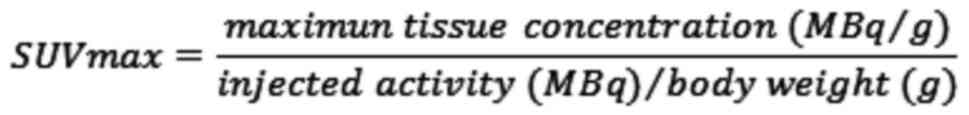Introduction
Esophageal cancer is associated with poor prognosis
and has a 5-year survival rate of 17-34% (1). Therefore, obtaining information on the
expected prognosis is important to ensure that more intensive
treatment can be provided to patients with poor prognoses. For
example, a phase III trial (NEOCRTEC5010) compared the safety and
survival outcomes of surgery alone with those of neoadjuvant
chemoradiotherapy (CRT) followed by surgery. The results showed
improved overall survival (OS) and disease-free survival (DFS) in
patients who underwent the combined treatment (2). Nevertheless, esophagectomy after CRT
has been linked to a high risk of complications and
treatment-related mortality (3,4).
Therefore, the availability of additional information regarding the
prediction of patient prognosis would allow the provision of more
suitable treatments.
The maximum standardized uptake value
(SUVmax) is broadly used for the semiquantitative
measurement of the maximum 18F-2-fluorodeoxyglucose
(18F-FDG) uptake. This value is determined using
positron emission tomography (PET) with computed tomography (CT).
Since the 18F-FDG reflects tumor glucose metabolism, the
SUV is used as a surrogate marker for tumor metabolism (5). Several studies have documented the
value of a PET scan for assessing the prognosis of esophageal, head
and neck, and non-small-cell lung cancer (1,6-8).
It is established that chronic inflammation induces
carcinogenesis and progression of cancer (9). C-reactive protein (CRP) belongs to a
family of acute-phase proteins whose plasma concentrations increase
in response to inflammation. Following the occurrence of
inflammation in tissue cells, CRP is secreted from the liver into
the blood (10). Notably, it is
upregulated by pro-inflammatory cytokines, such as interleukin-6
(IL-6), IL-8, and tumor necrosis factor-α (TNF-α) (11). Several studies have shown that the
elevation of pre-treatment CRP levels is a significant prognostic
indicator in patients with esophageal cancer and tends to correlate
with TNM staging (12,13). Jurisic et al (14) have shown that TNF-α is also
increased in certain types of tumors. Although other markers, such
as IL-1β, IL-6, IL-8, IL-10, and monocyte chemoattractant protein-1
(MCP-1), are used in the diagnosis of cancer (15), CRP appears to be a rapid, simple,
and cost-effective predictor in clinical practice.
Moreover, Chen et al (16) revealed that elevated levels of CRP
are associated with a high metabolic rate and proliferative
activity (measured according to the SUVmax) in head and
neck carcinoma.
In this study, we aimed to retrospectively evaluate
the prognostic values of pre-treatment SUVmax of
18F-FDG-PET and CRP in radiotherapy (RT) of esophageal
cancer.
Materials and methods
Study participants
We retrospectively (March 2013-December 2016)
researched patients with esophageal squamous cell cancer, which was
detected with CT scans at the University of Tokyo Hospital. The
clinical TNM stage was determined according to the 7th edition of
the American Joint Committee on Cancer staging in esophageal
cancer. All patients underwent RT with or without chemotherapy.
Treatment
RT was performed using 6-10 MV photon linear
accelerators at doses of 50-60 Gy. The irradiation method was
either three-dimensional conformal RT or intensity-modulated RT.
The gross tumor volume was defined based on the results of the CT
scan, endoscopy, and PET scan, if available. The treatment fields
encompassed the tumor bed with 3-5 cm proximal and distal margins
and 2 cm lateral margins. Involved-field RT (IFRT) was
conducted.
Measurement of CRP
Serum CRP levels were measured in peripheral venous
blood samples using a latex turbidimetric immunoassay on day 1 of
RT.
Measurement of SUVmax
PET-CT was conducted within 2 weeks prior to the
initiation of RT using Aquiduo PCA-7000B (Toshiba Medical Systems
Corp.). This system consists of a 16-detector row CT scanner and a
lutetium oxyorthosilicate-based PET scanner. Patients, fasted for
≥5 h, received 4.5 MBq/kg (minimum: 180 MBq; maximum: 405 MBq) of
18F-FDG. Data were acquired 60 min after injection. The
PET images were captured in the three-dimensional acquisition mode
at eight bed positions from the knee to the skull. Transmission
imaging was performed using CT (120 kV, 50 mA, 0.5 sec, helical
scan) with an axial field of view of 50 cm and matrix size of
512x512. The CT images were reconstructed using true cone-beam
tomography. The PET images were iteratively reconstructed using
Fourier rebinning and ordered subset expectation maximization for
14 subsets and four iterations.
Regions of interests, representing the areas in the
lesions showing the highest accumulation of 18F-FDG,
were drawn on the fused PET/CT image. The SUVmax was
measured in the regions of interest. The SUVmax was
calculated using the following formula:
Statistical analysis
OS and DFS were set as clinical outcomes.
Statistical analysis was performed using the EZR version 1.38
software (Saitama Medical Center, Jichi Medical University,
Saitama, Japan). The χ2 test and Fisher's exact
probability test were used to compare data between the two groups.
The Receiver Operating Characteristic was employed to determine the
positive predictive value (PPV) and negative predictive lavue (NPV)
in patients who had OS event. Univariate analysis was conducted
using the Kaplan-Meier method. The statistical significance of
differences between survival curves was examined using the log-rank
test. Multivariate analysis was performed using the Cox
proportional hazards regression model. Carriable selection of
step-wise method with Bayesian information criterion was conducted.
Univariate and multivariate analyses were considered significant at
P<0.05.
Results
Patient characteristics
A total of 69 consecutive patients were included in
this analysis. Patient characteristics are shown in Table I. The median age of patients was 65
years (range: 44-95 years); 53 and 16 patients were males (76.8%)
and females (23.2%), respectively. Concurrent chemotherapy
consisted mainly of nedaplatin (NDP) and tegafur/gimeracil/oteracil
(TS-1); another regimen included cisplatin (CDDP) and
5-fluorouracil (5-FU). Two patients (2.9%) underwent only RT. A
total of 62 patients (89.8%) received radiation doses of 50.4 Gy/28
Fr or 50 Gy/25 Fr, and seven patients (10.1%) received doses of 60
Gy/30 Fr. The majority of patients (N=58; 84%) had clinical T stage
3 or 4 disease. The most common primary site was the middle
thoracic esophagus in 39 patients (56.5%).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Value |
|---|
| Age, years | |
|
Range | 44-95 |
|
Median | 65 |
|
<75, n
(%) | 58 (84.1) |
|
≥75, n
(%) | 11 (15.9) |
| Sex, n (%) | |
|
Male | 53 (76.8) |
|
Female | 16 (23.2) |
| Chemotherapy, n
(%) | |
|
NDP/TS1 | 62 (89.8) |
|
CDDP/5-FU | 5 (7.2) |
|
None | 2 (2.9) |
| RT dose, n (%) | |
|
60 Gy | 7 (10.1) |
|
50.4 Gy | 53 (76.8) |
|
50 Gy | 9 (13.0) |
| cStage, n (%) | |
|
I | 7 (10.1) |
|
II | 4 (5.8) |
|
III | 41 (59.4) |
|
IV | 17 (24.6) |
| Primary site, n
(%) | |
|
Ce | 10 (14.5) |
|
Ut | 9 (13.0) |
|
Mt | 39 (56.5) |
|
Lt | 8 (11.6) |
|
EGJ | 3 (4.3) |
According to the χ2 test and Fisher's
exact probability test, patients with advanced T stage showed
higher CRP (P=0.03) and SUVmax (P<0.01) (Table II).
 | Table IIχ2 test and Fisher's exact
probability test. |
Table II
χ2 test and Fisher's exact
probability test.
| Variable | CRP ≥1 mg/dl,
n | CRP <1 mg/dl,
n | P-value |
|---|
| Age, years | | | 0.28a |
|
<75 | 15 | 43 | |
|
≥75 | 5 | 6 | |
| Sex | | | 0.39b |
|
Male | 14 | 39 | |
|
Female | 6 | 10 | |
| Stage | | | 0.03a |
|
I-II | 0 | 11 | |
|
III-IV | 20 | 38 | |
| Primary site | | | 0.77b |
|
Ce-Ut | 6 | 13 | |
|
Mt-EGJ | 14 | 36 | |
Cut-off levels
Data on the pre-therapeutic SUVmax were
available in 56 patients (81.2%). The median SUVmax was
12.85 (0-31). Therefore, the patients were divided into two groups:
SUVmax >12.85 (28 patients, 50%) and
SUVmax ≤12.85 (28 patients, 50%). Data on the
pre-therapeutic levels of CRP were available for all patients. The
median CRP was 0.18 mg/dl (<0.02-31 mg/dl). According to the ROC
curve (Fig. 1), 0.790 mg/dl would
be suitable cut-off with sensitivity of 67%, specificity of 78%,
PPV of 52% and NPV of 87%. But we classified patients into two
groups: CRP ≥1 mg/dl (20 patients, 29%) and CRP <1 mg/dl (49
patients, 71%). The reason we chose CRP level of 1 mg/dl as cut-off
level of CRP, which is close to 0.790 mg/dl, is to make it easier
for clinical use.
Survival
The median follow-up for censored cases was 45.7
months (3.1-68.9 months). The 2-year OS and DFS rates for all
enrolled patients were 61.6% [95% confidence interval (CI):
48.6-72.3%] and 49.5% (95% CI: 37.1-60.8%), respectively. According
to the comparison of the Kaplan-Meier curves using a log-rank test
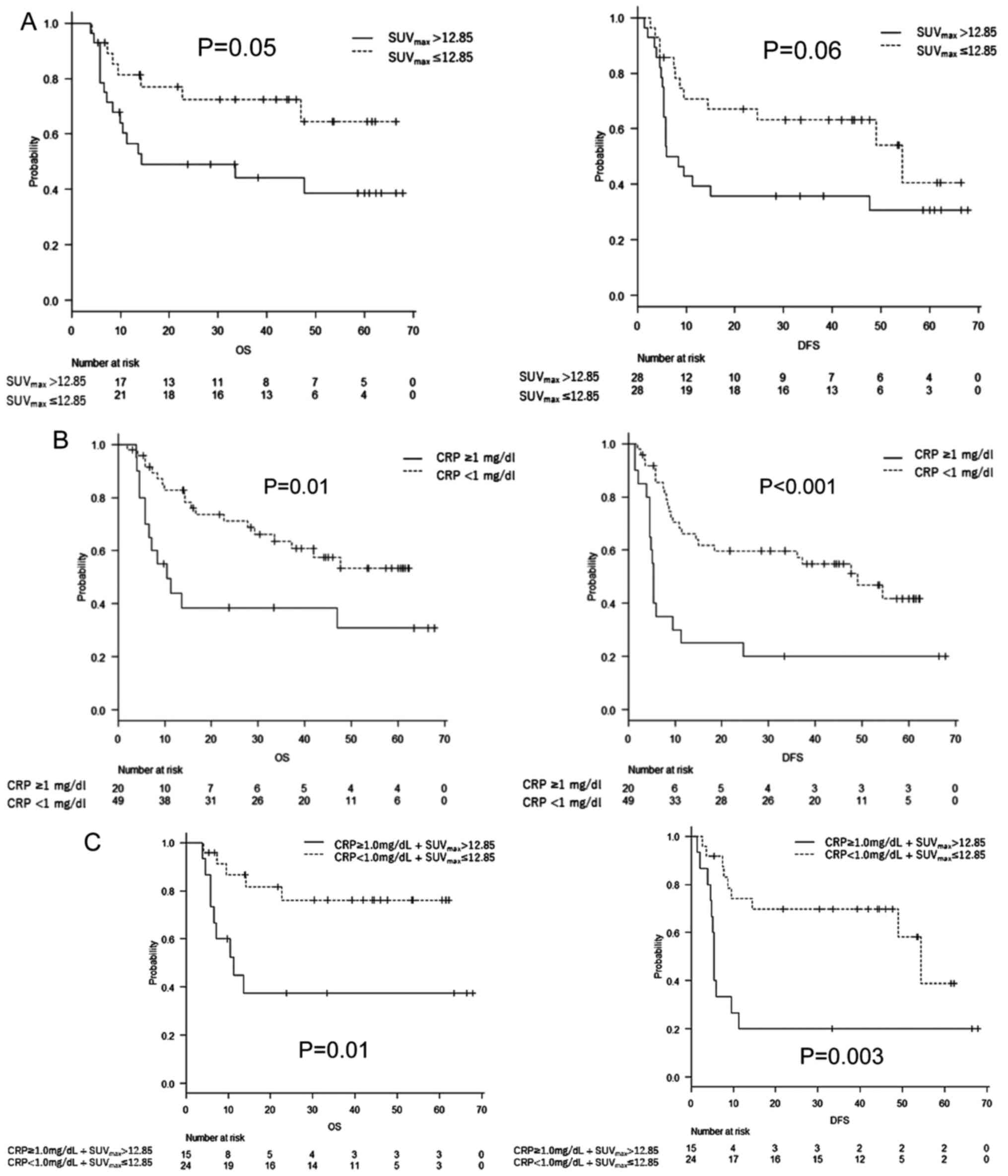
(P=0.05), the OS was significantly worse in the SUVmax
>12.85 group than in the SUVmax ≤12.85 group, with
median survivals of not applicable versus 14.3 months,
respectively. The DFS was worse in the elevated SUVmax
group; however, the difference was not statistically significant
(P=0.06) (Fig. 2A). The OS and DFS
were significantly worse in the CRP ≥1 mg/dl group than in the CRP
<1 mg/dl group (P=0.01 and P<0.001, respectively) (Fig. 2B). Similarly, the OS and DFS were
significantly worse in the elevated CRP and SUVmax
groups (P=0.01 and 0.003, respectively) (Fig. 2C).
Correlation between CRP
levels/SUVmax levels and survival
In a univariate analysis, age, CRP,
SUVmax, and CRP+SUVmax were prognostic
factors for OS (Table III). Age,
CRP, and CRP+SUVmax were also prognostic factors for DFS
(Table IV). A multivariate
analysis revealed that the pre-treatment serum CRP levels remained
an independent prognostic factor for both OS and DFS (Table V). CRP levels also remained in the
final model employing carriable selection of step-wise method with
Bayesian information criterion for both OS and DFS (OS: Hazard
ratio [HR]; 0.25, 95% CI; 0.08-0.76, P=0.01, DFS: HR; 0.28, 95% CI;
0.12-0.69, P<0.01).
 | Table IIIUnivariate analysis of OS. |
Table III
Univariate analysis of OS.
| Variable | 2-year OS, % (95%
CI) | P-value
(log-lank) |
|---|
| Age, years | | <0.001 |
|
<75 | 69.3
(55.3-79.7) | |
|
≥75 | 20.0
(3.1-47.5) | |
| CRP, mg/dl | | 0.013 |
|
<1 | 71.2
(55.4-82.2) | |
|
≥1 | 38.5
(17.7-59.0) | |
|
SUVmax | | 0.048 |
|
≤12.85 | 72.4
(50.5-85.9) | |
|
>12.85 | 49.0
(29.5-65.9) | |
|
CRP/SUVmax | | 0.008 |
|
CRP <1
mg/dl/SUVmax ≤12.85 | 76.2
(51.6-89.4) | |
|
CRP ≥1
mg/dl/SUVmax >12.85 | 37.5
(14.1-61.2) | |
| Stage | | 0.298 |
|
I-II | 67.5
(29.1-88.2) | |
|
III-IV | 45.1
(32.1-57.3) | |
| Primary site | | 0.579 |
|
Ce-Ut | 62.6
(36.3-79.8) | |
|
Mt-EGJ | 61.6
(46.1-73.9) | |
 | Table IVUnivariate analysis of DFS. |
Table IV
Univariate analysis of DFS.
| Variable | 2-year DFS, % (95%
CI) | P-value
(log-lank) |
|---|
| Age, years | | 0.019 |
|
<75 | 54.6
(40.9-66.4) | |
|
≥75
years | 20.5
(3.2-48.2) | |
| CRP, mg/dl | | <0.001 |
|
<1 | 59.7
(44.4-72.1) | |
|
≥1 | 25.0
(9.1-44.9) | |
|
SUVmax | | 0.057 |
|
≤12.85 | 67.1
(46.2-81.3) | |
|
>12.85 | 35.7
(18.9-53.0) | |
|
CRP/SUVmax | | 0.003 |
|
CRP <1
mg/dl/SUVmax ≤12.85 | 69.8
(46.9-84.3) | |
|
CRP ≥1
mg/dl/SUVmax >12.85 | 20.0
(4.9-42.4) | |
| Stage | | 0.186 |
|
I-II | 65.6
(26.0-87.6) | |
|
III-IV | 45.1
(32.1-57.3) | |
| Primary site | | 0.365 |
|
Ce-Ut | 57.9
(33.2-76.3) | |
|
Mt-EGJ | 46.2
(31.8-59.5) | |
 | Table VMultivariate analysis. |
Table V
Multivariate analysis.
| | OS | DFS |
|---|
| Cox proportional
hazards model | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<75 vs. ≥75
years) | 0.24 | 0.08-0.69 | 0.01 | 0.33 | 0.12-0.93 | 0.04 |
| CRP (<1 vs. ≥1
mg/dl) | 0.54 | 0.21-1.37 | 0.20 | 0.43 | 0.19-0.96 | 0.04 |
| SUVmax
(≤12.85 vs. >12.85) | 0.50 | 0.18-1.39 | 0.18 | 0.64 | 0.27-1.51 | 0.31 |
| cStage (I-II vs.
III-IV) | 1.15 | 0.22-5.94 | 0.87 | 0.95 | 0.24-3.68 | 0.94 |
Discussion
In this study, a univariate analysis revealed that
pre-treatment CRP is a prognostic factor for OS/DFS, while
SUVmax is a prognostic factor for OS.
The CRP may be a prognostic factor due to following
mechanism. IL-6 is thought to correlate with CRP (11), and it has been confirmed that an
IL-6 signaling pathway stimulates cancer progression through the
IL-6 receptor on the surface of prostate cancer cells (17). It is possible that a similar process
occurs in esophageal cancer. However, further investigation is
warranted to confirm this hypothesis. It has been demonstrated that
the SUVmax correlates with tumor aggressiveness in
patients with head and neck cancer (18). This may explain its prognostic value
in esophageal cancer.
Although a few studies have shown a correlation
between the serum CRP levels/SUVmax and OS/DFS in
esophageal cancer, esophagectomy was performed in most of them
(Table VI) (6,12,13,19,20).
This is one of a few studies showing the prognostic value of the
pre-treatment SUVmax of 18F-FDG-PET and serum
CRP levels in RT of esophageal cancer.
 | Table VIPrevious studies showing the
association between serum CRP levels/SUVmax and overall
survival/disease-free survival in esophageal cancer. |
Table VI
Previous studies showing the
association between serum CRP levels/SUVmax and overall
survival/disease-free survival in esophageal cancer.
| A,
SUVmax |
|---|
| | MST, months | |
|---|
| First author,
year | No. of
patients | Treatment
modality | Threshold | High | Low | P-value | (Refs.) |
|---|
| Van Westreenen
et al, 2005 | 40 | Esophagectomy or
BSC | 6.7 | 8.7 | 20.4 | 0.016 | (19) |
| Shum et al,
2012 | 26 | Esophagectomy with
or without RT | 16 mla | 15 | NA | 0.018 | (20) |
| Brown et al,
2012 | 46 | Esophagectomy | 5.5 | 14 | 39 | 0.72 | (6) |
| Present study | 56 | Radiotherapy with
or without chemotherapy | 12.85 | 14 | NA | 0.048 | - |
| B, CRP |
| | MST, months | |
| First author,
year | No. of
patients | Treatment
modality | Threshold | High | Low | P-value | (Refs.) |
| Wang et al,
2009 | 123 | CRT with or without
esophagectomy | 5
mg/dlb | 11 | NA | <0.001 | (12) |
| Huang et al,
2019 | 552 | Esophagectomy | 5
mg/dlb | 40 | NA | 0.044 | (13) |
| Present study | 69 | Radiotherapy with
or without chemotherapy | 1 mg/dl | 10 | NA | 0.013 | - |
Accurate prediction of prognosis before treatment
would permit the provision of more intensive care. This would
include the addition of more cycles of chemotherapy as adjuvant
treatment, use of more intensive concurrent chemotherapy regimens
(e.g., docetaxel/CDDP/5-FU [DCF]), more careful observation after
CRT (e.g., monthly endoscopy or CT), and consideration of salvage
esophagectomy.
Higuchi et al (21) reported high effectiveness of
concurrent CRT using DCF (DCF-R) in a phase II study. This study
showed a favorable response, with a clinical response rate of 52.4%
(37.3-67.5%) and a partial response rate of 33.3%. The
investigators concluded that DCF-R frequently caused
myelosuppression and esophagitis. However, it was highly
efficacious and suggested to be a promising regimen in the
treatment of advanced esophageal cancer. Another retrospective
study revealed improved OS and complete response in a DCF-R group
compared with a CDDP/5-FU-R group (22). The researchers also reported that
the incidence of grade 3/4 leukopenia was significantly higher in
the DCF-R group. Notably, there were no significant intergroup
differences in neutropenia, anemia, thrombocytopenia,
radiation-induced dermatitis, radiation esophagitis, or late
adverse events.
Based on the study conducted by Yamashita et
al (23), we used NDP/S-1 in
combination with RT in 89.8% of patients. In that study, the
investigators reported that a complete response was achieved in 85%
of patients who received CRT with NDP/S-1. The 3-year OS rate in
those who received definitive CRT or salvage CRT was 54.4 and
39.8%, respectively; 70% received treatment as outpatients.
In this study, we conducted IFRT based on PET. A
phase II study reported that, of 63 patients who were treated with
IFRT based on PET, only two patients experienced out-of-field
loco-regional nodal recurrence (24). The same investigators have
retrospectively reported that tendencies toward improved
loco-regional progression-free survival and a significantly
increased OS rate favored the IFRT arm over the elective nodal
irradiation arm (25).
In the Radiation Therapy Oncology Group 9405 study,
Minsky et al (26) revealed
that high-dose RT does not improve local/regional control or
survival. Furthermore, other studies have shown that doses >55
or >60 Gy are associated with higher rates of morbidity after
salvage surgery (27,28). Therefore, we suggest that 60 Gy is
not necessary even in patients with high CRP/SUVmax.
Regarding the cut-off levels of SUVmax,
Huang et al (1) used ROC
analysis, Shum et al (20)
used MTV 2.5 and MTV 20% (volume higher than a fixed threshold of
20% of the maximum intra-tumoral activity), and Van Westreenen
et al (19) used the median
SUVmax. We performed an ROC analysis; we found that 10.4
would be the most appropriate threshold, and the area under the
curve was 0.64 (95% CI: 0.49-0.8). On the other hand, the median
SUVmax was 12.85. We thought that the median
SUVmax would be more reliable, and decided to use a
SUVmax of 12.85 as the threshold.
Concerning the cut-off levels of CRP, although this
study used a latex turbidimetric immunoassay, most published
studies have used the immunonephelometry method. The median CRP was
0.18 mg/dl, which did not seem to have any clinical significance.
Hence, we decided to use the ROC curve. Since survival in the
present study was comparable to that reported in other published
studies, we think that the thresholds used in this study are
reasonable.
The clinical stage did not remain significant in
either the univariate or multivariate analysis. This may be
attributed to the inclusion of only CT-visible tumors; patients
with disease at an earlier stage may also have poor prognoses.
There were a few limitations in this study. Firstly,
we included only CT-visible tumors. Otherwise, the
SUVmax would not be precise, and we would have been
unable to distinguish whether the levels of CRP were elevated
because of the tumor or other infectious causes. Thus, it is
uncertain whether these prognostic factors are meaningful in
CT-invisible tumors. Furthermore, the CT-visible tumors evaluated
in this study included primary sites and metastatic lymph nodes.
However, we are unsure whether primary sites and metastatic lymph
nodes share similar characteristics. We did not search for adverse
effects since we aimed to determine the correlation between
SUVmax/CRP and survival. Other limitations of this study
are the various types of prescribed doses (we mainly prescribed 60
Gy before 2014); various types of adjuvant chemotherapy (we are
currently planning to compare CDDP/5-FU versus NDP/TS-1); the
retrospective design; and limited number of patients included.
In conclusion, prognostic prediction based on
pre-treatment SUVmax of 18F-FDG-PET and serum
CRP levels is possible in RT of esophageal cancer. It is important
to consider the provision of more intensive treatment to patients
with poor prognoses for better treatment outcome.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJ designed the study, analyzed data and wrote the
initial draft of the manuscript. TK, YM and AK recruited the
patients and collected their clinical data. HY also made
substantial contributions to conception of the study and revised
the manuscript critically for important intellectual content. KN
and OA made substantial contributions to analysis and
interpretation of data, were involved in revising the manuscript
critically and gave final approval of the version to be published.
All the raw data have been assessed by HJ and HY to ensure their
legitimacy. All authors read and approved the final version of the
manuscript, and agreed to be accountable for all aspects of the
work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Written informed consent was provided by all
individuals included in the study at the time of initial data
collection. The study was approved by the Institutional Review
Board of the University of Tokyo Hospital (Tokyo, Japan) and
performed in accordance with the ethical guidelines of the
institution and Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang YC, Lu HI, Huang SC, Hsu CC, Chiu
NT, Wang YM, Chiu YC and Li SH: FDG PET using SUVmax for
preoperative T-staging of esophageal squamous cell carcinoma with
and without neoadjuvant chemoradiotherapy. BMC Med Imaging.
17(1)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu
Z, Mao W, Xiang J, Han Y, Chen Z, et al: Neoadjuvant
chemoradiotherapy followed by surgery versus surgery alone for
locally advanced squamous cell carcinoma of the esophagus
(NEOCRTEC5010): A phase III multicenter, randomized, open-label
clinical trial. J Clin Oncol. 36:2796–2803. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sewnaik A, Keereweer S, Al-Mamgani A,
Baatenburg De Jong RJ, Wieringa MH, Meeuwis CA and Kerrebijn JDF:
High complication risk of salvage surgery after chemoradiation
failures. Acta Otolaryngol. 132:96–100. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vellayappan BA, Soon YY, Ku GY, Leong CN,
Lu JJ and Tey JC: Chemoradiotherapy versus chemoradiotherapy plus
surgery for esophageal cancer. Cochrane Database Syst Rev.
8(CD010511)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Paidpally V, Chirindel A, Lam S, Agrawal
N, Quon H and Subramaniam RM: FDG-PET/CT imaging biomarkers in head
and neck squamous cell carcinoma. Imaging Med. 4:633–647.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brown C, Howes B, Jamieson GG,
Bartholomeusz D, Zingg U, Sullivan TR and Thompson SK: Accuracy of
PET-CT in predicting survival in patients with esophageal cancer.
World J Surg. 36:1089–1095. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Suzuki H, Kato K, Nishio M, Tamaki T,
Fujimoto Y, Hiramatsu M, Hanai N, Kodaira T, Itoh Y, Naganawa S, et
al: FDG-PET/CT predicts survival and lung metastasis of
hypopharyngeal cancer in a multi-institutional retrospective study.
Ann Nucl Med. 31:514–520. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dong M, Liu J, Sun X and Xing L:
Prognositc significance of SUVmax on pretreatment
18F-FDG PET/CT in early-stage non-small cell lung cancer
treated with stereotactic body radiotherapy: A meta-analysis. J Med
Imaging Radiat Oncol. 61:652–659. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pepys MB and Hirschfield GM: C-reactive
protein: A critical update. J Clin Invest. 111:1805–1812.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Erlinger TP, Platz EA, Rifai N and
Helzlsouer KJ: C-reactive protein and the risk of incident
colorectal cancer. JAMA. 291:585–590. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang CY, Hsieh MJ, Chiu YC, Li SH, Huang
HW, Fang FM and Huang YJ: Higher serum C-reactive protein
concentration and hypoalbuminemia are poor prognostic indicators in
patients with esophageal cancer undergoing radiotherapy. Radiother
Oncol. 92:270–275. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Huang W, Wu L, Liu X, Long H, Rong T and
Ma G: Preoperative serum C-reactive protein levels and
postoperative survival in patients with esophageal squamous cell
carcinoma: A propensity score matching analysis. J Cardiothorac
Surg. 14(167)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jurisic V, Terzic T, Colic S and Jurisic
M: The concentration of TNF-alpha correlate with number of
inflammatory cells and degree of vascularization in radicular
cysts. Oral Dis. 14:600–605. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen HH, Wang HM, Fan KH, Lin CY, Yen TC,
Liao CT, Chen IH, Kang CJ and Huang SF: Pre-treatment levels of
C-reactive protein and squamous cell carcinoma antigen for
predicting the aggressiveness of pharyngolaryngeal carcinoma. PLoS
One. 8(e55327)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scheller J, Chalaris A, Schmidt-Arras D
and Rose-John S: The pro- and anti-inflammatory properties of the
cytokine interleukin-6. Biochim Biophys Acta. 1813:878–888.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liao CT, Wang HM, Chang JT, Lin CY, Ng SH,
Huang SF, Chen IH, Hsueh C, Lee LY, Lin CH, et al: Influence of
pathological nodal status and maximal standardized uptake value of
the primary tumor and regional lymph nodes on treatment plans in
patients with advanced oral cavity squamous cell carcinoma. Int J
Radiat Oncol Biol Phys. 77:421–429. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Van Westreenen HL, Plukker JT, Cobben DC,
Verhoogt CJ, Groen H and Jager PL: Prognostic value of the
standardized uptake value in esophageal cancer. Am J Roentgenol.
185:436–440. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shum WY, Ding HJ, Liang JA, Yen KY, Chen
SW and Kao CH: Use of pretreatment metabolic tumor volumes on
PET-CT to predict the survival of patients with squamous cell
carcinoma of esophagus treated by curative surgery. Anticancer Res.
32:4163–4168. 2012.PubMed/NCBI
|
|
21
|
Higuchi K, Komori S, Tanabe S, Katada C,
Azuma M, Ishiyama H, Sasaki T, Ishido K, Katada N, Hayakawa K, et
al: Definitive chemoradiation therapy with docetaxel, cisplatin,
and 5-fluorouracil (DCF-R) in advanced esophageal cancer: A phase 2
trial (KDOG 0501-P2). Int J Radiat Oncol Biol Phys. 89:872–879.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tamaki Y, Hieda Y, Nakajima M, Kitajima K,
Yoshida R, Yoshizako T, Ue A, Tokudo M, Hirahara N, Moriyama I, et
al: Concurrent chemoradiotherapy with docetaxel, cisplatin, and
5-fluorouracil improves survival of patients with advanced
esophageal cancer compared with conventional concurrent
chemoradiotherapy with cisplatin and 5-fluorouracil. J Cancer.
9:2765–2772. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yamashita H, Haga A, Takenaka R, Kiritoshi
T, Okuma K, Ohtomo K and Nakagawa K: Efficacy and feasibility of
ambulatory treatment-based monthly nedaplatin plus S-1 in
definitive or salvage concurrent chemoradiotherapy for early,
advanced, and relapsed esophageal cancer. Radiat Oncol.
11(4)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yamashita H, Omori M, Takenaka R, Okuma K,
Kobayashi R, Ohtomo K and Nakagawa K: Involved-field irradiation
concurrently combined with nedaplatin/5-fluorouracil for inoperable
esophageal cancer on basis of 18FDG-PET scans: A phase II study.
Radiother Oncol. 113:182–187. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yamashita H, Takenaka R, Omori M, Imae T,
Okuma K, Ohtomo K and Nakagawa K: Involved-field radiotherapy
(IFRT) versus elective nodal irradiation (ENI) in combination with
concurrent chemotherapy for 239 esophageal cancers: A single
institutional retrospective study. Radiat Oncol.
10(171)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky
TM, Martenson J, Komaki R, Okawara G, Rosenthal SA and Kelsen DP:
INT 0123 (radiation therapy oncology group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: High-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cohen C, Tessier W, Gronnier C, Renaud F,
Pasquer A, Théreaux J, Gagnière J, Meunier B, Collet D, Piessen G,
et al: Salvage surgery for esophageal cancer: How to improve
outcomes? Ann Surg Oncol. 25:1277–1286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sugimura K, Miyata H, Shinno N, Ushigome
H, Asukai K, Hara H, Hasegawa S, Yamada D, Yamamoto K, Haraguchi N,
et al: Prognostic impact of postoperative complications following
salvage esophagectomy for esophageal cancer after definitive
chemoradiotherapy. Oncology. 98:280–288. 2020.PubMed/NCBI View Article : Google Scholar
|