Introduction
Prostate cancer (PC) is the second most commonly
diagnosed malignancy (excluding non-melanoma skin cancer) and the
fifth leading cause of cancer-associated mortality in men worldwide
in 2018(1). Overall, 1.28 million
men were diagnosed with PC (accounting for 15% of all cancer cases
in men) in 2018, with ~70% of cases (759,000) occurring in
developed countries, including the United States of America,
Australia, New Zealand and Europe (1). In current medical practice, prognostic
markers for PC include serum prostate-specific antigen (PSA)
levels, tumour Gleason score (GS) and clinical tumour grading
(2). Predictive accuracy may be
improved by introducing better biomarkers into clinical practice
(3). Previously, molecular
biomarkers such as TNF-α, IL-1, IL-6, cyclin E and
metallothionein-2A, have been evaluated for their efficiency in
predicting disease progression, response to therapy and survival in
patients with PC (4-7).
Certain pro-inflammatory cytokines, including IL-6,
TNF-α, IL-1 and IL-17, serve an essential role in radiotherapy (RT)
resistance and enable tumour progression, invasion and angiogenesis
(7-9).
Cytokines are water-soluble, low molecular weight proteins that
transport signals between cells (10). Rubin et al (11) were among the first to describe the
role of cytokines in mediating RT-induced toxicity: They reported
that levels TGF-β, IL-1 and TNF-α increase immediately following RT
exposure and that elevated TGF-β levels are associated with
increased risk of pulmonary fibrosis (11). Christensen et al (12) reported that interferon-γ (IFN-γ) and
IL-6 levels are significantly increased during prostate RT and are
associated with increased acute gastrointestinal and genitourinary
toxicity.
In addition to higher serum PSA levels and other
preliminary assessments, histopathological investigations of PC in
needle biopsy specimens predict tumour behaviour and assist with
therapeutic decision-making (13).
In clinical practice, the pathology report of PC includes the grade
of tissue differentiation according to GS and a quantitative
assessment of tumour volume per biopsy in either length in mm or
percentage of a tumour (14,15).
GS is based on the histological pattern of arrangement of carcinoma
cells in hematoxylin-stained prostatic tissue (16). The final GS is obtained by summing
of pattern-numbers of the primary and secondary tissue grade,
ranging from 2 to 10(16). GS
quantifies pathological aggressiveness and is also one of the key
factors in treatment decision-making, together with TNM staging,
age and pre-treatment blood PSA levels (17). However, histological examination has
several limitations, such as morphological mimics of prostate
carcinoma, including adenosis (a non-cancerous condition), atypical
adenomatous hyperplasia and very low- or high-grade carcinoma,
which hinder the interpretation of tumour biopsy (13,18).
The present clinical study evaluated expression
levels of pro-inflammatory TNF-α and IL-6 and anti-inflammatory
TGF-β1 in prostatic needle biopsy and blood plasma specimens. The
study also aimed to analyse the correlation between
pro-inflammatory TNF-α and IL-6 and TGF-β1 expression levels with
GS, pre-operative serum PSA and pre-RT plasma cytokine levels.
Materials and methods
Patients and clinical data
Between July 2015 and April 2016, a total of 18 male
patients with PC were recruited at Alan Walker Cancer Care Centre
(Darwin, Australia) for this prospective clinical study. Eligible
patients were ≥18 years old, had histologically confirmed prostate
adenocarcinoma and Eastern Cooperative Oncology Group performance
status of 0 to 1 and had not received prior prostate surgery.
Exclusion criteria included metastatic disease at presentation,
prior history of malignancy (excluding non-melanoma skin cancer)
and serious illness precluding safe administration of RT. These
patients were naïve to RT or androgen deprivation therapy (ADT)
before transrectal ultrasound (TRUS) biopsy and clinical data were
collected. All PC cases were classified into as follows:
Low-[clinical (c)T stage ≤2a; PSA<10 ng/ml; GS≤6];
intermediate-(cT=2b; PSA, 10-20 ng/ml; GS=7) and high-risk (cT≥2c;
PSA>20 ng/ml; GS=8-10) (2). The
present study was approved by the Human Research and Ethics
Committee of the Northern Territory (approval no. 2015-2385)
Department of Health and Menzies School of Health Research. Written
informed consent was obtained from all participants to provide
access to prostate tissue biopsies, blood samples collected at
various time intervals before, during and after therapy and medical
and pathology records from Royal Darwin Hospital and Alan Walker
Cancer Care Centre.
Immunohistochemistry (IHC)
staining
Tissue samples were fixed in 10% formalin overnight
at room temperature before being embedded in paraffin. The tissue
was sectioned to 4 µm and mounted on poly-lysine-coated slides
(Dako; Agilent Technologies, Inc.). All tissue sections were stored
in a 50˚C water bath. Slides were dried for 30 min in a thermostat
at 60˚C. All sections were deparaffinised using xylene and
subsequently rehydrated with a series of graded ethanol dilutions.
Then, antigen retrieval was performed by placing slides in a Coplin
jar with target retrieval solution (Dako; Agilent Technologies,
Inc.; pH, 9.0) for 20 min at 90-95˚C in a hot water bath. All
sections were marked using a Dako PEP pen (Agilent Technologies,
Inc.) for accuracy.
Sections were incubated in methanol containing 3%
hydrogen peroxide for 30 min at room temperature and washed twice
(3 min/wash) with TBS washing buffer. Goat serum (Dako; Agilent
Technologies, Inc.) was applied to all sections and incubated at
room temperature for 10 min. Primary antibodies (Novus Biologicals,
LLC) were used to determine expression levels of pro-inflammatory
TNF-α and IL-6 and TGF-β1 in tumour biopsy samples from patients
with PC. All tissues were incubated at room temperature for 1 h
using the following primary antibodies: Anti-mouse monoclonal TNF-α
(1:50; cat. no. NB600-1422) and TGF-β1 (1:100; cat. no.
NBP2-22114SS) and anti-rabbit polyclonal IL-6 (1:100; cat. no.
NB600-1131SS). Antibody diluent was substituted with primary
antibody for negative control sections (Dako; Agilent Technologies,
Inc.). All tissue sections were rinsed in TBS as aforementioned.
Then, 3-4 drops of secondary antibody (REAL Link-biotinylated
secondary Ab2; cat. no. K5001; Dako; Agilent Technologies, Inc.)
were applied to all tissue sections and incubated for 10 min at
room temperature. All tissue sections were rinsed twice with TBS
then incubated at room temperature with horseradish
peroxidase-conjugated streptavidin for 10 min by adding 3-4 drops
to the slides (Dako; Agilent Technologies, Inc.). Finally, all
sections were developed with 3'-diaminobenzidine for 5 min at room
temperature and counterstained with Mayer's haematoxylin at room
temperature for another 2 min. All tissue sections were dehydrated
via a graded series of ethanol dilutions and washed with xylene.
After staining, coverslips were applied and sealed using permanent
mounting medium.
Microscopic analysis
IHC-stained slides were evaluated for expression of
pro-inflammatory TNF-α and IL-6 and TGF-β1 by light microscopy
(magnification, x20) in a blinded manner by two clinical pathology
consultants. Expression levels of pro-inflammatory TNF-α and IL-6
and TGF-β1 were evaluated using a semi-quantitative scale based on
the proportion of positive-stained cells as follows: -, <10; +,
10-50; ++, 51-80; +++, >80% (6,19,20).
ELISA
Levels of pro-inflammatory TNF-α and IL-6 and TGF-β1
in pre-RT plasma were assessed. For plasma cytokine analysis, ELISA
kits were used including human TNF-α (cat. no. KAC1751), TGF-β1
(cat. no. EHTGFBI), IL-6 (cat. no. KAC1261) and IL-8 (cat. no.
KAC1301; all Thermo Fisher Scientific, Inc.). Assay kits were
chromogen-based and cytokine concentration (colour) was quantified
using a Titertek Multiskan MCC/340 plate reader according to the
manufacturer's instructions. Each assay was calibrated against a
standard curve with a full range predetermined for each cytokine
and sample source.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 7 software (GraphPad Software, Inc.). Established clinical
variables included in the study were age, pre-operative PSA, risk
stratification, c and pathological (p)TNM stage and GS. Data are
presented as the mean ± SD (n=18). Spearman's correlation test was
performed to assess the correlation between pro-inflammatory TNF-α
and IL-6 and anti-inflammatory TGF-β1 expression levels and the
aforementioned variables. Spearman's correlation test was also used
to determine the linear correlation between cytokine expression
levels in pre-RT plasma and corresponding prostatic needle biopsy
specimens. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics of patients
with PC
The clinicopathological information of participants,
such as age, pre-operative PSA levels, risk stratification, c and
pTNM stage, and GS, are summarised in Table I.
 | Table IClinicopathological characteristics
of patients with prostate cancer. |
Table I
Clinicopathological characteristics
of patients with prostate cancer.
| Characteristic | N | Percentage, % |
|---|
| Age, years (mean ±
SD) | 66.83±7.93
(53.00-80.00) | |
| Pre-operative PSA,
ng/ml | | |
|
<10 | 6.00 | 33 |
|
10-20 | 9.00 | 50 |
|
>20 | 3.00 | 17 |
| Risk
stratification | | |
|
Low | 0.00 | 0 |
|
Intermediate | 5.00 | 28 |
|
High | 13.00 | 72 |
| cTNM stage | | |
|
T1a-cN0M0 | 4.00 | 23 |
|
T2a-cN0M0 | 6.00 | 33 |
|
T3a-cN0M0 | 8.00 | 44 |
| pTNM stage | | |
|
T1a-cN0M0 | 0.00 | 0 |
|
T2a-cN0M0 | 16.00 | 89 |
|
T3a-cN0M0 | 2.00 | 11 |
| Gleason score | | |
|
6 | 1.00 | 6 |
|
7 | 8.00 | 44 |
|
8-10 | 9.00 | 50 |
The mean age of patients with PC at the time of
diagnosis was 66.83±7.93 years (range, 53.00-80.00 years). The mean
pre-operative serum PSA levels were 16.03±15.81 ng/ml (range,
4.00-71.00 ng/ml); 33% of patients (6/18) exhibited PSA<10.00,
50% (9/18) exhibited 10.00-20.00 ng/ml PSA and 17% (3/18) exhibited
PSA>20.00 ng/ml. The mean GS was 7.88±1.14 (range, 6.00-10.00);
6% of patients (1/18) exhibited GS=6.00, 44% (8/19) exhibited
GS=7.00 and 50% (9/18) exhibited GS=8.00-10.00. Tumour staging was
divided into c and pT stage. For cT stage, 23% of patients (4/18)
were T1a-c, 33% (6/18) were T2a-c and 44% (8/18) were T3a-c. For pT
stage, 0% of patients (0/18) were T1a-c, 89% (16/18) were T2a-c and
11% (2/18) were T3a-c. Only one patient (6%) exhibited one
ipsilateral pelvic node involved before therapy.
Cytokine expression in prostatic
needle biopsy specimens
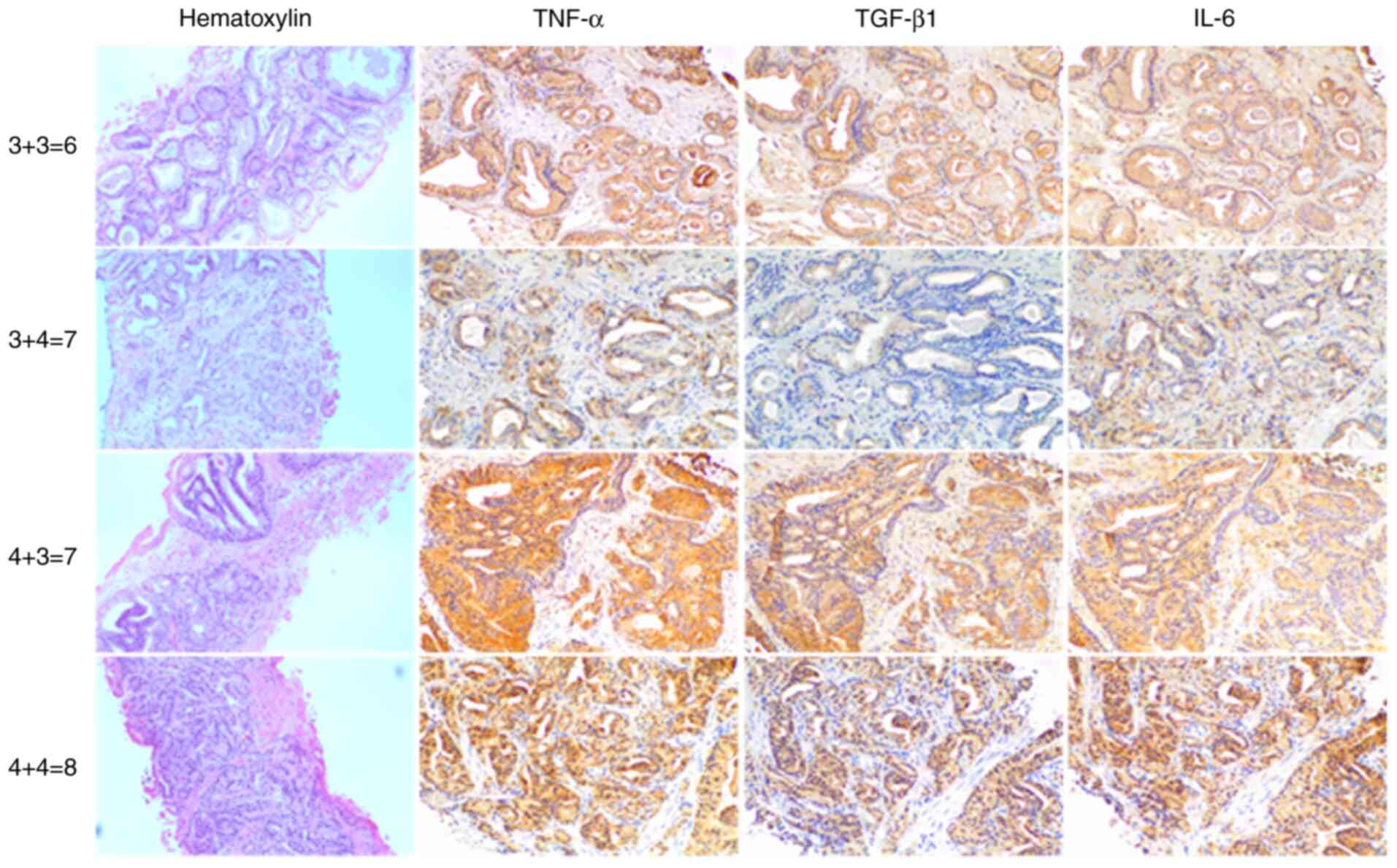
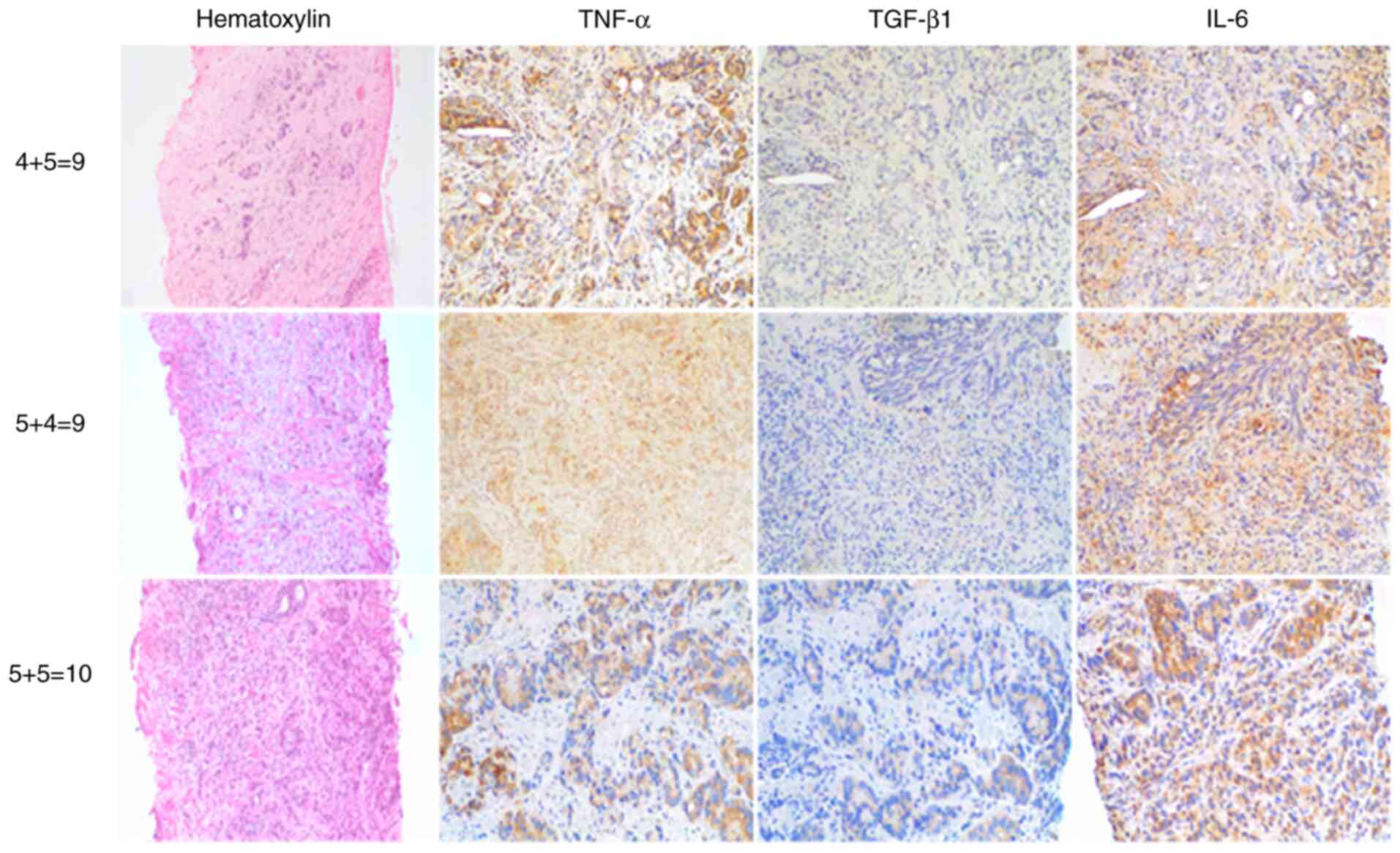
IHC staining for pro-inflammatory TNF-α, IL-6 and
anti-inflamatory TGF-β1 revealed elevated levels of these cytokines
in most tumour tissue samples compared with healthy tissue
(Figs. 1 and 2). Malignant prostate cells exhibited
brown cytoplasmic staining, indicating expression of
pro-inflammatory TNF-α and IL-6 and TGF-β1 in prostatic needle
biopsy specimens from patients with PC.
Correlation between cytokine
expression and pre-operative serum PSA levels
Serum PSA is used as a guide to initiate prostatic
biopsies and to monitor men older than 50 years for PC (21). Serum PSA level is the most commonly
used tumour biomarker for PC There was no correlation between
expression levels of pro-inflammatory TNF-α and IL-6 and
anti-inflammatory TGF-β1 and pre-operative serum PSA levels (data
not shown).
Correlation between cytokine
expression levels and GS
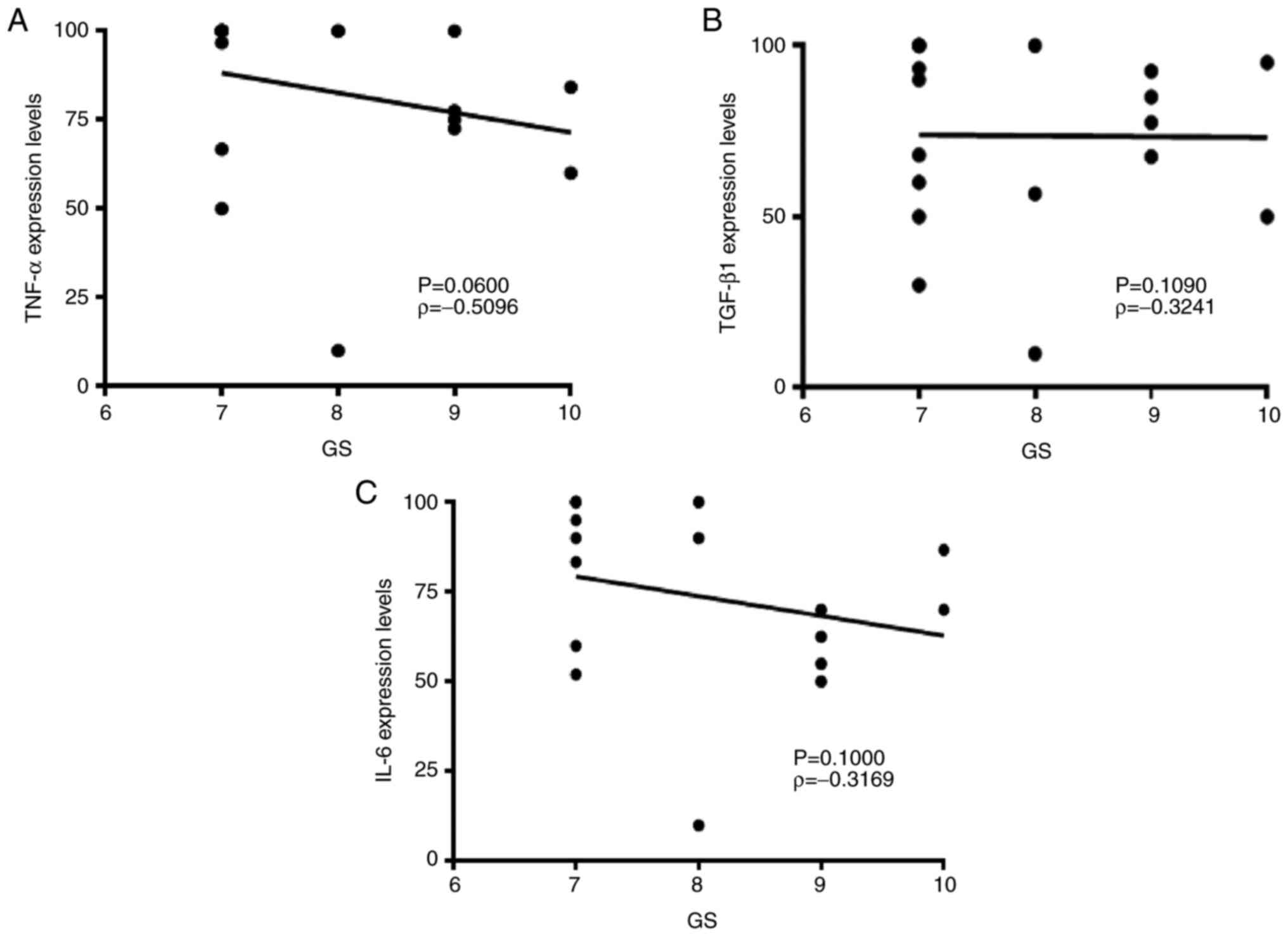
Spearman's correlation test was performed to assess
the association between cytokine expression levels and GS. GS
ranges from 1-5 and describes how much cancer from a biopsy
resembles healthy (lower score) or abnormal tissue (higher score).
Most cancers score ≥3 in anatomical pathology practice depending on
aggressiveness (22). Figs. 1 and 2 show H&E and IHC staining in biopsy
samples with GS as follows: 3+3=6, 3+4=7, 4+3=7, 4+4=8, 4+5=9,
5+4=9 and 5+5=10. Lower expression levels of pro-inflammatory TNF-α
and IL-6 were associated with high GS; however, no statistically
significant association was found (TNF-α, ρ=-0.5096; IL-6,
ρ=-0.3169; Fig. 3).
Anti-inflammatory TGF-β did not show any association with GS
(ρ=-0.3241).
Correlation between cytokine
expression levels in biopsy samples and pre-RT plasma
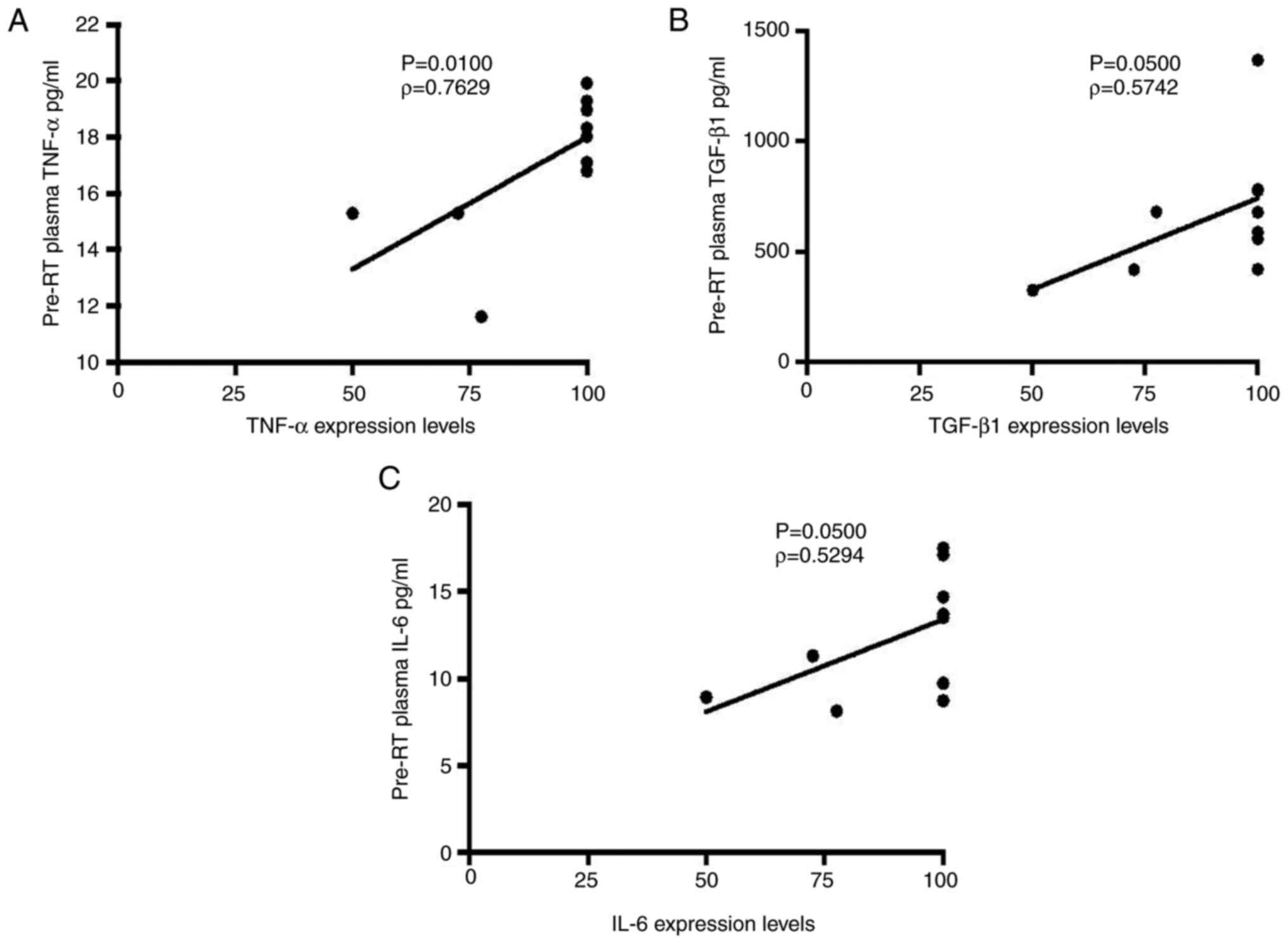
The present study evaluated the potential impact of
tumour-derived cytokine production on circulating plasma levels.
IHC expression levels of pro-inflammatory TNF-α and IL-6 and
anti-inflammatory TGF-β1 were increased in prostatic needle-biopsy
specimens with increased pre-RT plasma cytokines levels detected by
ELISA (Fig. 4). A statistically
significant association was found between staining intensity of
proinflammatory TNF-α and IL-6 and anti-inflammatory TGF-β1 in
prostatic needle biopsy specimens and concentration in pre-RT
plasma (TNF-α, ρ=0.7629; TGF-β1, ρ=0.5742; IL-6, ρ=0.5294; Fig. 4).
Discussion
Histopathological analysis and GS can predict
outcomes of PC (23). A number of
clinical studies have reported the significance of novel biomarkers
that may be used in future as predictors of prognosis and tumour
development (24,25). Numerous biomarkers, such as
cytokines, hormone receptors, oncogenes and tumour suppressor
genes, are well-established in clinical scientific literature
(26). The role of pro-inflammatory
cytokines, including TNF-α, IL-1 or IL-6, in cancer development has
been established in PC (26,27).
The primary clinical challenge in PC is the lack of
diagnostic tests, including PSA screening and histopathological
grading, to differentiate between aggressive and indolent tumours
(28). PSA is present in normal
prostatic secretions and its levels are often elevated in patients
with PC (29,30). Rodriguez-Berriguete et al
demonstrated an association between elevated stromal expression of
IL-1 receptor-associated kinase 1 (IRAK-1) and high pre-operative
serum PSA levels. IL-1β expression in PC tumours and IL-1 receptor,
type II and IRAK-1 expression levels in tumour stroma have
prognostic value after adjusting for the effects of pT stage, GS
and total pre-operative serum PSA (6). There is a significant association
between positive p27 expression and lower mean serum PSA levels
(P=0.091) (31). Shariat et
al (32) reported that
pre-treatment serum levels of TGF-β1, IL-6 and soluble IL-6
receptor levels are positively correlated with pre-operative PSA
levels (P=0.004, P<0.001 and P=0.011, respectively). Also,
patients with elevated expression of IL-1α exhibit higher serum PSA
levels (>20 ng/ml) (33). In the
present clinical study, no association between expression levels of
pro-inflammatory TNF-α and IL-6 and TGF-β1 in prostatic needle
biopsy specimens and pre-operative serum PSA levels was
detected.
GS histopathological grading is an important
prognostic indicator of PC (34,35).
GS quantifies pathological aggressiveness of PC and is one of the
principal factors in treatment decision-making, along with TNM
stage, age and presenting PSA levels. GS of 8-10 represents a
clinically aggressive form of the disease and is used to classify
patients as high-risk (36).
High-grade cancer poses increased risk of biochemical, locoregional
and distant recurrence with subsequent detrimental effects on
overall survival (36). Michalaki
et al (37) demonstrated
that serum levels of IL-6 are significantly higher in patients with
metastatic disease and GS>6. Another clinical study reported
that elevated levels of IL-6 are associated with GS≥7 and
metastases in regional lymph nodes (32). Gomes et al (38) reported that high six-transmembrane
epithelial antigen of the prostate 1 (STEAP1) expression is
significantly associated with GS=7-9; patients with higher GS
(7-9)
exhibited elevated STEAP1 expression, whereas those with lower GS
(5-6) showed moderate STEAP1 expression. The
data in current study are opposite to those of the aforementioned
studies: We identified lower expression levels of pro-inflammatory
TNF-α and IL-6 were associated with high GS, however, this was not
statistically significant.
The present clinical study also evaluated
pro-inflammatory TNF-α and IL-6 and TGF-β1 plasma cytokine levels
in patients with PC. Rube et al (39) reported a statistically significant
correlation between pre-RT plasma IL-6 and TGF-β1 cytokine levels
and staining intensity of corresponding tumour biopsy. The present
study revealed elevated levels of pro-inflammatory TNF-α and IL-6
and TGF-β1 in prostatic needle biopsy specimens of patients with
increased pre-RT plasma cytokine levels using ELISA. Furthermore, a
statistically significant correlation was detected between IHC
staining intensity of pro-inflammatory TNF-α and IL-6 and TGF-β1 in
prostatic needle biopsy specimens and expression levels in pre-RT
plasma (TNF-α, P=0.01; TGF-β1, P=0.05 and IL-6, P=0.05). Our
previous study demonstrated pre-RT plasma cytokine expression
levels in patients with PC. Further experiments will investigate
correlation between cytokine expression in prostatic needle biopsy
specimens and concentration in pre-RT plasma (40).
The present clinical study identified a correlation
between cytokine expression levels in biopsy samples with GS and
pre-RT plasma cytokine levels. However, a statistically significant
difference was only found between pre-RT plasma and biopsy sample
cytokine levels. Further clinical studies are required to validate
these findings and identify biomarkers in the clinical setting to
predict patient outcomes and improve treatment success.
Acknowledgements
The authors would like to thank Ms Beryl Edward
(Anatomical Pathology Department) and Ms Ruby Hilario (Royal Darwin
Hospital) for assistance with tissue selection and sectioning for
IHC analysis and clinical data collection.
Funding
The present clinical study was supported by grants from the
College of Health and Human Sciences, Charles Darwin University,
Northern Territory, Australia.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JS, SSS, TT, HD and PDI designed the study,
collected and analysed patient data and wrote the manuscript. JS,
TT, HD and PDI recruited patients and collected blood and tissue
samples. AL, WS and RR interpreted and graded the IHC images. MSE
and SSS statistically analysed experimental data. All authors read
and approved the final manuscript. JS and SSS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Human Research
and Ethics Committee of the Northern Territory (approval no.
2015-2385) and Department of Health and Menzies School of Health
Research. Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mattiuzzi C and Lippi G: Current cancer
epidemiology. J Epidemiol Glob Health. 9:217–222. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
D'Amico AV, Whittington R, Malkowicz SB,
Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA,
Kaplan I, Beard CJ and Wein A: Biochemical outcome after radical
prostatectomy, external beam radiation therapy, or interstitial
radiation therapy for clinically localized prostate cancer. JAMA.
280:969–974. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Burke HB: Predicting clinical outcomes
using molecular biomarkers. Biomark Cancer. 8:89–99.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Milicevic N, Mrcela M, Galic J and
Marjanovic K: Expression of proinflammatory cytokine interleukin-6
in tissue samples of human prostate obtained by needle biopsy.
Pathol Res Pract. 211:865–870. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ma D, Zhou Z, Yang B, He Q, Zhang Q and
Zhang XH: Association of molecular biomarkers expression with
biochemical recurrence in prostate cancer through tissue microarray
immunostaining. Oncol Lett. 10:2185–2191. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rodriguez-Berriguete G, Sanchez-Espiridion
B, Cansino JR, Olmedilla G, Martinez-Onsurbe P, Sanchez-Chapado M,
Paniagua R, Fraile B and Royuela M: Clinical significance of both
tumor and stromal expression of components of the IL-1 and TNF-α
signaling pathways in prostate cancer. Cytokine. 64:555–563.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Deorukhkar A and Krishnan S: Targeting
inflammatory pathways for tumor radiosensitization. Biochem
Pharmacol. 80:1904–1914. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Steiner GE, Newman ME, Paikl D, Stix U,
Memaran-Dagda N, Lee C and Marberger MJ: Expression and function of
pro-inflammatory interleukin IL-17 and IL-17 receptor in normal,
benign hyperplastic, and malignant prostate. Prostate. 56:171–182.
2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Poutahidis T, Rao VP, Olipitz W, Taylor
CL, Jackson EA, Levkovich T, Lee CW, Fox JG, Ge Z and Erdman SE:
CD4+ lymphocytes modulate prostate cancer progression in
mice. Int J Cancer. 125:868–878. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
George DJ, Halabi S, Shepard TF, Sanford
B, Vogelzang NJ, Small EJ and Kantoff PW: The prognostic
significance of plasma interleukin-6 levels in patients with
metastatic hormone-refractory prostate cancer: Results from cancer
and leukemia group B 9480. Clin Cancer Res. 11:1815–1820.
2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rubin P, Johnston CJ, Williams JP,
McDonald S and Finkelstein JN: A perpetual cascade of cytokines
postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol
Biol Phys. 33:99–109. 1995.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Christensen E, Pintilie M, Evans KR,
Lenarduzzi M, Menard C, Catton CN, Diamandis EP and Bristow RG:
Longitudinal cytokine expression during IMRT for prostate cancer
and acute treatment toxicity. Clin Cancer Res. 15:5576–83.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hoogland AM, Kweldam CF and van Leenders
GJ: Prognostic histopathological and molecular markers on prostate
cancer needle-biopsies: A review. Biomed Res Int.
2014(341324)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: ISUP Grading Committee. The 2005 International society
of urological pathology (ISUP) consensus conference on gleason
grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Van der Kwast T, Bubendorf L, Mazerolles
C, Raspollini MR, Van Leenders GJ, Pihl CG and Kujala P: Guidelines
on processing and reporting of prostate biopsies: The 2013 update
of the pathology committee of the European randomized study of
screening for prostate cancer (ERSPC). Virchows Arch. 463:367–377.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gleason DF: Histologic grading of prostate
cancer: A perspective. Hum Pathol. 23:273–279. 1992.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tagai EK, Miller SM, Kutikov A, Diefenbach
MA, Gor RA, Al-Saleem T, Chen DYT, Fleszar S and Roy G: Prostate
cancer patients' understanding of the gleason scoring system:
Implications for shared decision-making. J Cancer Educ. 34:441–445.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Epstein JI, Feng Z, Trock BJ and
Pierorazio PM: Upgrading and downgrading of prostate cancer from
biopsy to radical prostatectomy: Incidence and predictive factors
using the modified Gleason grading system and factoring in tertiary
grades. Eur Urol. 61:1019–1024. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zimmermann AK, Camenisch U, Rechsteiner
MP, Bode-Lesniewska B and Rossle M: Value of immunohistochemistry
in the detection of BRAF(V600E) mutations in fine-needle aspiration
biopsies of papillary thyroid carcinoma. Cancer Cytopathol.
122:48–58. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kapoor P and Deshmukh R: VEGF: A critical
driver for angiogenesis and subsequent tumor growth: An IHC study.
J Oral Maxillofac Pathol. 16:330–337. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu YS, Wu XB, Zhang N, Jiang GL, Yu Y,
Tong SJ, Jiang HW, Mao SH, Na R and Ding Q: Evaluation of PSA-age
volume score in predicting prostate cancer in Chinese population.
Asian J Androl. 20:324–329. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pessoa R, Werahera PN and Kim FJ: Chapter
102-Prostate Cancer. In Abernathy's Surgical Secrets, 7th edition.
Harken AH and Moore EE (eds). Elsevier, Philadelphia, PA,
pp450-451, 2018.
|
|
23
|
Kır G, Seneldir H and Gumus E: Outcomes of
Gleason score 3+4=7 prostate cancer with minimal amounts (<6%)
vs. ≥6% of Gleason pattern 4 tissue in needle biopsy specimens. Ann
Diagn Pathol. 20:48–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Freedland SJ and Moul JW: Prostate
specific antigen recurrence after definitive therapy. J Urol.
177:1985–1991. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Doganavsargil B, Simsir A, Boyacioglu H,
Cal C and Hekimgil M: A comparison of p21 and p27 immunoexpression
in benign glands, prostatic intraepithelial neoplasia and prostate
adenocarcinoma. BJU Int. 97:644–648. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pfitzenmaier J, Vessella R, Higano CS,
Noteboom JL, Wallace D Jr and Corey E: Elevation of cytokine levels
in cachectic patients with prostate carcinoma. Cancer.
97:1211–1216. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mauri D, Pentheroudakis G, Tolis C,
Chojnacka M and Pavlidis N: Inflammatory prostate cancer: An
underestimated paraneoplastic clinical manifestation. Urol Oncol.
23:318–322. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Romero Otero J, Garcia Gomez B, Campos
Juanatey F and Touijer KA: Prostate cancer biomarkers: An update.
Urol Oncol. 32:252–260. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu D, Ni J, Beretov J, Cozzi P, Willcox M,
Wasinger V, Walsh B, Graham P and Li Y: Urinary biomarkers in
prostate cancer detection and monitoring progression. Crit Rev
Oncol Hematol. 118:15–26. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lilja H, Ulmert D and Vickers AJ:
Prostate-specific antigen and prostate cancer: Prediction,
detection and monitoring. Nat Rev Cancer. 8:268–278.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Nassif AE and Tambara Filho R:
Immunohistochemistry expression of tumor markers CD34 and P27 as a
prognostic factor of clinically localized prostate adenocarcinoma
after radical prostatectomy. Rev Col Bras Cir. 37:338–344.
2010.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
32
|
Shariat SF, Kattan MW, Traxel E, Andrews
B, Zhu K, Wheeler TM and Slawin KM: Association of pre- and
postoperative plasma levels of transforming growth factor beta(1)
and interleukin 6 and its soluble receptor with prostate cancer
progression. Clin Cancer Res. 10:1992–1999. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cansino Alcaide JR, Vera San Martin R,
Rodriguez de Bethencourt Codes F, Bouraoui Y, Rodriguez Berriguete
G, Oueslati R, Perez-Utrilla M, De la Pena Barthel J, Paniagua
Gomez-Alvarez R and Royuela Garcia M: Prostatic specific antigen
(PS), pro-inflammatory cytokines, and prostatic pathology (benign
prostatic hyperplasia and cancer). Relationship with malignancy.
Arch Esp Urol. 62:359–366. 2009.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
34
|
Epstein JI: An update of the Gleason
grading system. J Urol. 183:433–440. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Shen MM and Abate-Shen C: Molecular
genetics of prostate cancer: New prospects for old challenges.
Genes Dev. 24:1967–2000. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Stock RG, Cesaretti JA, Hall SJ and Stone
NN: Outcomes for patients with high-grade prostate cancer treated
with a combination of brachytherapy, external beam radiotherapy and
hormonal therapy. BJU Int. 104:1631–1636. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Michalaki V, Syrigos K, Charles P and
Waxman J: Serum levels of IL-6 and TNF-alpha correlate with
clinicopathological features and patient survival in patients with
prostate cancer. Br J Cancer. 90:2312–2316. 2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gomes IM, Arinto P, Lopes C, Santos CR and
Maia CJ: STEAP1 is overexpressed in prostate cancer and prostatic
intraepithelial neoplasia lesions, and it is positively associated
with Gleason score. Urol Oncol. 32:53.e23–29. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rube CE, Palm J, Erren M, Fleckenstein J,
Konig J, Remberger K and Rube C: Cytokine plasma levels: reliable
predictors for radiation pneumonitis? PLoS One.
3(e2898)2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Singh J, Sohal SS, Ahuja K, Lim A, Duncan
H, Thachil T and De Ieso P: Levels of plasma cytokine in patients
undergoing neoadjuvant androgen deprivation therapy and external
beam radiation therapy for adenocarcinoma of the prostate. Ann
Transl Med. 8(636)2020.PubMed/NCBI View Article : Google Scholar
|