Introduction
Galectin-1, an endogenous lectin found at
immune-privileged sites, has a critical role in the regulation of
the immune response in the tumor microenvironment (1). Wide varieties of biological phenomena
are related to galectins, i.e., development, differentiation,
morphogenesis, tumor metastasis, apoptosis, RNA splicing, and
immunoregulatory function (2,3). In
different cancer types, galectin-1 is abundantly expressed
(4,5) and is noted as one of the key players
in tumor-mediated immune escape (6). Intra-tumoral high galectin-1 protein
expression can be a poor prognostic biomarker in cancers given its
immuno-suppressive function (7).
Although galectin-1 protein in cancer tissues is an
immune-escape biomarker, its analysis in the serum may be
preferable in clinical practice (8,9). Serum
galectin-1 levels has been reported to associated with tumor
progression and poor prognosis in various types of cancer (10-12).
While, serum autoantibodies against various tumor antigens were
reported to be useful biomarkers for early detection and for
predicting cancer biology (13,14)
rather than serum tumor antigen. The prevalence of such
autoantibodies (15) should be a
fundamental data in designing clinical trials targeting tumor
antigens. Previously, we reported that serum galectin-1
autoantibody (s-Gal-1-Abs) is a useful biomarker in hepatocellular
carcinoma (16) which might be
associated with galectin-1 protein expression. However, there was
no data to compare positive rates of s-Gal-1-Abs in various cancer
types.
Therefore, in the present study, 1,833 patients with
seven different cancer types were evaluated for the presence of
s-GAL-1-Abs using the ELISA system. Such fundamental information of
s-GAL-1-Abs in a wide range of tumor types may be useful for
further clinical studies of immune-therapy or immune-diagnosis.
Materials and methods
Collection of sera
Before the onset of treatment, sera were obtained
from 1,833 patients at Chiba Cancer Center with different cancer
types involving the esophagus (n=172), stomach (n=317), large
intestine (n=262), liver (n=91), prostate (n=358), breast (n=364),
lung (n=269), and 72 healthy donors. Age and stages were shown in
Table I. Each serum sample was
centrifuged at 3,000 x g for 5 min, and the resulting supernatant
was stored at -80˚C until analyzed at Toho University. We avoided
repeated thawing and freezing of the samples. This study was
approved by the institutional ethics committee of the Chiba Cancer
Center (#21-26) and the Toho University School of Medicine
(#22-112, #22-047). Additionally, written informed consent was
obtained from all patients. Patient recruitment was conducted from
July 2008 to March 2010.
 | Table ICharacteristics of the patients
analyzed in the current study. |
Table I
Characteristics of the patients
analyzed in the current study.
| | Stage (n) |
|---|
| Type of cancer | Age, years (mean ±
SD) | I | II | III | IV |
|---|
| Esophageal
cancer | 68.3±7.7 | 33 | 38 | 57 | 44 |
| Gastric cancer | 67.0±10.6 | 193 | 60 | 21 | 43 |
| Colorectal
cancer | 63.4±10.2 | 108 | 75 | 41 | 38 |
| Hepatocellular
carcinoma | 65.2±9.0 | 14 | 40 | 22 | 15 |
| Prostate cancer | 68.5±7.1 | 26 | 225 | 60 | 47 |
| Breast cancer | 56.3±12.5 | 164 | 157 | 31 | 12 |
| Lung cancer | 66.7±8.6 | 98 | 64 | 36 | 71 |
| Healthy control | 37.5±9.3 | - | - | - | - |
Enzyme-linked immunosorbent assay to
detect s-Gal-1-Abs and other conventional tumor markers
Serum samples were analyzed using an enzyme-linked
immunosorbent assay, as previously described (16). Purified recombinant GAL-1 protein
was coated onto 96-well microtiter plates (Maxisorp; Nunc). The
absorbance was measured at 450 nm using a SUNRISE Microplate Reader
(Tecan Japan Co., Ltd.). Gal-1 signals were evaluated by
calculating the difference in absorbance between the wells
containing galectin-1 and phosphate-buffered saline. Since the
antibody titers are displayed in numerical value of absorbance,
there is no unit of the protein amount.
Statistical analyses
Fisher's exact (two-sided) probability test was used
to determine the differences between the two groups. All
statistical analyses were performed using EZR (Saitama Medical
Centre, Jichi Medical University; Saitama, Japan) (17), which is a graphical user interface
for R (The R Foundation for Statistical Computing; version 2.13.0).
A P-value <0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
In the present study, 1,833 patients with different
cancer types were evaluated for the presence of s-GAL-1-Abs.
Patients with HCC and Lung cancer demonstrated significantly higher
positive rates for s-GAL-1-Abs.
Serum titers of anti-galectin-1
antibodies
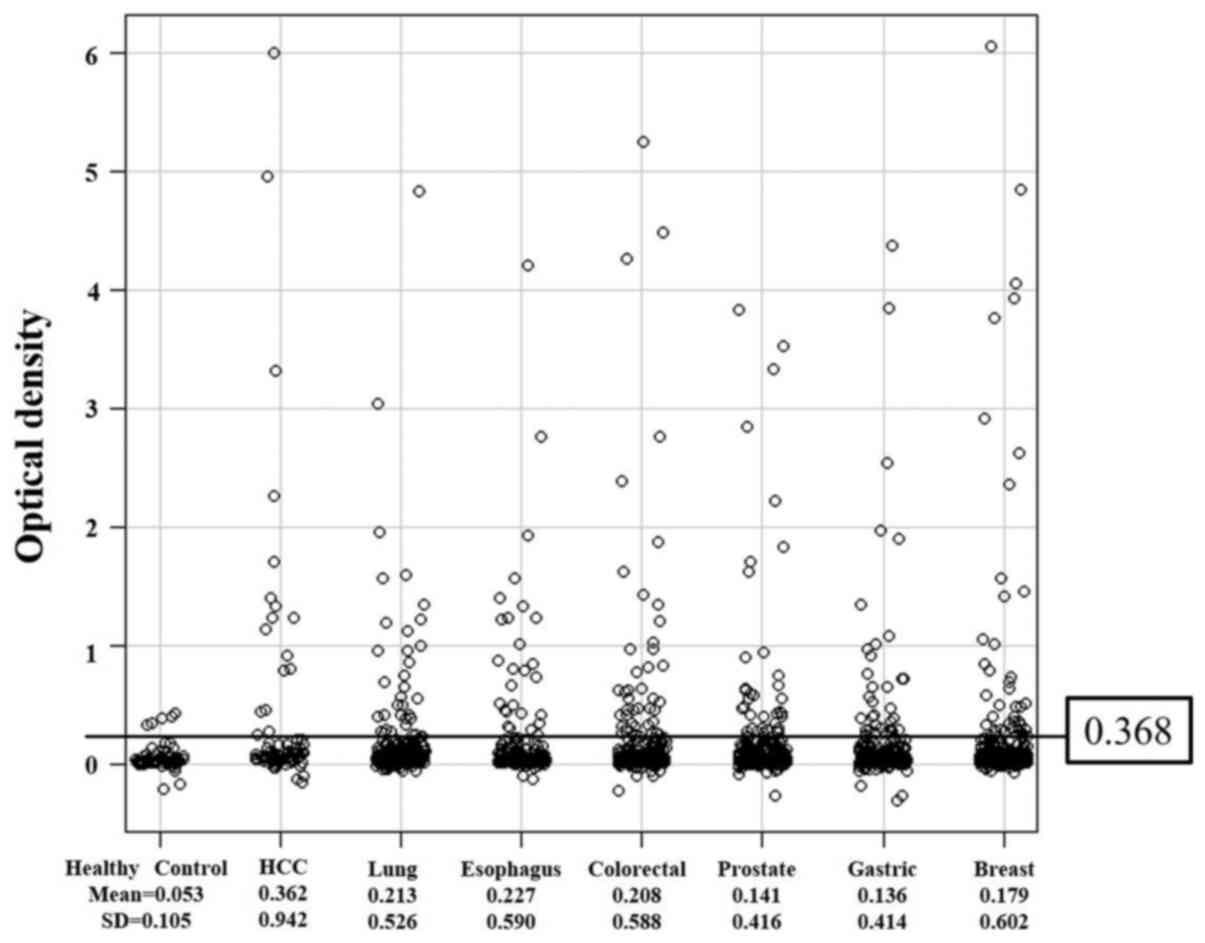
s-GAL-1-Abs levels were divided into two groups;
normal optical density (OD) values that were below the border level
of 0.368 (calculated as the mean (0.053) + 3 standard deviations
(0.105) of the values in healthy donors) and abnormal, or positive,
values that were higher than 0.368 (Fig. 1). Mean OD values for each cancer
type were as follows: 0.362 in HCC, 0.213 in lung cancer, 0.227 in
esophageal cancer, 0.208 in colorectal cancer, 0.141 in prostate
cancer, 0.136 in gastric cancer, and 0.179 in breast cancer.
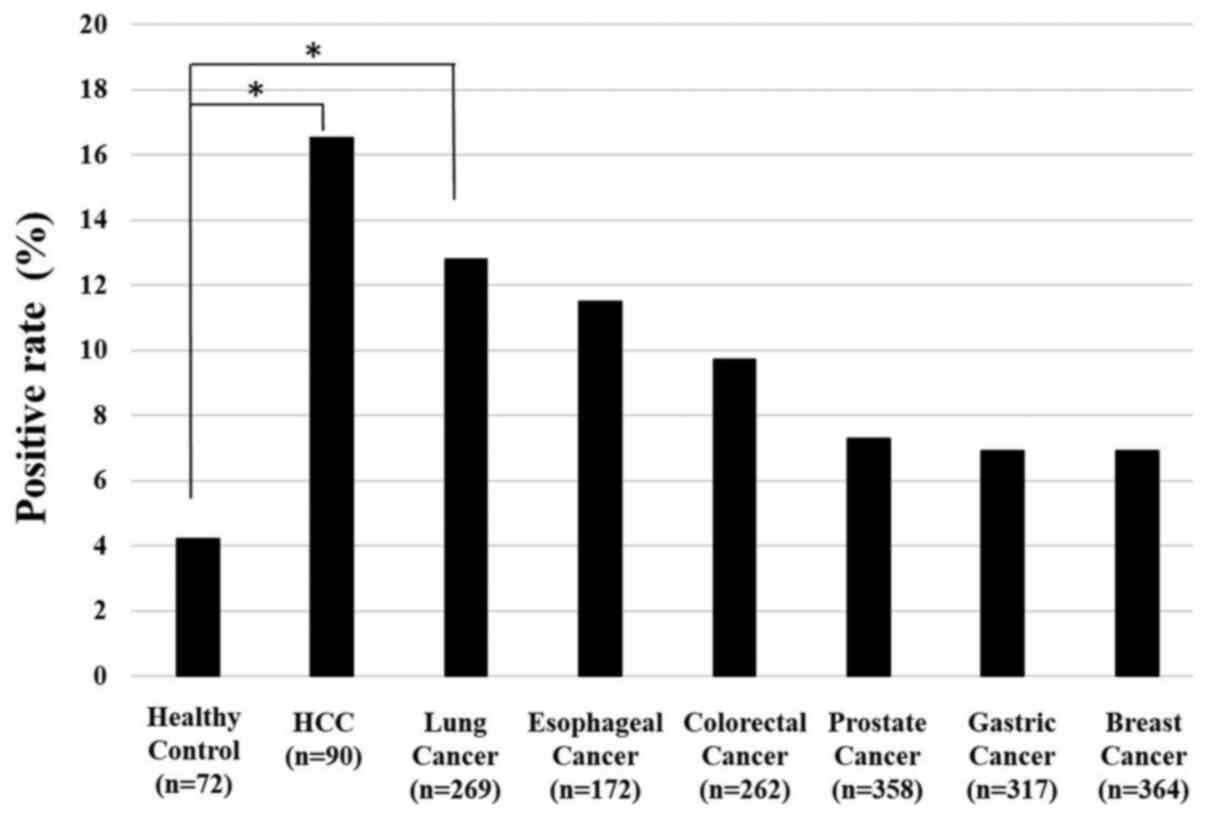
Positive rates of serum
anti-galectin-1 antibodies in various types of tumors
The positive rate of serum anti-GAL-1 antibodies
were greater than 10% in patients with HCC (16.7%), lung cancer
(13.8%), esophageal squamous cell carcinoma (11.6%), and colorectal
cancer (11.5%) (Fig. 2). Other
cancer types demonstrated similar positive rates of around 6 to 7%.
Although the overall positive rate of s-GAL-1-Abs was not higher
than that of conventional tumor markers (13,18-21),
it was found to be positive in various cancer types.
The differences of the positive rates of s-GAL-1-Abs
among various cancer types might be associated with the positive
rates of galectin-1 protein expression in the tumor tissues.
Actually, the positive rates of galectin-1 protein expression were
57% in HCC (22), 51.5% in lung
cancer (23), 30% in colorectal
cancer (24), and 60.6% in gastric
cancer (25). There were no reports
on esophageal cancer, prostate cancer, and breast cancer. Although
the information of protein expression was limited, there were no
association with the positive rate of serum autoantibody.
Unlike conventional secretory tumor markers,
autoantibody markers have been reported to be positive even in
early stage cancer. However, the positive rates of advanced stages
were not always higher than the positive rates of early stages. The
exact reason why such paradoxical positive rates was unknow but
potential immunosuppression in advanced stages might affect
antibody reaction (26-28).
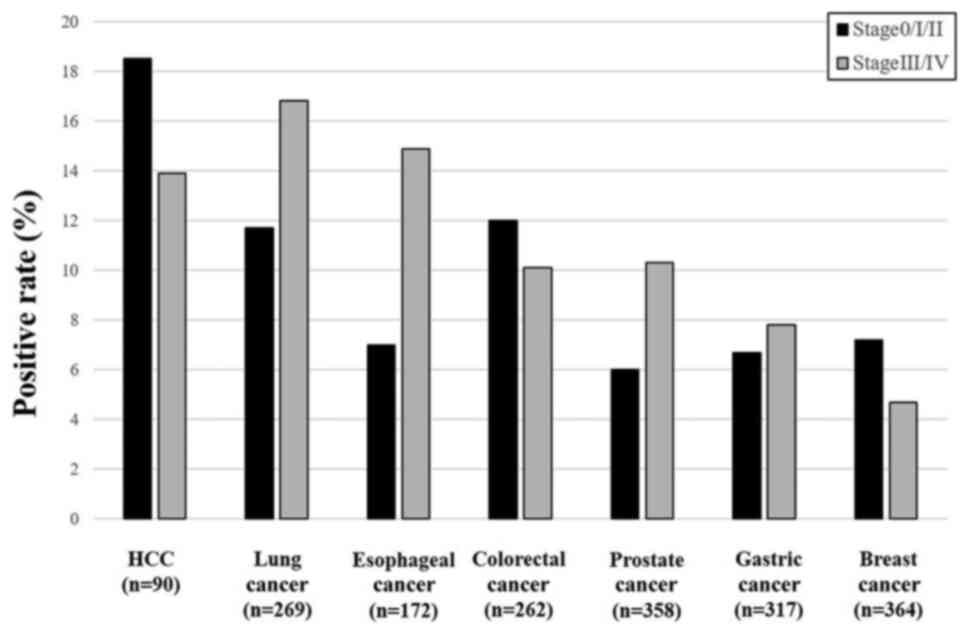
Comparison of positive rates of serum
anti-galectin-1 antibodies according to tumor stages
For the comparison of s-GLA-Abs according to the
stages, each tumor was divided into Stage 0/I/II and III/VI
(Fig. 3). HCC, colorectal cancer,
and breast cancer showed higher positive rates in stage 0/I/II than
in stage III/IV. Lung cancer, esophageal cancer, prostate cancer,
and gastric cancer showed higher positive rates in stage III/IV
than in stage 0/I/II.
Recent reports showed that galectin-1 targeting
therapy may have a potential role to modify anti-cancer immune
response (6,29). Galectin-1 vaccination or knockdown
therapy could be one of the options for s-GAL-1-Abs positive cancer
patients.
Figs. 2 and 3 showed just percentage of positive rates
in each cancer types. These percentages were calculated by dividing
the positive cases by all cases in Fig.
2. In Fig. 3, the percentages
were calculated by dividing the positive cases by total cases at
each stage.
In summary, the present study, of the 1,833 patients
with seven different cancer types, showed that HCC and lung cancer
patients showed relatively higher positive rates of s-GAL-1-Abs
than the other cancer types. Based on its low sensitivity, the
s-GAL-1-Abs may not be the first choice of a serum marker in other
cancers. Such fundamental information of s-GAL-1-Abs in a wide
range of tumor types could be a little help for further clinical
studies of immune-therapy or immune-diagnosis.
Limitations
Our present study did not include any survival data.
Further studies are required in prospective manner to evaluate the
prognostic significance and effects on treatment response of serum
Galectin-1autoantibodies.
Acknowledgements
The authors would like to acknowledge Ms. Seiko
Ohtsuka (Research Secretary of Toho University Hospital) for
sampling and preparing the database for this study.
Funding
The current study was supported by a Grant-in-Aid for Scientific
Research from the Ministry of Education, Culture, Sports, Science
and Technology of Japan (grant nos. 24591961 and 21591717).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TN, IH, MI and HS conceived and designed the current
study. SY, YO, TS, and FS analyzed the data. AK developed the ELISA
system. TN, TH and HS analyzed patient data and drafted the
manuscript. HS and TN confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the institutional
Ethics Committees of the Chiba Cancer Center (approval no. #21-26)
and the Toho University School of Medicine (approval nos. #22-112
and #22-047). Additionally, written informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
HS received a research grant from Medical &
Biological Laboratories Co., Ltd.. The other authors declare that
they have no competing interests.
References
|
1
|
Martínez-Bosch N and Navarro P: Galectins
in the tumor microenvironment: Focus on galectin-1. Adv Exp Med
Biol. 1259:17–38. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Elola MT, Chiesa ME, Alberti AF, Mordoh J
and Fink NE: Galectin-1 receptors in different cell types. J Biomed
Sci. 12:13–29. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thijssen VL, Heusschen R, Caers J and
Griffioen AW: Galectin expression in cancer diagnosis and
prognosis: A systematic review. Biochim Biophys Acta. 1855:235–247.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chung LY, Tang SJ, Sun GH, Chou TY, Yeh
TS, Yu SL and Sun KH: Galectin-1 promotes lung cancer progression
and chemoresistance by upregulating p38 MAPK, ERK, and
cyclooxygenase-2. Clin Cancer Res. 18:4037–4047. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Holst JM, Ludvigsen M, Hamilton-Dutoit SJ,
Bendix K, Plesner TL, Nørgaard P, Møller MB, Steiniche T,
Rabinovich GA, d'Amore F and Pedersen MB: High intra-tumoural
galectin-1 expression predicts adverse outcome in ALK-
ALCL and CD30+ PTCL-NOS. Hematol Oncol. 38:59–66.
2020.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Verschuere T, Toelen J, Maes W, Poirier F,
Boon L, Tousseyn T, Mathivet T, Gerhardt H, Mathieu V, Kiss R, et
al: Glioma-derived galectin-1 regulates innate and adaptive
antitumor immunity. Int J Cancer. 134:873–884. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gajiwala S, Torgeson A, Garrido-Laguna I,
Kinsey C and Lloyd S: Combination immunotherapy and radiation
therapy strategies for pancreatic cancer-targeting multiple steps
in the cancer immunity cycle. J Gastrointest Oncol. 9:1014–1026.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Petrovic S, Radosavljevic GD, Pantic J,
Jovanovic I, Jankovic N and Arsenijevic N: Circulating and tissue
galectin-1 and galectin-3 in colorectal carcinoma: Association with
clinicopathological parameters, serum CEA, IL-17 and IL23. J BUON.
21:941–949. 2016.PubMed/NCBI
|
|
9
|
Chen L, Yao Y, Sun L, Zhou J, Liu J, Wang
J, Li J and Tang J: Clinical implication of the serum galectin-1
expression in epithelial ovarian cancer patients. J Ovarian Res.
8(78)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen K, Cai Y, Zhang M, Wu Z and Wu Y:
Both serum and tissue galectin-1 levels are associated with adverse
clinical features in neuroblastoma. Pediatr Blood Cancer.
65(e27229)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gurel Cayir E, Demir L, Varol U, Atahan
MK, Salman T, Oflazoglu U, Yildiz Y, Taskaynatan H, Saray S,
Kucukzeybek Y, et al: Preliminary study of serum galectin-1 in
breast cancer carcinogenesis [Izmir Oncology Group (IZOG) study]. J
BUON. 25:675–680. 2020.PubMed/NCBI
|
|
12
|
Wu KL, Chen HH, Pen CT, Yeh WL, Huang EY,
Hsiao CC and Yang KD: Circulating galectin-1 and 90K/Mac-2BP
correlated with the tumor stages of patients with colorectal
cancer. Biomed Res Int. 2015(306964)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shimada H, Ochiai T and Nomura F: Japan
p53 Antibody Research Group. Titration of serum p53 antibodies in
1,085 patients with various types of malignant tumors: A
multi-institutional analysis by the Japan p53 antibody research
group. Cancer. 97:682–689. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shimada H, Takeda A, Arima M, Okazumi S,
Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T, et
al: Serum p53 antibody is a useful tumor marker in superficial
esophageal squamous cell carcinoma. Cancer. 89:1677–1683.
2000.PubMed/NCBI
|
|
15
|
Oshima Y, Shimada H, Yajima S, Nanami T,
Matsushita K, Nomura F, Kainuma O, Takiguchi N, Soda H, Ueda T, et
al: NY-ESO-1 autoantibody as a tumor-specific biomarker for
esophageal cancer: Screening in 1969 patients with various cancers.
J Gastroenterol. 51:30–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shiratori F, Shimada H, Nagata M, Kubota
Y, Otsuka Y and Kaneko H: Serum galectin-1 autoantibodies in
patients with hepatocellular carcinoma. Toho J Med. 2:67–72.
2016.
|
|
17
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ushigome M, Shimada H, Miura Y, Yoshida K,
Kaneko T, Koda T, Nagashima Y, Suzuki T, Kagami S and Funahashi K:
Changing pattern of tumor markers in recurrent colorectal cancer
patients before surgery to recurrence: Serum p53 antibodies, CA19-9
and CEA. Int J Clin Oncol. 25:622–632. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shimada H, Noie T, Ohashi M, Oba K and
Takahashi Y: Clinical significance of serum tumor markers for
gastric cancer: A systematic review of literature by the task force
of the Japanese gastric cancer association. Gastric Cancer.
17:26–33. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sengupta S and Parikh ND: Biomarker
development for hepatocellular carcinoma early detection: Current
and future perspectives. Hepat Oncol. 4:111–122. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nakamura H and Nishimura T: History,
molecular features, and clinical importance of conventional serum
biomarkers in lung cancer. Surg Today. 47:1037–1059.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yeh CC, Hsu CH, Shao YY, Ho WC, Tsai MH,
Freg WC and Chow LP: Integrated stable isotope labeling by
aminoacids in cell culture (SILAC) and isobaric tags for relative
and absolute quantitation (iTRAQ) quantitative proteomic analysis
identifies galectin-1 as a potential biomarker for predicting
sorafenib resistance in liver cancer. Mol Cell Proteomics.
14:1527–1545. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Carlini MJ, Roitman P, Nuñez M, Pallotta
MG, Boggio G, Smith D, Salatino M, Joffé ED, Rabinovich GA and
Puricelli LI: Clinical relevance of galectin-1 expression in
non-small cell lung cancer patients. Lung Cancer. 84:73–78.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nagy N, Legendre H, Engels O, André S,
Kaltner H, Wasano K, Zick Y, Pector JC, Decaestecker C, Gabius HJ,
et al: Refined prognostic evaluation in colon carcinoma using
immunohistochemical galectin fingerprinting. Cancer. 97:1849–1858.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
He XJ, Tao HQ, Hu ZM, Ma YY, Xu J, Wang
HJ, Xia YJ, Li l, Fei BY, Li YQ and Chen JZ: Expression of
galectin-1 in carcinoma-associated fibroblasts promotes gastric
cancer cell invasion through upregulation of integrin β1. Cancer
Sci. 105:1402–1410. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Okada R, Otsuka Y, Wakabayashi T, Shinoda
M, Aoki T, Murakami M, Arizumi S, Yamamoto M, Aramaki O, Takayama
T, et al: Six autoantibodies as potential serum biomarkers of
hepatocellular carcinoma: A prospective multicenter study. Int J
Cancer. 47:2578–2586. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Takashi S, Satoshi Y, Akihiko O, Naoya Y,
Yusuke T, Kentaro M, Yu O, Yasuaki N, Koichi Y, Takashi F, et al:
Clinical impact of preoperative serum p53 antibody titers in 1,487
patients with surgically treated esophageal squamous cell
carcinoma: A multi-institutional study. Esophagus. 18:65–71.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shimada H, Nagata M, Nabeya Y, Yajima S,
Oshima Y and Itami M: Paradoxical changing of serum p53 antibody
titers during chemotherapy for a stage IV esophageal squamous cell
carcinoma. Int Cancer Conf J. 3:232–236. 2014.
|
|
29
|
Van Woensel M, Mathivet T, Wauthoz N,
Rosière R, Garg AD, Agostinis P, Mathieu V, Kiss R, Lefranc F, Boon
L, et al: Sensitization of glioblastoma tumor micro-environment to
chemo- and immunotherapy by galectin-1 intranasal knock-down
strategy. Sci Rep. 7(1217)2017.PubMed/NCBI View Article : Google Scholar
|