Introduction
Neuroendocrine tumors (NETs) comprise a
heterogeneous group of solid malignancies that frequently occur in
the intestine, but have also been found to originate from other
organs. The incidence of NETs is increasing worldwide; however, the
associated cause remains unknown (1-5).
The clinical manifestations of NETs vary widely depending on the
location of the tumor and, in some cases, the type of hormones
secreted, which promotes non-specific symptoms, such as diarrhea,
bronchospasms, flushing, cardiac valve disease and other
non-specific debilitating symptoms (6). When the NETs secrete hormones
(functional tumors), clinical manifestations are common (4). Patients who present with a functional
NET may complain of abdominal discomfort, which may be mistaken for
irritable bowel syndrome, or other non-specific symptoms that can
lead to misdiagnosis of dyspepsia and other benign conditions
(6). When the NET is
non-functional, the patient may be asymptomatic and the tumor is
frequently left undetected. This causes a delay in diagnosis and a
worse prognosis (7).
Research groups in Latin America have described the
epidemiology of NET cases in their countries. One group from
Argentina published a study in 2014 in which 532 cases were
included to describe the epidemiology, prevalence, demography,
symptoms and diagnostic methods for NETs (8). A similar study was conducted in Chile
(9) published in 2019, that
consisted in 166 patients with NETs. Interestingly, the demographic
data from these two studies showed that patient age at diagnosis
and sex were similar, but with a predominance of small-bowel NETs
in the Chilean cohort. To the best of our knowledge, there have
been no comprehensive studies firmly establishing the incidence,
clinical characteristics, diagnostics procedures used or overall
survival for NET cases in Panama to date. The present study
represents the first comprehensive effort to determine the
epidemiological characteristics, associated epidemiological factors
and survival of patients with NETs in the Republic of Panama.
Through a collaborative nationwide network that included surgical
and medical oncologists, interventional radiologists and
radio-oncologists, the present study was undertaken to establish a
Panamanian NET database with the aim of improving our understanding
of this rare condition.
Patients and methods
Study design
Both retrospective studies were conducted using the
hospital medical records of patients from all over the Republic of
Panama who were referred to the three largest national referral
hospitals: The Complejo Hospitalario Metropolitano (CHM), Hospital
Santo Tomas (HST) and Instituto Oncologico Nacional (ION). All
patients, regardless of the age, with complete diagnosis record of
NET were included. Patients with unconfirmed diagnosis by
histopathology were not included. All three hospitals are located
near the country's capital, Panama City. The study proposal was
submitted to the Institutional Ethics Board Committee at the ION
(Panama City, Panama), and was granted both national and
institutional approval. The retrospective part of the study
included 64 patients, who were treated between January 2016 and
December 2017. The second group comprised 93 patients, for whom
data were collected continuously between January 2018 and December
2019. A total of 157 cases were included in both cohorts. Data
collection was concluded in January 2020. Cases were evaluated by a
multidisciplinary team including surgical oncology, medical
oncology, interventional radiology and radiation oncology
specialists. Treatment was conducted based on international
guidelines; details are not included, since this was outside the
scope of the present study.
Histopathological diagnosis, classification and
grading of NETs were determined based on paraffin-embedded block
results reported for tissues analyzed at ION, which included
biopsies and/or resection samples. The nomenclature and
classification used for these tumors was based on the 2010
guidelines of the World Health Organization (10). The analysis of the levels of the
biomarkers CD56, chromogranin A (CgA), synaptophysin (SYN) and
Ki-67 were performed using immunohistochemistry and tumor behavior
assessment. Fixation and staining of samples had been conducted
using institutional protocols that included 4-µm sections and
immunohistochemistry assays using anti-SYN (cat. no. MRQ-40; Merck
KGaA), anti-CgA (cat. no. LK2H10; Ventana Medical Systems, Inc.;
Roche Diagnostics), CD-56 (cat. no. 123C3; Dako; Agilent
Technologies, Inc.) and anti-human Ki-67 (cat. no. M7240; clone
MIB-1; Dako, Agilent Technologies, Inc.) antibodies, and did not
constitute part of the present study. The collected data were
curated and anonymized prior to analysis by removing all personal
identifiable information.
Data for both arms of the study were independently
collected. The data from the two groups had similar variables, and
were combined in a single Excel spreadsheet, followed by manual
curation of entries to confirm consistency, elimination of
duplicate records and anonymization of the data. The final data
were compiled into a database with 157 unique identifiers. Cases
from the retrospective arm of the study are referred to as cohort
1, whereas cases from the second group, for which data were
continuously collected, were identified as cohort 2. The unified
database contained information on the epidemiology, diagnosis,
treatment, levels of tumor markers and patient age at
diagnosis.
Statistical analysis
Statistical analysis was performed using EpiInfo
v7.2.4.0 software (Centers for Disease Control) and a 95%
confidence interval (CI) estimation. SPSS v23.0 software (IBM
Corp.) was used to perform descriptive univariate analysis of the
frequencies and to determine the statistical significance of the
geographical distribution of cases (11,12).
For the Kaplan-Meier survival curves, data were stratified by age
groups as follows: <40, 40-59, 60-79 and ≥80 years. Mantel Cox
tests were applied to detect statistically significant differences
between groups. The overall survival (OS) by tumor grade and
anatomical site of the primary tumor was also determined. For
variables presented on all tables, 95% CIs were determined.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Age and sex distribution of patients
with NETs
Data from a total of 157 NET cases were collected
from the three main tertiary referral hospitals in the Republic of
Panama. The age was similar between cohort 1 (mean, 61 years;
range, 21-93 years) and cohort 2 (mean, 59 years; range, 20-90
years) (Table I). Male:female sex
distribution was different between the two groups (1:1,
respectively, in cohort 1; and 1:2, respectively, in cohort 2).
Since the Republic of Panama has a population of ~4.14 million, the
incidence of NETs in the country between 2016 and 2019 was
calculated to be 0.92 cases for every 100,000 individuals, using
National Registry Census data for those years (13).
 | Table ICombined characteristics of all 157
patients in the present study. |
Table I
Combined characteristics of all 157
patients in the present study.
| Characteristics | Value, n | Value, % | 95% CI |
|---|
| Mean age, years | 60 | | - |
| Age range, years | 20-93 | | - |
| Sex | | | |
|
Male | 65 | 41.40 | 33.61-49.53 |
|
Female | 92 | 58.60 | 50.47-66.39 |
| Age, years | | | |
|
<40 | 16 | 10.19 | 5.94-16.02 |
|
40-59 | 58 | 36.94 | 29.39-45.00 |
|
60-80 | 73 | 46.50 | 38.51-54.62 |
|
>80 | 10 | 6.37 | 3.10-11.40 |
| Metastasis | | | |
|
Yes | 72 | 45.86 | 37.89-53.99 |
|
No | 85 | 54.14 | 46.01-62.11 |
| Tumor grade | | | |
|
G1 | 73 | 46.50 | 38.51-54.62 |
|
G2 | 31 | 19.75 | 13.83-26.84 |
|
G3 | 45 | 28.66 | 21.74-36.41 |
|
Unspecified | 8 | 5.10 | 2.23-9.79 |
Age and tumor grade of patients with
NETs
The mean age of patients with NETs was 60 years, and
the majority of the cases were identified in the 60-80 years
(46.50%) and 40-59 years (36.94%) age groups. In total, <17% of
all cases were observed in patients aged <40 or >80 years. A
total of 72 patients (45.86%) also exhibited metastasis. The
majority of the tumors (46.50%) were classified as grade G1,
followed by G3 (28.66%) and G2 (19.75%). In 5.10% of the cases,
tumor grade was undetermined or not available. Tumor grading
information for each primary tumor location is presented in
Table SI.
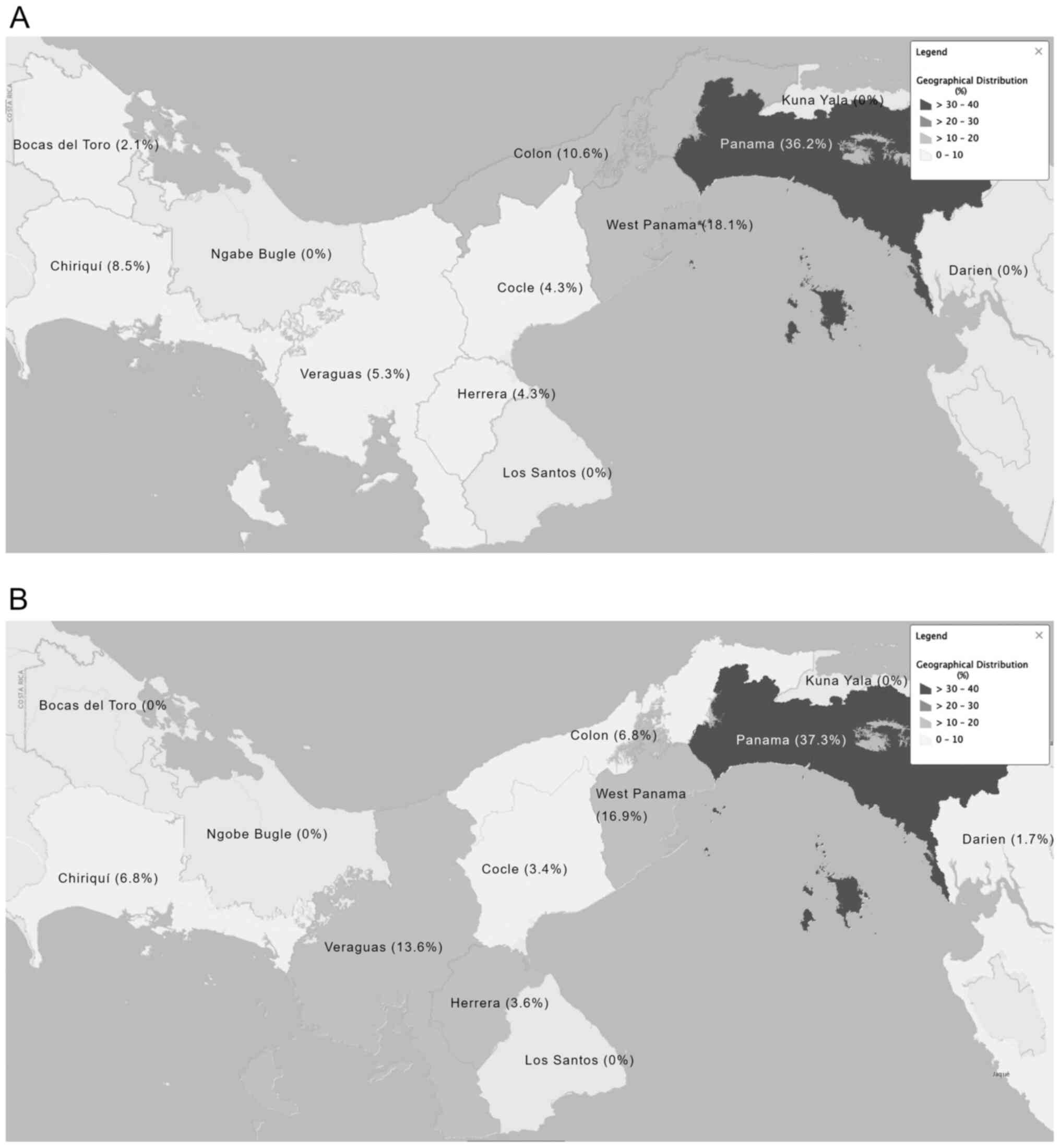
Geographical distribution of patients
with NETs
The geographical distribution of cases before
referral throughout the Republic of Panama is presented in Fig. 1. In total, 37.3% of all cases were
found to have originated from the Panama province, 16.9% from the
West Panama province and 13.6% originated from the Veraguas
province.
Diagnosis, staging, classification and
tumor grading of patients with NETs
The diagnosis, staging, classification and tumor
grading of patients with NETs were performed using non-invasive and
invasive procedures, imaging techniques and biomarker expression
levels (Table II). The most common
imaging modality used was contrast-enhanced CT scans, in which
contrast was reported in 59.38% of cases. Colonoscopies and
endoscopies were performed in 13.28 and 10.94% of cases,
respectively. In addition, 9.38% of the patients underwent an
ultrasound examination. Other diagnostic methods, such as
mammography, radiography, magnetic resonance imaging, bone scan,
cystoscopy and colposcopy accounted for 7.02% of all cases included
in both cohorts.
 | Table IIFrequency of diagnostic procedures
for patients with neuroendocrine tumors. |
Table II
Frequency of diagnostic procedures
for patients with neuroendocrine tumors.
| Procedures | Value, n | Value, % | 95% CI |
|---|
| CT with
contrast | 76 | 59.38 | 50.34-67.96 |
| Colonoscopy | 17 | 13.28 | 7.93-20.41 |
| Endoscopy | 14 | 10.94 | 6.11-17.67 |
| Ultrasound | 12 | 9.38 | 4.94-15.8 |
| Mammography | 2 | 1.56 | 0.19-5.53 |
| Radiography | 2 | 1.56 | 0.19-5.53 |
| Magnetic resonance
imaging | 2 | 1.56 | 0.19-5.53 |
| Bone scan | 1 | 0.78 | 0.02-4.28 |
| Cystoscopy | 1 | 0.78 | 0.02-4.28 |
| Colposcopy | 1 | 0.78 | 0.02-4.28 |
| Total | 128a | 100.00 | - |
The expression levels of markers, including Ki-67,
CgA, SYN and CD56, were analyzed in the majority of cases in both
study groups for grading and diagnostic purposes. The test yield is
shown in Table III, but this did
not include cases with undetermined or unavailable results. SYN was
the most frequent marker used for diagnostic purposes (109 cases)
and provided a test yield of 67.52%. In addition, CgA was used for
cytological evaluation in 104 cases, demonstrating a test yield of
55.41%; none of the patients were using proton-pump inhibitors at
the time of measuring CgA levels. The % of Ki-67 staining was used
for tumor grading and prognostic purposes, providing a test yield
of 52.20%. The expression levels of the less frequently used
marker, CD56, were analyzed in 31 cases and showed a test yield of
19.11%.
 | Table IIIMarkers used to diagnose and classify
neuroendocrine tumor cases ordered by test yield. |
Table III
Markers used to diagnose and classify
neuroendocrine tumor cases ordered by test yield.
| Markers | Value, n | Test yield, % |
|---|
| Synaptophysin | 109 | 67.52 |
| Chromogranin A | 104 | 55.41 |
| Ki-67 | 136 | 52.20 |
| CD56 | 31 | 19.11 |
Anatomical location of primary and
metastatic NETs
The anatomical location of the primary NET was
determined and the frequency of lesions at each location is
summarized in Table IV. The data
revealed that the three most frequent locations of the primary
tumor were the colorectum (17.20% of cases), pancreas (12.74% of
cases) and stomach (12.10% of cases) in the combined cohort. In
7.0% of cases, the location of the primary tumor was unknown or
undetermined.
 | Table IVFrequency of primary neuroendocrine
tumor location. |
Table IV
Frequency of primary neuroendocrine
tumor location.
| Sites | Value, n | Value, % | 95% CI |
|---|
| Colorectum | 27 | 17.20 | 11.65-24.03 |
| Pancreas | 20 | 12.74 | 7.96-18.99 |
| Stomach | 19 | 12.10 | 7.45-18.25 |
| Jejunum-ileum | 14 | 8.92 | 4.96-14.51 |
| Lungs | 12 | 7.64 | 4.01-12.97 |
| Appendix | 11 | 7.01 | 3.55-12.19 |
| Breast | 9 | 5.73 | 2.65-10.60 |
| Duodenum | 5 | 3.18 | 1.04-7.28 |
| Ovaries | 4 | 2.55 | 0.70-6.39 |
| Skin | 3 | 1.91 | 0.40-5.48 |
| Others | 15 | 9.55 | 5.45-15.27 |
| Undetermined | 18 | 7.01 | 6.94-17.51 |
| Total | 157 | 100.00 | - |
The number of cases for each metastatic location was
also determined (Table V). The
liver was the most frequent metastatic site in both study groups
(data not shown), as 53 cases (33.76%) presented with hepatic
metastasis. Within this group, patients with liver metastasis also
had other affected organs, such as the peritoneum in 4.46% of cases
and the lungs in 2.55% of cases. The brain followed as the second
most common single metastatic site, with 2.55% of cases, followed
by bone and peritoneal metastases in 1.27% of cases for each.
Overall, other organs were identified as metastatic sites (7.01%)
but these occurred less frequently. Metastasis was not determined
or specified in 54.14% of cases.
 | Table VFrequency of metastasis and
metastatic sites. |
Table V
Frequency of metastasis and
metastatic sites.
| Sites | Value, n | Value, % | 95% CI |
|---|
| Liver | 53 | 33.76 | 26.41-41.73 |
|
Liver
only | 36 | 22.93 | - |
|
Liver and
peritoneum | 7 | 4.46 | - |
|
Liver and
lungs | 4 | 2.55 | - |
|
Others | 6 | 3.82 | - |
| Brain | 4 | 2.55 | 0.70-6.39 |
| Bone | 2 | 1.27 | 0.15-4.53 |
| Peritoneum | 2 | 1.27 | 0.15-4.53 |
| Other sites | 11 | 7.01 | 3.55-12.19 |
|
Undetermineda | 85 | 54.14 | 46.01-62.11 |
| Total | 157 | 100.00 | - |
Survival analysis of patients with
NETs
The patients were treated according to the degree of
tumor differentiation. Patients with differentiated tumors mainly
received treatment with the somatostatin analogue, octreotide, and
second-line treatment with targeted therapies, such as mTOR
inhibitors, regardless of whether the tumors were functional or
non-functional, due to the lack of functional imaging facilities in
the country (data not shown).
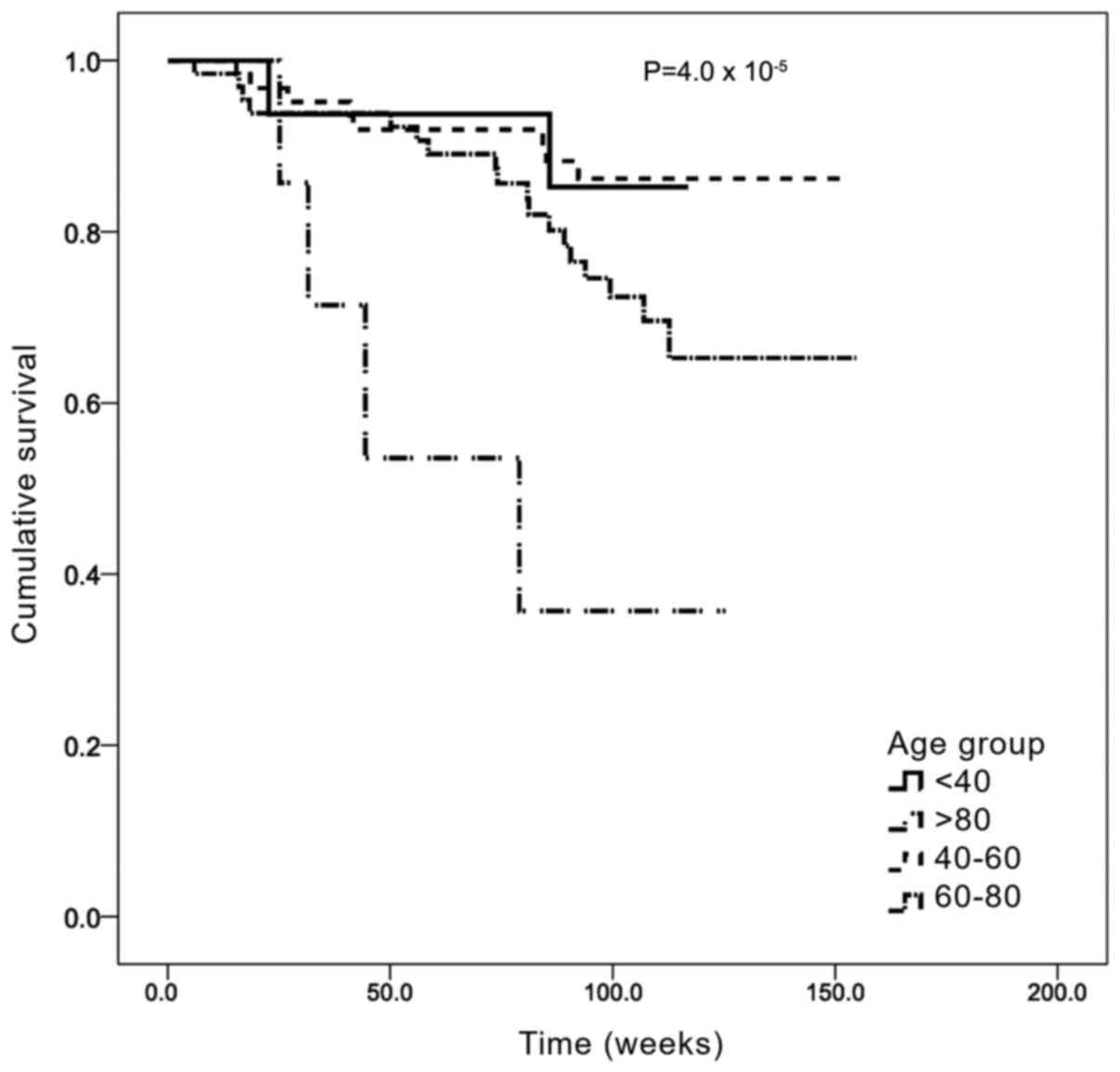
Survival analysis was first stratified by age group
(Fig. 2). The median OS time was
132.3 weeks (95% CI: 125.2-139.1) and the median follow-up time was
100 weeks (~2 years). The 40-59 years age group had the longest OS,
with a mean OS of 139.4 weeks (95% CI: 130.2-148.5), while the
>80 years age group had the shortest OS, with a mean of 68.8
weeks (95% CI: 41.4-96.2). The differences between these groups
were statistically significant (Mantel Cox χ2 =23.0;
P=0.00004).
The OS according to the tumor grade is presented in
Fig. 3. Study subjects had a mean
survival of 132.2 weeks (95% CI: 124.8-139.5). Patients in the G1
group had the longest OS, with a mean OS of 142.9 weeks (95% CI:
135.1-150.8), while patients in the G3 group had the shortest OS
survival, with a mean OS of 96.8 weeks (95% CI: 84.0-109.7). The
differences between these groups were also found to be
statistically significant (Mantel Cox χ2=10.755;
P=0.005).
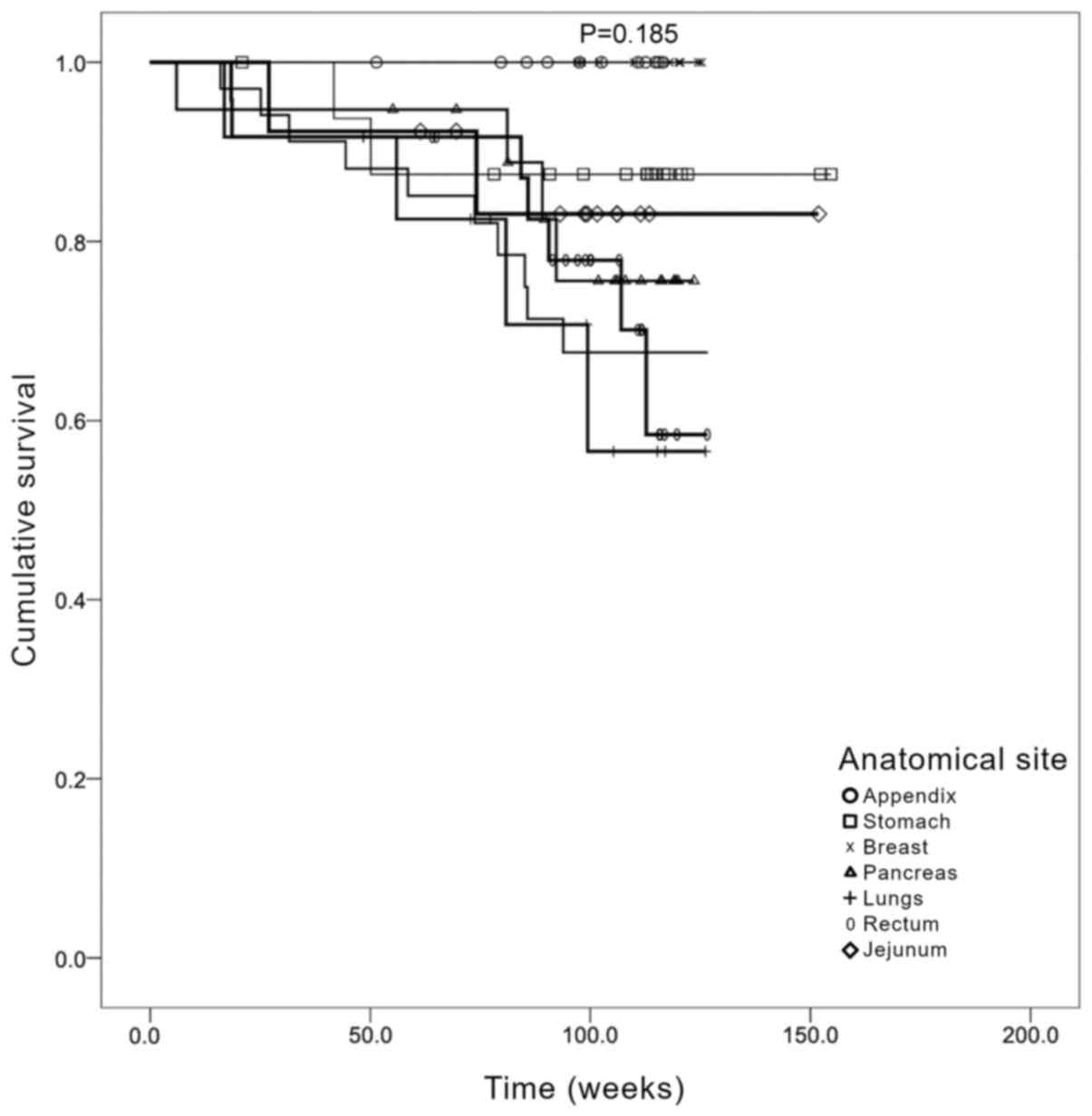
OS was subsequently determined according to the
anatomical site of the primary tumor (Fig. 4). The data revealed that those cases
in which the primary tumor was located in the lung had the worse
OS. This was followed by cases that had tumors localized in the
colorectal region and pancreas. However, no statistically
significant differences were identified between the different
primary locations when stratified by treatment (Mantel Cox
χ2=3.27; P=0.185).
Discussion
NETs comprise a group of rare neoplasms that
frequently affect the gastrointestinal and bronchopulmonary tissues
(5); however, they can also develop
in other tissues and organs, including the breast, ovaries and
skin. Since NETs are of endocrine and neurological origin, they may
secrete hormones that are associated with specific symptoms
(6,7). In the earliest stages of the disease,
patients with NETs may by asymptomatic or have non-functional
tumors (14); thus, the absence of
signs of disease may delay diagnosis (6). For this reason, it is paramount to
understand the epidemiology of NET cases and raise awareness of
this disease in Panama. To the best of our knowledge, the present
study was the first comprehensive nationwide study in the Republic
of Panama to characterize patients with NETs in two cohorts,
between 2016 and 2017 and between 2018 and 2019.
The Panama and West Panama provinces are located in
close proximity to each other and have the largest populations in
the country (15). In the current
study, these two provinces received all suspected cases from the
other provinces, which partially explains the higher number of
cases in these provinces. For example, it was reported herein that
Panama province had 37.3% of all NET cases, while West Panama
province had 16.9%. The largest tertiary referral hospitals are
located in these two provinces, on which the remaining provinces
rely for referring patients with NETs. However, there is currently
no complete explanation for the higher prevalence of NETs in these
two provinces.
Although the two cohorts differed in size and date
of investigation, each group displayed similarities in the mean age
and age range of the patients diagnosed with NETs. The findings of
the present study revealed that the majority of NETs were diagnosed
within the study population with a mean age of 60 years, and almost
all cases were diagnosed within the 40-80 years age group. In
contrast to these findings, previous studies revealed that the
median age for diagnosis of NETs in Chile was 53 years and in
Argentina 53.2 years, which are slightly lower compared with the
age in the present study (8,9). The
differences in the incidence of NETs by sex in the present study
were not statistically significant; however, there was a higher
prevalence in women in cohort 2, whereas the same frequency was
observed between the two sexes in cohort 1. The majority of cases
did not present with metastasis and the tumors were graded as G1.
These results are consistent with the findings of previous studies
and may be explained by the increase in early diagnosis and
improved diagnostic tools (16,17).
Classifying and staging NETs requires multiple
invasive or non-invasive procedures, imaging techniques,
immunohistochemistry and the detection of biomarkers such as SYN,
Ki-67 and CgA (18-20).
In the current study, the most frequent procedure for determining
the characteristics of the NETs in both groups was a CT scan with
contrast, followed by several invasive procedures, including
colonoscopies, endoscopies and ultrasounds. CT scans with contrast
are readily available in the participating hospitals, CHM, HST and
ION, but access to this diagnostic method is limited in the other
provinces of Panama. It is important to mention that other
functional imaging tools, such as positron emission tomography and
octreotide scans, are currently unavailable in the Republic of
Panama. Confirmation of diagnosis was performed using
histopathological analysis.
The identification of established diagnostic and
prognostic markers of NETs in the present cases was performed at
the ION. SYN is considered as the most sensitive biomarker, whereas
CgA is the most specific for NETs (21,22).
In addition, the percentage of Ki-67 staining is often used as a
proliferation marker (23,24). Test yields were calculated for each
of the immunohistology markers detected; the results revealed that,
on average, SYN had the highest yield (67.0%), followed by CgA
(54.8%) and CD56 (19.1%). These results are consistent with
previous studies reporting that CD56 has the lowest yield (25).
The data of the present study revealed that the most
frequent primary sites of NETs were the colorectum, pancreas and
stomach. It has been reported that the location of the primary site
varies significantly in different countries, which may be
associated with undetermined factors (2). For example, the most frequent primary
sites for NETs in a study involving patients from Taiwan are the
rectum, lungs and stomach (26). By
contrast, data from Latin American countries, such as Argentina,
report that the small intestine, pancreas and colorectum are the
three most frequent sites (8). In
the USA, particularly in the state of Kentucky, which has a
population size similar to Panama, the most frequent primary tumor
site is the lung, followed by the small intestine and colorectum
(2). These observed differences
among countries support the requirement for further investigations
to identify the factors underlying the anatomical location of
NETs.
Similar to other populations worldwide, the most
frequent organ affected by the metastasis of NETs is the liver
(27,28), accounting for 33.8% of all cases in
the present study. Other metastatic organs were also reported;
however, all other sites had a frequency of <10%. In addition,
the presence of metastasis was not reported or remained
undetermined in 54.1% of all cases. This may be due to a
combination of factors, such as the lack of early diagnosis,
technological limitations in determining the presence of small
lesions, or the lack of available data from clinical records.
Survival analysis for the present cohorts were
adjusted for age. Following the determination of OS by age group,
elderly patients (>80 years old) had a worse prognosis, with a
median survival of 68.8 weeks compared with 139.4 weeks for the
40-60 years age group. These results indicated that age may
contribute to worse prognosis, consistent with previous findings
(29). Other confounding factors
were not included in the present analysis. By contrast, analysis of
the OS according to sex revealed no statistically significant
difference between the two sexes. Following the determination of OS
by anatomical location of the primary tumor, the data suggested
that patients with a primary lesion located in the appendix or
stomach had a longer OS, whereas the OS of patients with primary
tumors located in the jejunum and pancreas was shorter. By
contrast, patients with lung and colorectal tumors exhibited a
longer OS. These data are consistent with other previous studies
reporting that the shortest OS was observed among patients with
primary pancreatic and intestinal cancers (4,16,26).
There were several limitations to the present study,
which are related to the current legislation that states that it is
not mandatory for patients with NETs to be referred to the ION;
therefore, not all NET cases may have been registered. Moreover,
since cases originated from different geographical locations using
a wide range of diagnostic resources, the identification of cases
is more likely to occur in the main provinces with more complex,
developed healthcare infrastructures. Finally, during the process
of data collection and analysis, the World Health Organization 2010
classification (10) for NET tumors
was used instead of the 2019 guidelines (29), which may also limit our ability to
compare our data to recent publications that use the 2019
classification. This is due to the implementation of the new
guidelines after data collection for the present study had been
completed.
In conclusion, the incidence of NETs has been
reported to be increasing in the Republic of Panama. From an
epidemiological perspective, the present study found that NETs
behave in a similar manner compared with reports from other
countries. As the first nationwide NET database, the present study
aimed to further understand the characteristics of NETs in the
Republic of Panama, and provided important epidemiological findings
from patients and further OS data. These results may benefit the
Republic of Panama by providing evidence to peers and healthcare
authorities in order to allocate more financial resources to obtain
additional equipment and training to enable the early diagnosis of
NETs. The present study also highlights the strengths and
weaknesses of the healthcare system in the Republic of Panama, and
it is evident that the coordination between provinces in referring
NET cases should be improved throughout the country.
Supplementary Material
Primary tumor site and grading.
Acknowledgements
The authors would like to thank Dr Arturo Rebollon
(University of South Florida, Panamá, dr.arebollon@gmail.com) for
his contribution to the integration of the databases and the
generation of the overall survival plots.
Funding
The present study was funded by an educational grant from
Novartis© (grant no. 615268).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC and RV designed the study, acquired, analyzed and
interpreted the data and drafted the manuscript. JDMR analyzed and
interpreted the data and drafted the manuscript. IB, ET, YL, OEA,
HT, GP, MP and OC designed the study, acquired the data and drafted
the manuscript. MC and RV confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study proposal was submitted to the
Institutional Ethics Board Committee at the ION, and was granted
both national and institutional approval. The requirement for
informed patient consent was waived by the Institutional Ethics
Board Committee at ION.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao LL, Lu J, Lin JX, Zheng CH, Li P, Xie
JW, Wang JB, Chen QY, Lin M, Tu RH and Huang CM: Incidence and
survival trends for gastric neuroendocrine neoplasms: An analysis
of 3523 patients in the SEER database. Eur J Surg Oncol.
44:1628–1633. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chauhan A, Yu Q, Ray N, Farooqui Z, Huang
B, Durbin EB, Tucker T, Evers M, Arnold S and Anthony LB: Global
burden of neuroendocrine tumors and changing incidence in Kentucky.
Oncotarget. 9:19245–19254. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
United States. JAMA Oncol. 3:1335–1342. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fraenkel M, Faggiano A and Valk GD:
Epidemiology of neuroendocrine tumors. Front Horm Res. 44:1–23.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hallet J, Law CH, Cukier M, Saskin R, Liu
N and Singh S: Exploring the rising incidence of neuroendocrine
tumors: A population-based analysis of epidemiology, metastatic
presentation, and outcomes: Neuroendocrine tumor epidemiology.
Cancer. 121:589–597. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Basuroy R, Bouvier C, Ramage JK, Sissons
M, Kent A and Srirajaskanthan R: Presenting symptoms and delay in
diagnosis of gastrointestinal and pancreatic neuroendocrine
tumours. Neuroendocrinology. 107:42–49. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Basuroy R, Bouvier C, Ramage JK, Sissons M
and Srirajaskanthan R: Delays and routes to diagnosis of
neuroendocrine tumours. BMC Cancer. 18(1122)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
O'Connor JM, Marmissolle F, Bestani C,
Pesce V, Belli S, Dominichini E, Mendez G, Price P, Giacomi N,
Pairola A, et al: Observational study of patients with
gastroenteropancreatic and bronchial neuroendocrine tumors in
Argentina: Results from the large database of a multidisciplinary
group clinical multicenter study. Mol Clin Oncol. 2:673–684.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pinto MP, Muñoz Medel M, Carrillo D,
Retamal IN, Bravo ML, Valenzuela Y, Nervi B, Sánchez C, Galindo H,
Ibañez C, et al: Chilean registry for neuroendocrine tumors: A
Latin American perspective. Horm Cancer. 10:3–10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rindi G, Arnold R, Bosman FT, Capella C,
Klimstra DS, Klöppel G, Komminoth P and Solcia E: Nomenclature and
classification of neuroendocrine neoplasms of the digestive system.
In: WHO Classification of Tumours of the Digestive System. 4th
edition. WHO Press, Lyon, 2010.
|
|
11
|
Palepu J, Shrikhande SV, Bhaduri D, Shah
RC, Sirohi B, Chhabra V, Dhar P, Sastry R and Sikora S: Trends in
diagnosis of gastroenteropancreatic neuroendocrine tumors
(GEP-NETs) in India: A report of multicenter data from a web-based
registry. Indian J Gastroenterol. 36:445–451. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Maric M, de Haan E, Hogendoorn SM, Wolters
LH and Huizenga HM: Evaluating statistical and clinical
significance of intervention effects in single-case experimental
designs: An SPSS method to analyze univariate data. Behav Ther.
46:230–241. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
United Nations, Department of Economic and
Social Affairs, Population Division. World Population Prospects,
2019. [cited 2020 Dec 6]. Available from: https://population.un.org/wpp/.
|
|
14
|
Modlin IM, Oberg K, Chung DC, Jensen RT,
de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA,
Krenning EP, et al: Gastroenteropancreatic neuroendocrine tumours.
Lancet Oncol. 9:61–72. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Instituto Nacional de Estadística y
Censo-Panamá: Estimación de la población total en la República, por
provincia y comarca indígena, según sexo y grupos de edad: Al 1 de
julio de, 2018. Available from: https://www.inec.gob.pa/publicaciones/Default3.aspx?ID_PUBLICACION=990&ID_CATEGORIA=17&ID_SUBCATEGORIA=45.
|
|
16
|
Korse CM, Taal BG, van Velthuysen ML and
Visser O: Incidence and survival of neuroendocrine tumours in the
Netherlands according to histological grade: Experience of two
decades of cancer registry. Eur J Cancer. 49:1975–1983.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kourie HR, Ghorra C, Rassy M, Kesserouani
C and Kattan J: Digestive neuroendocrine tumor distribution and
characteristics according to the 2010 WHO classification: A single
institution experience in lebanon. Asian Pac J Cancer Prev.
17:2679–2681. 2016.PubMed/NCBI
|
|
18
|
Caplin ME, Baudin E, Ferolla P, Filosso P,
Garcia-Yuste M, Lim E, Oberg K, Pelosi G, Perren A, Rossi RE, et
al: Pulmonary neuroendocrine (carcinoid) tumors: European
neuroendocrine tumor society expert consensus and recommendations
for best practice for typical and atypical pulmonary carcinoids.
Ann Oncol. 26:1604–1620. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dias AR, Azevedo BC, Alban LBV, Yagi OK,
Ramos MFKP, Jacob CE, Barchi LC, Cecconello I, Ribeiro U Jr and
Zilberstein B: Gastric neuroendocrine tumor: Review and update. Arq
Bras Cir Dig. 30:150–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee L, Ito T and Jensen RT: Prognostic and
predictive factors on overall survival and surgical outcomes in
pancreatic neuroendocrine tumors: Recent advances and
controversies. Expert Rev Anticancer Ther. 19:1029–1050.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Al-Risi ES, Al-Essry FS and Mula-Abed WS:
Chromogranin A as a biochemical marker for neuroendocrine tumors: A
single center experience at royal hospital, Oman. Oman Med J.
32:365–370. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim JY and Hong SM: Recent updates on
neuroendocrine tumors from the gastrointestinal and
pancreatobiliary tracts. Arch Pathol Lab Med. 140:437–448.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jin M, Zhou X, Yearsley M and Frankel WL:
Liver metastases of neuroendocrine tumors rarely show overlapping
immunoprofile with hepatocellular carcinomas. Endocr Pathol.
27:253–258. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu E, Telem DA, Warner RR, Dikman A and
Divino CM: The role of Ki-67 in predicting biological behavior of
goblet cell carcinoid tumor in appendix. Am J Surg. 202:400–403.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
O'Toole D, Grossman A, Gross D, Delle Fave
G, Barkmanova J, O'Connor J, Pape UF and Plöckinger U: Mallorca
Consensus Conference participants; European Neuroendocrine Tumor
Society. ENETS consensus guidelines for the standards of care in
neuroendocrine tumors: Biochemical markers. Neuroendocrinology.
90:194–202. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT
and Chang JS: The epidemiology of neuroendocrine tumors in Taiwan:
A nation-wide cancer registry-based study. PLoS One.
8(e62487)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Edfeldt K, Björklund P, Åkerström G,
Westin G, Hellman P and Stålberg P: Different gene expression
profiles in metastasizing midgut carcinoid tumors. Endocr Relat
Cancer. 18:479–489. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Riihimäki M, Hemminki A, Sundquist K,
Sundquist J and Hemminki K: The epidemiology of metastases in
neuroendocrine tumors. Int J Cancer. 139:2679–2686. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Klimstra D, Kloppell G, La Rosa S and
Rindi G: Classification of neuroendocrine neoplasms of the
digestive system. In: WHO Classification of Tumours: Digestive
System Tumours. 5th edition. WHO Classification of Tumours
Editorial Board (Ed), International Agency for Research on Cancer,
Lyon, pp16, 2019.
|