally
asymptomatic until the metastatic mass has reached a certain size,
and such tumors frequently manifest as bilateral, solid, small
ovarian masses. Some patients may present with other symptoms, such
as ascites, gastrointestinal symptoms, pelvic pain and vaginal
bleeding (22-24).
Metastatic ovarian cancer can be diagnosed based on pathological
examination, as was seen in the present case. In cases of breast
cancer, both ILC and IDC have been reported to metastasize to the
ovaries. In a study of 29 cases of OM from breast cancer, 12 of the
29 cases involved ILC (25). In
another study, 2,605 cases of ILC or IDC of the breast were
encountered during the 18-year study period. ILC and IDC accounted
for 359 (13.8%) and 2,246 (86.2%) of the cases, respectively. Among
those, there were 7 (1.9%) cases of OM from ILC and 13 (0.6%) cases
of OM from IDC (26). In several
studies, the pattern of metastasis from breast cancer was
investigated, and ILC was found to affect the internal reproductive
organs more often than IDC (26,27).
In the present case, the patient had simultaneous bilateral breast
carcinomas, namely ILC in the left breast and IDC in the right
breast, and the OM originated from the ILC in the left breast. Less
than 10% of patients with breast cancer display evidence of distant
metastasis at the initial diagnosis. On the other hand, patients
with a history of breast cancer are 3-7 times more likely to
develop primary ovarian cancer than OM (8,28,29).
As the present case involved simultaneous bilateral breast cancers,
it is possible that the patient had HBOC syndrome. In cases of
breast cancer involving mutations in the BRCA genes,
poly(ADP)-ribose polymerase (PARP) inhibitors are highly effective
when used as adjuvant treatment (30,31).
BRCA1 and BRCA2 germline mutations are identified in
~10% of all breast cancers, and PARP inhibitors and platinum-based
chemotherapies are considered as suitable treatments for such cases
(32). In addition, prophylactic
risk-reducing surgery (risk-reducing mastectomy and/or
salpingo-oophorectomy) should be considered for patients that
harbor BRCA1 or BRCA2 germline mutations. In cases in
which two or more primary breast cancers develop, it is recommended
that genetic testing of BRCA1/2 should be carried out to
identify any relevant genetic changes (33). Since our patient developed
simultaneous bilateral breast cancers, it cannot be ruled out that
other genetic factors may have been involved.
In conclusion, the differential diagnosis of OM and
primary ovarian cancer based on clinical findings alone may be
challenging, and clinicians should be aware that OM from breast
cancer may occasionally masquerade as primary ovarian cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported, in part, by the SHISEIKAI
Scientific Award 2019 to YA.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YA, TY, YN, TO and TT collaborated in the conception
and design of the study. TY and YN performed the pathological
diagnosis of the ovarian and breast lesions. YA and YN wrote the
manuscript. TK, YH, TY and YN were involved in figure preparation.
TK, YH, YS, EN, YA and YN reviewed the manuscript. TK, YH, YS, EN,
YA and YN analyzed the data and reviewed the manuscript. All the
authors were involved in the preparation of the manuscript. All the
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for the publication of the
clinical details of this case was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kubeček O, Laco J, Špaček J, Petera J,
Kopecký J, Kubečková A and Filip S: The pathogenesis, diagnosis,
and management of metastatic tumors to the ovary: A comprehensive
review. Clin Exp Metastasis. 34:295–307. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Omranipour R and Abasahl A: Ovarian
metastases in colorectal cancer. Int J Gynecol Cancer.
19:1524–1528. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alvarado-Cabrero I, Rodríguez-Gómez A,
Castelan-Pedraza J and Valencia-Cedillo R: Metastatic ovarian
tumors: A clinicopathologic study of 150 cases. Anal Quant
Cytopathol Histpathol. 35:241–248. 2013.PubMed/NCBI
|
|
4
|
Yadav BS, Sharma SC, Robin TP, Sams S,
Elias AD, Kaklamani V, Kelly Marcom P, Schaefer S and Morris GJ:
Synchronous primary carcinoma of breast and ovary versus ovarian
metastases. Semin Oncol. 42:e13–e24. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guerriero S, Alcazar JL, Pascual MA,
Ajossa S, Olartecoechea B and Hereter L: Preoperative diagnosis of
metastatic ovarian cancer is related to origin of primary tumor.
Ultrasound Obstet Gynecol. 39:581–586. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gagnon Y and Têtu B: Ovarian metastases of
breast carcinoma. A clinicopathologic study of 59 cases. Cancer.
64:892–898. 1989.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bigorie V, Morice P, Duvillard P, Antoine
M, Cortez A, Flejou JF, Uzan S, Darai E and Barranger E: Ovarian
metastases from breast cancer: Report of 29 cases. Cancer.
116:799–804. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tserkezoglou A, Kontou S, Hadjieleftheriou
G, Apostolikas N, Vassilomanolakis M, Sikiotis K, Salamalekis E,
Tseke P and Magiakos G: Primary and metastatic ovarian cancer in
patients with prior breast carcinoma. Pre-operative markers and
treatment results. Anticancer Res. 26:2339–2344. 2006.PubMed/NCBI
|
|
9
|
Yamanishi Y, Koshiyama M, Ohnaka M, Ueda
M, Ukita S, Hishikawa K, Nagura M, Kim T, Hirose M, Ozasa H and
Shirase T: Pathways of metastases from primary organs to the
ovaries. Obstet Gynecol Int. 2011(612817)2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
world. Asian Pac J Cancer Prev. 17:43–46. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ernst MF, van de Poll-Franse LV, Roukema
JA, Coebergh JW, van Gestel CM, Vreugdenhil G, Louwman MJ and Voogd
AC: Trends in the prognosis of patients with primary metastatic
breast cancer diagnosed between 1975 and 2002. Breast. 16:344–351.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stelow EB and Yaziji H:
Immunohistochemistry, carcinomas of unknown primary, and incidence
rates. Semin Diagn Pathol. 35:143–152. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Studzinski Z and Zajewski W: Bilateral
metastatic ovarian tumors (Krukenberg's tumors) in the course of
stomach cancer. Arch Gynecol Obstet. 267:95–97. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kawakubo N, Okido M, Tanaka R, Mitsugi K,
Fukuhara M, Aishima S, Kato M and Ichimiya H: Pseudo-Meigs'
syndrome associated with breast cancer metastasis to both ovaries:
Report of a case. Surg Today. 40:1148–1151. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Naito K, Oura S, Yasuoka H and Okamura Y:
A case of pseudo-meigs' syndrome associated with ovarian metastases
from breast cancer. J Breast Cancer. 15:474–477. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fujii M, Okino M, Fujioka K, Yamashita K
and Hamano K: Pseudo-Meigs' syndrome caused by breast cancer
metastasis to both ovaries. Breast Cancer. 13:344–348.
2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jung YE, Lee JW, Kim BG and Bae DS:
Ovarian metastasis from pulmonary adenocarcinoma. Obstet Gynecol
Sci. 56:341–344. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rosendahl M, Timmermans Wielenga V,
Nedergaard L, Kristensen SG, Ernst E, Rasmussen PE, Anderson M,
Schmidt KT and Andersen CY: Cryopreservation of ovarian tissue for
fertility preservation: No evidence of malignant cell contamination
in ovarian tissue from patients with breast cancer. Fertil Steril.
95:2158–2161. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peters IT, van Zwet EW, Smit VT, Liefers
GJ, Kuppen PJ, Hilders CG and Trimbos JB: Prevalence and risk
factors of ovarian metastases in breast cancer patients <41
years of age in the Netherlands: A nationwide retrospective cohort
study. PLoS One. 12(e0168277)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bastings L, Beerendonk CC, Westphal JR,
Massuger LF, Kaal SE, van Leeuwen FE, Braat DD and Peek R:
Autotransplantation of cryopreserved ovarian tissue in cancer
survivors and the risk of reintroducing malignancy: A systematic
review. Hum Reprod Update. 19:483–506. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Laifer S, Buscema J, Parmley TH and
Rosenshein NB: Ovarian cancer metastatic to the breast. Gynecol
Oncol. 24:97–102. 1986.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ayhan A, Guvenal T, Salman MC, Ozyuncu O,
Sakinci M and Basaran M: The role of cytoreductive surgery in
nongenital cancers metastatic to the ovaries. Gynecol Oncol.
98:235–241. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Eitan R, Gemignani ML, Venkatraman ES,
Barakat RR and Abu-Rustum NR: Breast cancer metastatic to abdomen
and pelvis: Role of surgical resection. Gynecol Oncol. 90:397–401.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Moore RG, Chung M, Granai CO, Gajewski W
and Steinhoff MM: Incidence of metastasis to the ovaries from
nongenital tract primary tumors. Gynecol Oncol. 93:87–91.
2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li CI, Anderson BO, Daling JR and Moe RE:
Trends in incidence rates of invasive lobular and ductal breast
carcinoma. JAMA. 289:1421–1424. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Borst MJ and Ingold JA: Metastatic
patterns of invasive lobular versus invasive ductal carcinoma of
the breast. Surgery. 114:637–641; discussion 641-632.
1993.PubMed/NCBI
|
|
27
|
Lamovec J and Bracko M: Metastatic pattern
of infiltrating lobular carcinoma of the breast: An autopsy study.
J Surg Oncol. 48:28–33. 1991.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Curtin JP, Barakat RR and Hoskins WJ:
Ovarian disease in women with breast cancer. Obstet Gynecol.
84:449–452. 1994.PubMed/NCBI
|
|
29
|
Simpkins F, Zahurak M, Armstrong D,
Grumbine F and Bristow R: Ovarian malignancy in breast cancer
patients with an adnexal mass. Obstet Gynecol. 105:507–513.
2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Robson M, Im SA, Senkus E, Xu B, Domchek
SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, et al:
Olaparib for metastatic breast cancer in patients with a germline
BRCA mutation. N Engl J Med. 377:523–533. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Economopoulou P, Dimitriadis G and Psyrri
A: Beyond BRCA: New hereditary breast cancer susceptibility genes.
Cancer Treat Rev. 41:1–8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
NCCN Clinical Practice Guidelines in
Oncology Genetic/Familial High-Risk Assessment; Breast, Ovarian and
Pancreatic. Version 2.2021-November 20,2020.
|