Introduction
Liver cancer is the fifth most common cancer and
second most common cause of cancer-related fatalities worldwide
(1). Hepatocellular carcinoma (HCC)
accounts for approximately 90% of primary liver cancers. HCC has
one of the worst prognoses among cancers due to late diagnosis,
resistance to chemotherapy, tumor recurrence, and metastasis
(2). In HCC, vascular invasion or
extrahepatic metastasis is considered as an advanced disease stage
and is classified as stage C in the Barcelona Clinic Liver Cancer
(BCLC) staging system (3). Although
the expected median survival of patients with advanced HCC is 7-9
months (4), the effectiveness of
molecular targeted agents (MTAs), including sorafenib, lenvatinib,
regorafenib, cabozantinib, and ramucirumab for treatment of
advanced HCC has been reported, and these MTAs are in clinical use
worldwide (1). The number of drugs
for the treatment of advanced HCC is expected to increase in the
future, and application of transcatheter arterial chemoembolization
(TACE), a crucial treatment approach currently, is expected to
decrease. However, TACE might be more effective in certain
situations, for example, in cases of large HCC where MTAs cannot
produce an immediate effect, or in cases of intrahepatic tumors
resistant to multiple MTAs. Thus, it is important to formulate a
sequential therapeutic strategy involving MTAs and TACE for
advanced HCC based on the pathological condition of the
patient.
Here, we present a case of a patient with advanced
HCC who had a large intrahepatic tumor along with extrahepatic
metastases. Sequential therapy with MTAs was effective after the
control of the large intrahepatic tumor through drug-eluting beads
(DEB)-TACE. Lenvatinib was effective for the extrahepatic
metastases including those in the lungs and spleen.
Conventional-TACE (C-TACE) was performed on demand for
lenvatinib-resistant intrahepatic radical tumors. This strategy
prolonged the long-term survival of the patient. Here, we discuss
the effectiveness of a multimodal treatment strategy including MTAs
and TACE for long-term survival in advanced HCC.
Case report
The patient was a 57-year-old Japanese woman with a
history of hepatitis C virus infection. Thirteen years prior to
this study, a sustained biological response was reported in her
after successful treatment with peginterferon alfa-2a. Although she
showed no disease symptoms, a large mass was noted in the right
lobe of the liver on abdominal ultrasonography performed at our
hospital for screening. Contrast-enhanced computed tomography
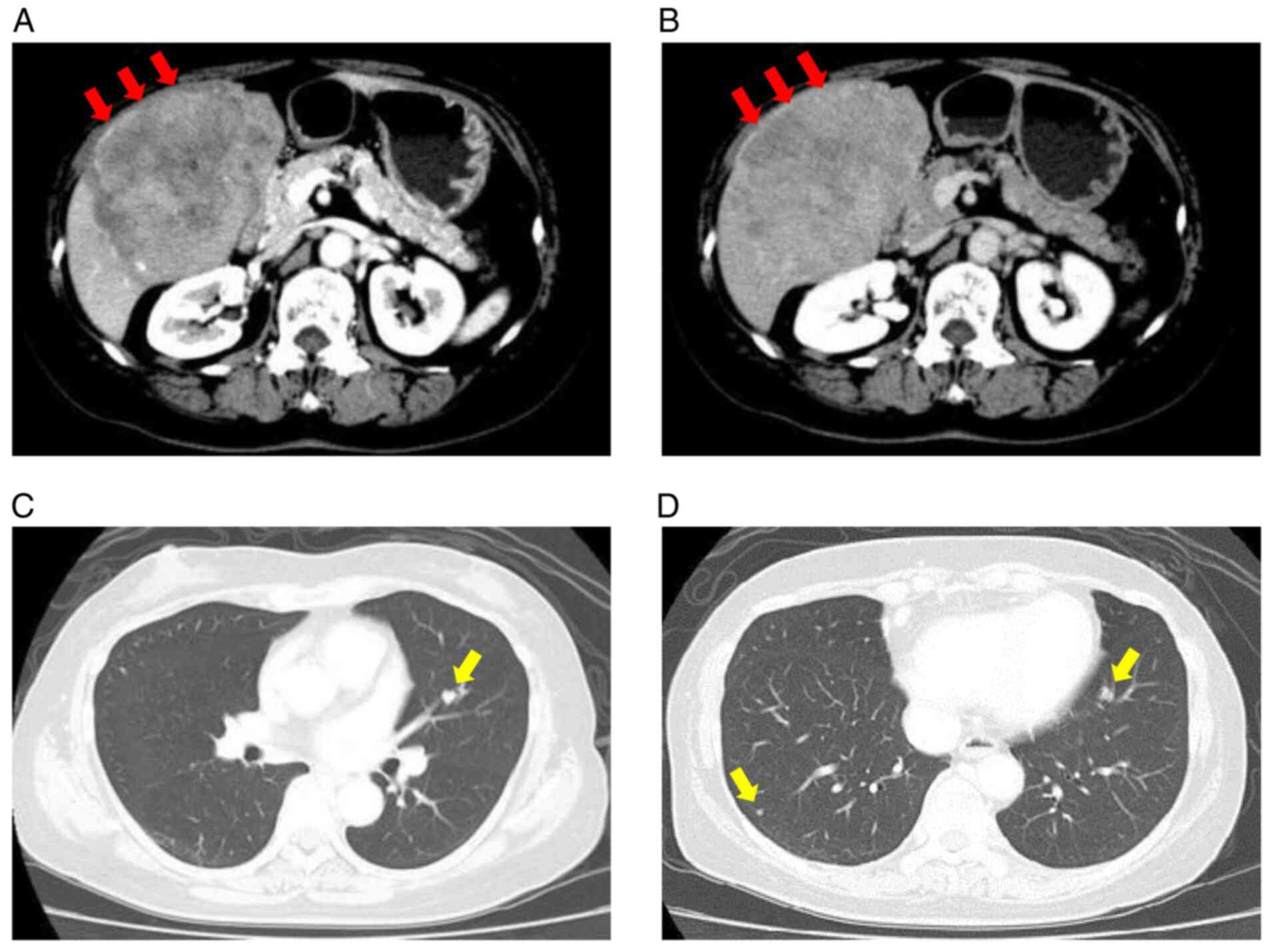
(CE-CT) examination revealed a tumor that was 140 mm in diameter
with an uneven high density in the early arterial phase (Fig. 1A). The tumor had a washout
appearance in the delayed phase, mainly in the posterior segment
(Fig. 1B). In addition, eight
metastatic lesions up to 10 mm in diameter were detected in both
lungs (Fig. 1C and D). The liver showed no signs of cirrhosis.
The level of the tumor marker des-γ-carboxy prothrombin (DCP) was
63,589 mAU/ml, which was considered to be remarkably high, whereas
that of α-fetoprotein (AFP) was 9 ng/ml, which was within the
prescribed range. The clinical diagnosis was stage IVb
hepatocellular carcinoma according to the Tumor-Nodule-Metastasis
classification based on the criteria of the Liver Cancer Study
Group of Japan (5). Laboratory data
showed no significant abnormalities (Table I). The Child-Pugh score was 5, which
indicated that liver function was well maintained, and the Eastern
Cooperative Oncology Group performance status (ECOG-PS) score of
the patient was 0. The carcinoma was classified as advanced stage C
with extrahepatic metastasis according to the BCLC criteria, and
the guidelines recommended systemic therapy, such as with MTAs, as
the initial treatment. As the large primary tumor in the posterior
segment of the liver was progressing rapidly and small lung
metastases were observed, TACE was planned to suppress the growth
of the large primary tumor.
 | Table ILaboratory data at the time of
diagnosis and at the start of lenvatinib treatment. |
Table I
Laboratory data at the time of
diagnosis and at the start of lenvatinib treatment.
| Laboratory data | Value at
diagnosis | Value at start of
lenvatinib treatment |
|---|
| Biochemistry | | |
|
Total
protein, g/dl | 7.2 | 6.8 |
|
Albumin,
g/dl | 4.7 | 4.0 |
|
Total
bilirubin, mg/dl | 0.4 | 0.5 |
|
Direct
bilirubin, mg/dl | 0.1 | 0.2 |
|
AST,
U/l | 28 | 21 |
|
ALT,
U/l | 21 | 18 |
|
LDH,
U/l | 220 | 282 |
|
ALP,
U/l | 238 | 188 |
|
GGTP,
U/l | 91 | 31 |
|
BUN,
mg/dl | 14.8 | 15.3 |
|
Creatinine,
mg/dl | 0.58 | 0.64 |
|
Sodium,
mmol/l | 143 | 142 |
|
Potassium,
mmol/l | 3.6 | 4.3 |
|
Chloride,
mEq/l | 108 | 108 |
|
Cholinesterase,
U/l | 347 | 339 |
|
CRP,
mg/dl | 0.05 | 0.42 |
| Coagulation | | |
|
PT, % | 147 | 109 |
|
APTT,
sec | 23.8 | 27.6 |
|
Fibrinogen,
mg/dl | 286 | |
|
Antithrombin
III, % | 111 | |
|
D-dimer,
µg/ml | 2.1 | |
|
FDP,
µg/ml | 5.9 | |
| Hematology | | |
|
White blood
cells, /µl | 4,120 | 4,400 |
|
Red blood
cells, x104/µl | 410 | 344 |
|
Hemoglobin,
g/dl | 12.5 | 10.9 |
|
Platelet
count, x104/µl | 18.6 | 16.0 |
| Endocrinology | | |
|
TSH,
µU/ml | 3.240 | 2.560 |
|
Free T3,
ng/dl | 2.96 | 2.45 |
|
Free T4,
pg/ml | 1.24 | 1.12 |
| Fibrosis
markers | | |
|
Hyaluronic
acid, ng/ml | 71.9 | |
|
Type IV
collagen, ng/ml | 4.1 | |
| Tumor markers | | |
|
AFP,
ng/ml | 9 | 3 |
|
AFP-L3,
% | 0.5 | 0.5 |
|
DCP,
mAU/ml | 68,036 | 873 |
| Hepatic virus | | |
|
HBs
antigen | (-) | |
|
HBc
antibody | (-) | |
|
HCV
antibody | (-) | |
|
HCV-RNA | Undetected | |
First, DEB-TACE with 82 mg epirubicin was performed
for the large primary tumor in the liver. After 6 weeks, C-TACE
with 62 mg cisplatin was additionally performed for the surrounding
residual lesion. Following the two sessions of TACE, DCP levels
were almost normalized and CE-CT revealed that most of the treated
intrahepatic tumors were necrotic (Fig.
2A). Considering that growth suppression of the primary
intrahepatic tumors was achieved, treatment with MTA was planned as
a systemic therapy.
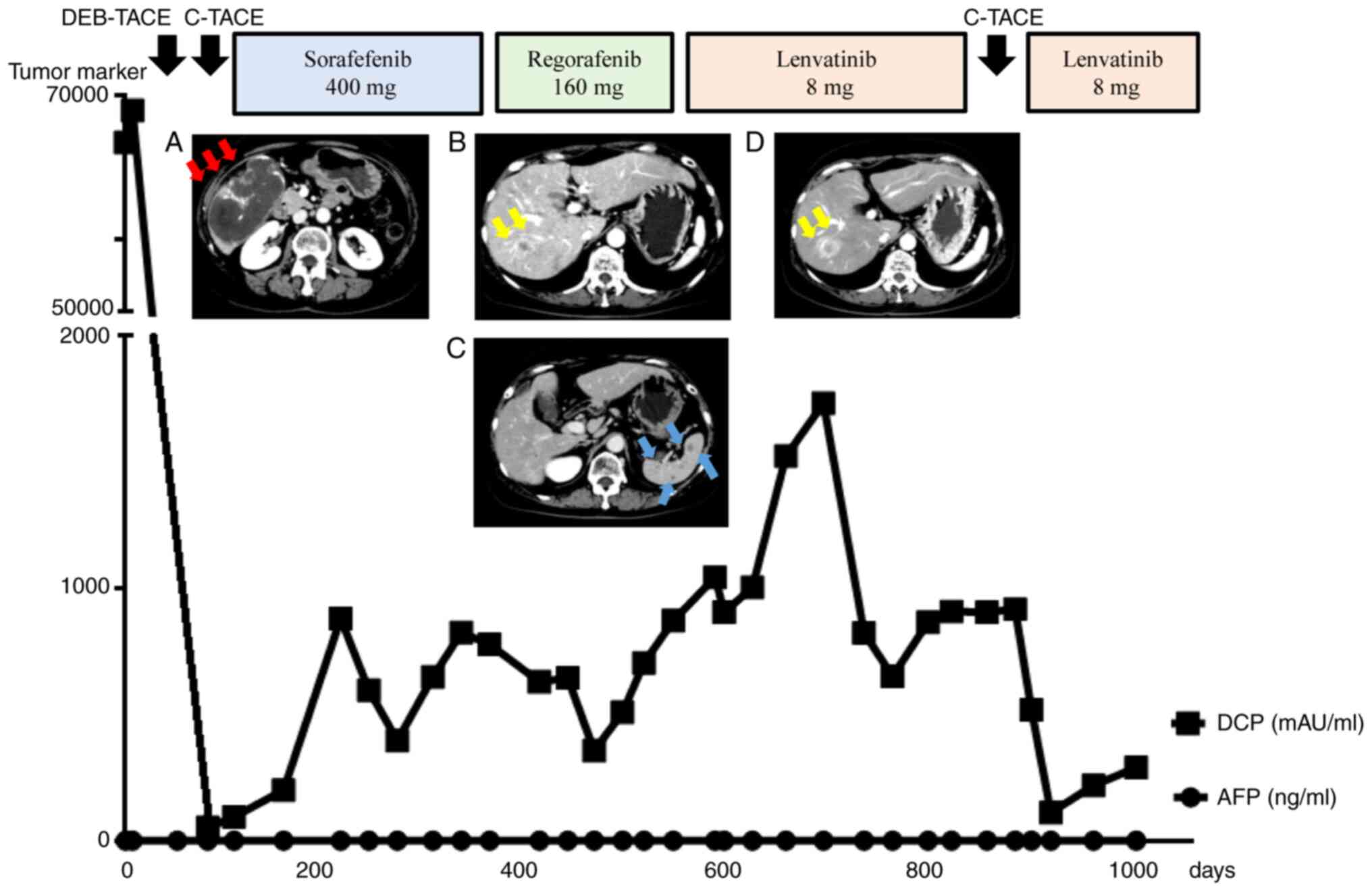 | Figure 2Clinical course with all treatment
regimens. (A) After two sessions of TACE, most of the treated
intrahepatic tumor was necrotic. (B) At 1.5 years post-diagnosis,
new intrahepatic metastatic lesions 20 mm in size appeared in S7,
and (C) multiple metastases 10 mm in size appeared in the spleen
during regorafenib treatment. (D) At 2.5 years post-diagnosis, the
S7 lesion progressed in the early arterial phase, and the tumor
size increased during lenvatinib treatment. Viable tumor growth was
also observed around the primary tumor in the posterior segment.
Red arrows indicate the primary lesion in the liver, yellow arrows
indicate other intrahepatic metastases, and blue arrows indicate
splenic metastases. TACE, transcatheter arterial chemoembolization;
DEB, drug-eluting beads; C, conventional; DCP, des-γ-carboxy
prothrombin; AFP, α-fetoprotein. |
At 2 months post-diagnosis (2 weeks after c-TACE),
serum transaminase decreased to normal levels, and liver reserve
was completely restored. The patient was administered 400 mg
sorafenib daily, which was the only MTA approved in Japan as
first-line therapy for advanced HCC at that time. After initiating
the sorafenib treatment, DCP levels, which had been almost
normalized earlier, increased gradually. However, tumor assessment
was performed via CE-CT every 8 weeks to confirm disease
progression.
At 1 year post-diagnosis (10 months after initiation
of sorafenib treatment), CE-CT revealed an increased
hyper-enhancement at the site of the primary intrahepatic tumor in
the early arterial phase, indicating tumor progression in the
marginal area; however, the lung metastases showed no change
relative to their states before the start of the treatment. Due to
the progression of HCC, the patient discontinued sorafenib for two
weeks, and she was administered 160 mg regorafenib daily for three
weeks, followed by no drug administration for one week.
At 1.5 years post-diagnosis (6 months after
initiation of regorafenib treatment), DCP levels increased slowly
and were in the range of 357-873 mAU/ml. Although no change was
observed in the lung metastases on the CE-CT, viable residual
marginal lesions of the primary liver tumor progressed. In
addition, new intrahepatic metastatic lesions 20 mm in size
appeared in the S7 segment (Fig.
2B), and multiple metastases 10 mm in size were observed in the
spleen (Fig. 2C). The therapeutic
effect was evaluated as progressive disease based on the modified
Response Evaluation Criteria in Solid Tumors (mRECIST) (6) and RECIST version 1.1 (v1.1) (7). At this point, there were no
significant abnormalities in the laboratory parameters (Table I), and the clinical data showed that
the Child-Pugh score was 5 and the ECOG-PS score was 0. Regorafenib
was discontinued for two weeks, and the patient was administered 8
mg of lenvatinib daily, which had just been approved for use in
Japan. During the clinical course, the patient suffered from mild
fatigue and anorexia equivalent to Grade 1 according to the Common
Terminology Criteria for Adverse Events (CTCAE) v5.0(8), with no serious adverse effects.
Although DCP levels gradually increased from 1,045 to 1,734 mAU/ml
over 2-5 months, CE-CT findings showed that the primary tumor in
the posterior segment and that in the S7 segment changed from a
high density to iso-density in the early arterial phase, suggesting
that lenvatinib could have a positive effect.
At 2.5 years post-diagnosis (one year after
initiating the lenvatinib treatment), DCP levels slightly decreased
(918 mAU/ml). However, CE-CT revealed that both intrahepatic tumors
had high density in the early arterial phase, and the tumor sizes
had also increased (Fig. 2D).
Notably, the lung and spleen metastases were downsized or
disappeared on CE-CT analysis. The intrahepatic metastases became
more resistant to lenvatinib later in the course of the treatment,
whereas lenvatinib was effective against extrahepatic metastases
even after 1 year. After discontinuing lenvatinib for 2 weeks,
C-TACE with 70 mg of cisplatin was performed for both intrahepatic
tumors, namely, the radical marginal lesions of the primary tumor
in the posterior segment and the growing tumor in the S7 segment.
Two weeks after TACE, recovery of the liver function was confirmed,
and lenvatinib administration was re-initiated because previous
lenvatinib treatment had been effective for extrahepatic metastases
in the lungs and spleen. During the course of this treatment, the
patient developed hypothyroidism equivalent to Grade 2 according to
the CTCAE v5.0 due to lenvatinib; hence, oral levothyroxine was
administered.
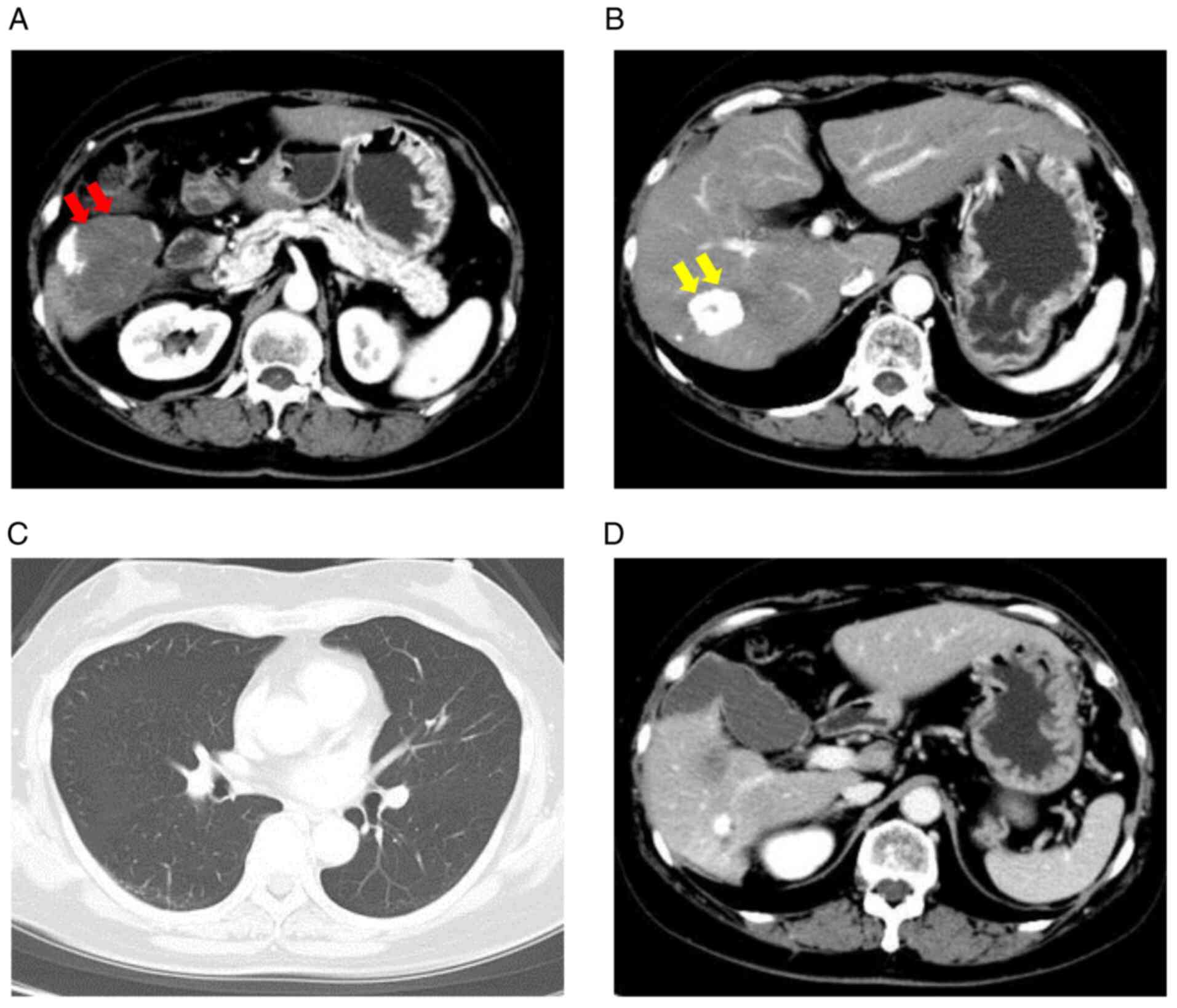
At 3 years post-diagnosis (5 months after
re-initiation of lenvatinib treatment), the patient's DCP levels
decreased to 290 mAU/ml, and AFP was 9 ng/ml, which was considered
to be within the prescribed range. CE-CT revealed that the primary
tumors in the posterior segment and S7 segment had downsized
(Fig. 3A and B), and no obvious intrahepatic lesions or
recurrence was observed. In addition, the extrahepatic metastases
in the lungs and spleen remained undetectable (Fig. 3C and D). Since good liver function and good
performance status were maintained, with a Child-Pugh score of 5
and ECOG-PS score of 0, the lenvatinib treatment was continued till
date (December 2020), without serious adverse effects during the
course of treatment of 3 years.
Discussion
For unresectable advanced HCC, sequential therapy
using multiple MTAs alongside TACE can confer long-term survival.
It is important to select an appropriate method from the multiple
treatment options available and switch to the next option at an
appropriate time to improve the prognosis. In addition to the many
effective clinical applications of various MTAs worldwide (1), several clinical studies on the use of
MTAs and immune checkpoint inhibitors (ICIs) are ongoing, and
combination therapies with MTAs and ICIs are likely to play a
central role in the treatment of advanced HCC (9).
For patients with HCC and extrahepatic metastases
classified as stage C according to the BCLC staging system,
administration of MTA as a systemic therapy is recommended.
However, in the present case, the large primary tumor in the
posterior segment of the liver progressed rapidly and small lung
metastases were observed; therefore, TACE was performed for the
large primary liver tumor to reduce its volume followed by
sorafenib treatment. Sorafenib prolongs survival by suppressing
tumor growth; however, reduction in tumor size cannot be expected,
and the complete response rate is 3% (10,11).
Therefore, even in cases of extrahepatic metastasis, prior
treatment with TACE to downsize a large intrahepatic tumor is
beneficial before administering an MTA such as sorafenib. In
contrast, although lenvatinib has a better effect on tumor
reduction than sorafenib (12), it
has been reported to cause hemorrhage in the tumor through necrosis
because of the rapid blockage of the feeding circulation. TACE
before lenvatinib might prevent tumor hemorrhage and rupture in
large HCC.
Maintaining good liver function is the most
important factor in improving the long-term prognosis of patients
with HCC because all MTAs are only recommended for patients with
good liver function. Losing further treatment opportunities with
MTAs due to decreased liver function is detrimental to patients
with HCC. Hence, excessive embolization causing widespread hepatic
ischemia should be avoided during TACE treatment. To maintain liver
function, it is important to perform contrast CT angiography with
the catheter placed in the hepatic artery during TACE treatment and
identify the exact location between the HCC and the feeding
arteries to be treated. Selective catheterization of the tumor
feeders and injection of embolic materials should be performed.
DEB-TACE, an endovascular treatment based on the use of
microspheres to release chemotherapeutic agents within the target
lesion, may downsize large intrahepatic tumors. As the antitumor
drug loaded in DEB remains in the tumor at a high concentration and
does not pass into the peripheral blood, DEB-TACE is less likely to
cause adverse effects such as liver damage and post-embolization
syndrome compared to C-TACE (13,14).
In the present case as well, sequential therapy with MTAs,
including sorafenib, regorafenib, and lenvatinib, could be
continued while maintaining good liver function after selective
DEB-TACE.
Sorafenib, which prolonged the survival of patients
with advanced HCC, was the first MTA used clinically and the only
systemic chemotherapeutic drug indicated for HCC for a long time
(11). In the later stages of the
RESORCE and REFLECT trials, regorafenib (15) and lenvatinib, respectively, were
found to be efficacious in patients with advanced HCC (12); these drugs were approved in Japan in
2017 and 2018, respectively. Shortly thereafter, the usefulness of
other MTAs, such as ramucirumab and cabozantinib, was also
demonstrated (16,17). Since the time when there were only
classical treatments such as radiofrequency ablation, TACE, and
hepatic arterial infusion chemotherapy, the medical treatment of
HCC has dramatically changed due to the increase in treatment
options.
Lenvatinib is a multi-kinase inhibitor targeting
vascular endothelial growth factor receptor (VEGFR) 1-3, fibroblast
growth factor receptor (FGFR) 1-4, platelet-derived growth factor
receptor α, rearranged during transfection receptor, and tyrosine
kinase receptor, thereby suppressing neo-vessel assembly and
maturation, and decreasing the vascular permeability of the tumor
microenvironment (18). In the
REFLECT trial mentioned above, lenvatinib was shown to be equally
effective as sorafenib. The overall survival of the patients
treated with lenvatinib was similar to that with sorafenib (13.6
vs. 12.3 months). Notably, the antitumor effect of lenvatinib
treatment vs. sorafenib treatment in mRECIST resulted in a response
rate, disease control rate, and progression-free survival of 40.6
vs. 12.4, 73.8 vs. 58.4%, and 7.3 vs. 3.6 months, respectively,
showing that lenvatinib was significantly better than sorafenib
(12). The high response rate of
lenvatinib also promises a therapeutic strategy for conversion to
resection, which is challenging with sorafenib. Among the adverse
events after lenvatinib treatment, hypertension, urinary protein
excretion, and hypothyroidism were more common, whereas hand-foot
syndrome and diarrhea were less frequently observed compared to
treatment with sorafenib. It is important to understand the
characteristics of each MTA and manage its adverse effects. In
addition, lenvatinib has a stronger inhibitory effect on the
angiogenic factors VEGFR1-3 and FGFR-1-4 than sorafenib (19), and it is expected to strongly
suppress angiogenetic factors that cause recurrence after TACE.
Drug resistance remains the major cause of failure
in MTA therapy (20) and is an
important issue when switching between MTAs. As in the present
case, even though the strongest antitumor effects are observed when
using multiple MTAs for advanced HCC, intrahepatic lesions,
especially primary tumors, often show resistance to MTAs as
indicated by a partial increase in size on CE-CT analysis. It is
necessary to understand the mechanisms underlying drug resistance.
In general, tumor heterogeneity causes primary resistance and
clonal evolution, eventually leading to drug resistance (21). According to recent findings, cancer
stem cells are implicated in drug resistance and are the main
factors associated with distant metastases through
epithelial-mesenchymal transition (EMT) (22). A study on patient-derived
sorafenib-resistant, poorly differentiated thyroid cancer cells has
revealed that lenvatinib alongside the histone deacetylase
inhibitor HNHA blocks EMT through interference with FGFR signaling
(23). Although the molecular
mechanism involved in EMT regulation has not been fully elucidated,
several studies focusing on EMT in stem cell models have indicated
that both p21 and p53 activation play key roles in suppressing EMT
(24). Although there are some
reports on the efficacy of lenvatinib in a multidrug-resistant
setting, most patients also develop resistance to lenvatinib,
particularly in cases of HCC. Although a few preclinical studies
have highlighted the role of the c-MET/PI3K/AKT cascade in thyroid
cancer (25) and mTOR in renal
cancer (26), the mechanism of
lenvatinib resistance in HCC remains unclear.
The treatment options for HCC resistant to multiple
MTAs are limited. In our case, TACE was performed on-demand for the
intrahepatic radical tumors that were resistant to multiple MTAs
and was successful after lenvatinib treatment. Despite the current
mainstream therapy with MTAs, on-demand TACE is an effective
treatment option for intrahepatic tumors resistant to sequential
therapies with multiple MTAs. However, it should be noted that TACE
might reduce liver function if a wide range of embolization or
frequent procedures are performed (27). Currently, the available MTAs for HCC
are recommended only for patients with good liver function,
especially Child-Pugh class A. To continue sequential treatment
with MTA later, unnecessary repetition of TACE should be avoided to
preserve liver function. In the present case, no progression of HCC
was observed after re-administration of lenvatinib. Furthermore,
the patient is still alive, showing no severe adverse effects or
decline in liver function. To the best of our knowledge, this is
the first report showing the responsiveness of metastatic advanced
HCC to re-initiation of lenvatinib treatment after TACE, although
the primary intrahepatic tumor had become resistant to initial
sorafenib, regorafenib, and lenvatinib regimens. However, one
limitation is that multimodal treatment of advanced HCC is often
based on the experience of the attending doctor, and the strategy
differs depending on the case and medical institute. There is still
ambiguity on many factors, such as the timepoints to change the
treatment course and to re-initiate MTA treatment after TACE.
Therefore, it is necessary to accumulate evidence over long
periods, including MTA treatment periods, in future studies.
In conclusion, although multiple MTAs introduced
into the clinic have been gradually replacing TACE, TACE can
improve the multimodal treatments for advanced HCC. In particular,
on-demand TACE in the multidisciplinary treatment of advanced HCC
is effective for intrahepatic hypervascular tumors resistant to
MTAs, including lenvatinib. By performing on-demand TACE with
adequate precautions to preserve liver function, it is possible to
re-initiate lenvatinib treatment with good efficacy against distant
metastatic lesions, thereby contributing to long-term survival.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KO and TM designed the study and wrote the
manuscript. KO, KT, MN and TT analyzed and interpreted the
patient's clinical data for the manuscript. KO, KF, SM, JT, AM, HK
and TM contributed to collecting the relevant literature and to
data analysis, and reviewed and critically interpreted the
information. KO and TM are responsible for confirming the
authenticity of the data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Approval was obtained from the Ethics Committee of
Kagawa University Hospital (Kagawa, Japan) (approval no. 2019-238).
Informed consent for participation in the study or use of the
clinical data was obtained from the patient.
Patient consent for publication
The patient provided written informed consent for
the publication of any data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
European Association for the Study of the
Liver. Electronic address simpleeasloffice@easloffice.eu;
European Association for the Study of the liver. EASL clinical
practice guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Altekruse SF, Henley SJ, Cucinelli JE and
McGlynn KA: Changing hepatocellular carcinoma incidence and liver
cancer mortality rates in the United States. Am J Gastroenterol.
109:542–553. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Llovet JM, Bustamante J, Castells A,
Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J and Bruix J: Natural
history of untreated nonsurgical hepatocellular carcinoma:
Rationale for the design and evaluation of therapeutic trials.
Hepatology. 29:62–67. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kudo M, Kitano M, Sakurai T and Nishida N:
General rules for the clinical and pathological study of primary
liver cancer, nationwide follow-up survey and clinical practice
guidelines: The outstanding achievements of the liver cancer study
group of Japan. Dig Dis. 33:765–770. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
National Cancer Institute (NCI): Common
Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017.
https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50.
Accessed May 18, 2020.
|
|
9
|
Kudo M: Combination cancer immunotherapy
with molecular targeted Agents/Anti-CTLA-4 antibody for
hepatocellular carcinoma. Liver Cancer. 8:1–11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Golfieri R, Giampalma E, Renzulli M, Cioni
R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R,
Gasparini D, et al: Randomised controlled trial of
doxorubicin-eluting beads vs conventional chemoembolisation for
hepatocellular carcinoma. Br J Cancer. 111:255–264. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
van Malenstein H, Maleux G, Vandecaveye V,
Heye S, Laleman W, van Pelt J, Vaninbroukx J, Nevens F and Verslype
C: A randomized phase II study of drug-eluting beads versus
transarterial chemoembolization for unresectable hepatocellular
carcinoma. Onkologie. 34:368–376. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu AX, Kang YK, Yen CJ, Finn RS, Galle
PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, et al:
Ramucirumab after sorafenib in patients with advanced
hepatocellular carcinoma and increased α-fetoprotein concentrations
(REACH-2): A randomised, double-blind, placebo-controlled, phase 3
trial. Lancet Oncol. 20:282–296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Abou-Alfa GK, Meyer T, Cheng AL,
El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park
JW, et al: Cabozantinib in patients with advanced and progressing
hepatocellular carcinoma. N Engl J Med. 379:54–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Koyama N, Saito K, Nishioka Y, Yusa W,
Yamamoto N, Yamada Y, Nokihara H, Koizumi F, Nishio K and Tamura T:
Pharmacodynamic change in plasma angiogenic proteins: A
dose-escalation phase 1 study of the multi-kinase inhibitor
lenvatinib. BMC Cancer. 14(530)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res.
2014(638747)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Marin JJG, Macias RIR, Monte MJ, Romero
MR, Asensio M, Sanchez-Martin A, Cives-Losada C, Temprano AG,
Espinosa-Escudero R, Reviejo M, et al: Molecular Bases of Drug
Resistance in Hepatocellular Carcinoma. Cancers (Basel).
12(1663)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McGranahan N and Swanton C: Clonal
heterogeneity and tumor evolution: Past, present, and the future.
Cell. 168:613–628. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Y and Weinberg RA:
Epithelial-to-mesenchymal transition in cancer: Complexity and
opportunities. Front Med. 12:361–373. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee YS, Kim SM, Kim BW, Chang HJ, Kim SY,
Park CS, Park KC and Chang HS: Anti-cancer effects of HNHA and
lenvatinib by the suppression of EMT-mediated drug resistance in
cancer stem cells. Neoplasia. 20:197–206. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Saji M and Ringel MD: The PI3K-Akt-mTOR
pathway in initiation and progression of thyroid tumors. Mol Cell
Endocrinol. 321:20–28. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ghidini M, Petrelli F, Ghidini A,
Tomasello G, Hahne JC, Passalacqua R and Barni S: Clinical
development of mTor inhibitors for renal cancer. Expert Opin
Investig Drugs. 26:1229–1237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hiraoka A, Kumada T, Kudo M, Hirooka M,
Koizumi Y, Hiasa Y, Tajiri K, Toyoda H, Tada T, Ochi H, et al:
Hepatic function during repeated TACE procedures and prognosis
after introducing sorafenib in patients with unresectable
hepatocellular carcinoma: Multicenter analysis. Dig Dis.
35:602–610. 2017.PubMed/NCBI View Article : Google Scholar
|