Introduction
For both sexes, colorectal cancer is the second
leading cause of cancer-related death, globally (9.2%) (1). The growing incidence, especially in
industrialized countries, can be attributed to a change in
lifestyle connected with obesity, physical inactivity, alcohol
consumption and high red meat intake (2). Colorectal cancer is the result of a
stepwise transition from normal mucosa to an invasive tumor,
comprising several intermediate stages of premalignant or invasive
lesions. As this process often drags on for years, cancer
prevention and early diagnosis through screening programs represent
a mainstay in colon cancer assessment and avoidance (3). Symptoms are generally associated with
large tumors or advanced disease stages, and in most cases are
relatively unspecific, so that the majority of colorectal cancers
go unnoticed in early stages (3).
Therapeutic options for the treatment of malignant tumors are
resection, radiation and/or chemotherapy, depending on tumor stage
and patient characteristics (3-5).
In the last 30 years, the survival of patients
suffering from colorectal cancer has increased markedly, owing
mainly to the introduction of screening programs and of new
therapeutic agents (6).
Conventional cytotoxic chemotherapy is the backbone of treatment
for colorectal cancer patients with lymph node positive disease
(7). Over the last decade, targeted
therapies came to the fore with genomic markers enabling the
selection of appropriate patients, who generally represent a
minority among the whole patient population (8). But with the latest results regarding
total neoadjuvant therapy (TNT), also cytotoxic chemotherapy gains
in importance again. Both, the RAPIDO-, as well as the PRODIGE
23-study showed a significant and clinically relevant extension of
disease-free survival after TNT instead of conventional,
neoadjuvant RCT (9,10). Although some prognostic indicators
for the probable response to conventional chemotherapy were
identified, most of the proposed biomarkers and predictive assays
are not currently used in the clinic, because of lacking
validation, practicability and scalability, and of long turnaround
times, or extensive costs (8,11-13).
Altogether, there is a great demand for analytical methods
easy-to-apply, which may support physicians with therapy decisions,
and help to protect patients from under- or over-treatment.
Circulating tumor cells, readily accessible from
blood samples of patients with solid tumors, are an important link
between primary tumors and metastases. A longitudinal monitoring of
their numbers and properties can provide valuable information on
therapy response and disease progression. Various studies
demonstrated a correlation between circulating tumor cells and
metastases, survival and therapy response for patients with
different types of cancer (14-18).
Ki-67 is a non-histone nuclear protein, which is
expressed in actively proliferating cells throughout the cell
cycle, but not in quiescent (G0) cells (19). Besides its detection in primary
tumors, Ki-67 was also shown to be expressed in circulating tumor
cells (20), and so might
constitute a biomarker for identifying patients at a high risk of
metastatic relapse.
While circulating tumor cells were shown to have
prognostic potential for tumors of different entities (14-18),
their clinical importance in colorectal cancer remained unclear.
Our study was designed to use the immunofluorescence-based
Maintrac® method to identify and quantify circulating
epithelial tumor cells (CETCs) in the blood of patients with
colorectal carcinoma (ICD10: C18/20) before and during neoadjuvant
and/or adjuvant R/CT. Moreover, the ratio of CETCs expressing the
proliferation marker Ki-67 was determined during the course of
therapy.
Materials and methods
Patient and inclusion criteria
A total of 22 patients, diagnosed with colorectal
cancer, were enrolled in this study between October 2018 und August
2020. Before treatment, all patients passed a complete clinical
evaluation including clinical history, physical examination,
rectoscopy/colonoscopy, relevant blood examination and
chest/abdominal computed tomography. Local stage was determined
according to the TNM classification of the UICC (21). The recruitment criteria were as
follows: Histologically confirmed, invasive colorectal carcinoma
(ICD10: C18/C20); primary diagnosis. The characteristics of all
patients enrolled in this study are shown in Table I. All patients were treated
according to current treatment guidelines for colon (ICD10: C18) or
rectal (ICD10: C20) cancer (22).
Long term R/CT for rectal cancer was performed as follows:
Radiation dose: 50.4 Gy (single dose 1.8 Gy); target volume: Rectal
cancer and region of pelvic lymphatic drainage; chemotherapy:
5-Fluorouracil (10 patients), Capecitabine (1 patient),
5-Fluouracil/Oxaliplatin (3 patients). Individual therapy decisions
were within the discretion of the attending physician and
independent of any data collected in the course of this study. For
patients receiving neoadjuvant therapy, 7.5 ml peripheral blood
samples were obtained 1-7 days before initiation of R/CT, 17±3 days
after the first cycle of R/CT, and after the completion of R/CT
(1-7 days before surgery). For patients with only or additional
adjuvant therapy, blood samples were obtained 1-7 days before
surgery, 6-8 weeks after surgery (before initiation of adjuvant
therapy), 17±3 days after the first cycle of adjuvant chemotherapy,
and on the last day of therapy, respectively.
 | Table IClinicopathological characteristics
of patients with colon (C18) and rectal (C20) cancer included in
this study. |
Table I
Clinicopathological characteristics
of patients with colon (C18) and rectal (C20) cancer included in
this study.
| Clinicopathological
characteristics | Number of patients
with colon cancer, n (%) | Number of patients
with rectal cancer, n (%) |
|---|
| Total | 6(27) | 16(73) |
| Age, years | | |
|
>60 | 5(23) | 11(50) |
|
≤60 | 1(4) | 5(23) |
| Sex | | |
|
Female | 3(14) | 6(27) |
|
Male | 3(14) | 10(45) |
| Tumor
sizea | | |
|
T1 | 0 (0) | 0 (0) |
|
T2 | 1(4) | 3(14) |
|
T3 | 4(18) | 11(50) |
|
T4 | 1(4) | 2(9) |
| Lymph node
statusa | | |
|
Positive | 6(27) | 4(18) |
|
Negative | 0 (0) | 12(54) |
| Distant
metastasis | | |
|
Positive | 1(4) | 1(4) |
|
Negative | 5(23) | 15(68) |
| Neoadjuvant
therapy | 1(17)b | 14(64) |
| Adjuvant
therapy | 6(27) | 3(14) |
The study was based on the Ethics Declaration of
Helsinki and was approved by the Ethics Committee of the University
of Bayreuth. Participants provided their written informed consent
to participate in this study.
Assessment of tumor regression after
neoadjuvant therapy
For all rectal cancer patients, treatment responses
were assessed according to the pathological results after surgery,
and graded by histological evaluation of the surgical specimens
according to the criteria described by Dworak et al
(23). The grade of tumor
regression was defined as follows: Grade 0: No regression; Grade 1:
Dominant tumor mass with obvious fibrosis and/or vasculopathy;
Grade 2: Dominantly fibrotic changes with few tumor cells or groups
(easy to find); Grade 3: Very few tumor cells (difficult to find
microscopically) in fibrotic tissue with or without mucous
substance; Grade 4: No tumor cells, only fibrotic mass (total
regression/response).
For a proper assessment of therapy response, we
additionally compared the tumor size and lymph node status as
assessed by computed tomography and/or endosonography of each
patient before and after neoadjuvant R/CT. According to Dworak
regression grade, as well as TNM re-staging, each patient was
individually assigned either to the group of good or poor
responders to neoadjuvant R/CT (Table
II).
 | Table IIResponses of patients with rectal
cancer (C20) after receiving neoadjuvant R/CT. |
Table II
Responses of patients with rectal
cancer (C20) after receiving neoadjuvant R/CT.
| | TNM | |
|---|
| Patient number | Before R/CT | After R/CT | Regression
grade | Response
category |
|---|
|
1 | µ, T2; µ, N0 | yp, T3b; yp,
N1 | 1 | Poor |
|
2 | µ, T2; c, T3; µ,
N1 | yp, T2; yp, N0 | 3 | Good |
|
3 | µ, T3; µ, N+ | yp, T2; yp, N0 | 2 | Good |
|
4 | µ, T3; µ, N0 | yp, T2; yp, N0 | 3 | Good |
|
5 | µ, T3; µ, N1; c,
M1HEP | yp, T3; yp, N0 | 3 | Good |
|
6 | c, T4; c, N2b | yp, T3b; yp,
N0 | 3 | Good |
|
7 | c, T3; c, N+ | yp, T3a; yp,
N1b | 3 | Good |
|
8 | µ, T3; µ, N+ | yp, T3a; yp,
N0 | 3 | Good |
|
9 | µ, T3; µ, N0 | yp, T3b; yp,
N0 | 1 | Poor |
| 10 | µ, T2; µ, N+ | yp, T3; yp, N0 | 2 | Poor |
| 11 | c, T3; c, N1 | yp, T4a; yp,
N1b | 3 | Poor |
| 12 | µ, T2; µ, N+ | yp, T3a; p, N0 | 1 | Poor |
| 13 | µ, T3; µ, N1 | yp, T3b; yp,
N0 | 1 | Poor |
| 14 | µ, T3; µ, N1; c,
M1aPUL | yp, T4a; yp, N0; c,
M1a | 1 | Poor |
Blood collection and
Maintrac® analysis
Peripheral blood (7.5 ml) from 22 patients with
colorectal cancer at different stages of disease was drawn into
blood count tubes containing ethylenediaminetetraacetic acid (EDTA)
as an anticoagulant and processed 24 h after collection.
The Maintrac® approach was used for
identification, quantification and further characterization of
CETCs (22). To this end, 1 ml of
EDTA-blood was subjected to red blood cells lysis at 4˚C for 15 min
using 14 ml erythrocyte lysis buffer (Qiagen GmbH). Remaining cells
were spun down at 700 x g for 7 min at rt and resuspended in 500 µl
of PBS/EDTA buffer. Immunostaining was performed by adding 4 µl
fluorescein-isothiocyanate (FITC)-conjugated anti-human epithelial
cell adhesion molecule (EpCAM) antibody (clone HEA-125; Miltenyi
Biotec GmbH) to 25 µl of the cell suspension (about 107
cells/100 µl) and incubation for 20 min at 4˚C in the dark. The
corresponding isotype control for EpCAM (mouse IgG1 K FITC;
Miltenyi Biotec) was used at the same final concentration. In case
of co-staining of Ki-67, additional 2.5 µl of phycoerythrin
(PE)-conjugated anti-Ki-67 antibody (clone B56; BD Biosciences) was
added prior to incubation. Subsequently, all samples were diluted
in PBS/EDTA buffer to a total volume of 250 µl. A defined volume of
the cell suspension and propidium iodide (PI; Sigma-Aldrich; Merck
KGaA) was transferred to the wells of ELISA-plates (Greiner
Bio-one). Co-staining of cells with Ki-67 was performed without PI.
Red and green fluorescence of the cells was examined using a
Fluorescence Scanning Microscope ScanR (Olympus), enabling
detection and relocation of cells for visual examination of EpCAM-,
PI- or Ki-67-positive cells. For quantification of CETCs, only
vital CETCs with intact cell morphology and without PI staining
were counted. For daily verification of optical components and
detectors of the microscope, fluorospheres (Flow-Check 770; Beckman
Coulter) were used.
Culture of spheres from peripheral
blood
Only a small subpopulation of CETCs possessing
additional stem cell properties is able to grow into metastases. By
enumeration of CETCs able to clonally grow into CETC microspheres
under specific conditions, we specified and quantified this
subpopulation. Therefore, CETCs and leukocytes were isolated from
peripheral blood as described earlier, plated at a density of
2x105 cells/ml in RPMI-1640 supplemented with
l-glutamine, HEPES, penicillin/streptomycin and growth factors such
as EGF, insulin and hydrocortisone, and incubated under standard
cell culture conditions (37˚C, 5% CO2) in a sterile
incubator. Every five days, the cultures were inspected under an
inverted light microscope (PrimoVert) and fresh culture medium was
added. Between days 21 and 28 of incubation, spheres were collected
from the culture flasks, pelleted (250 x g, 7 min), and resuspended
in 500 µl PBS. Immunostaining of spheres was performed using
FITC-conjugated mouse anti-human EpCAM-antibody (clone HEA-125;
Miltenyi Biotec GmbH), PE-conjugated mouse anti-human CD44-antibody
(BD Biosciences) or mouse anti-human CD133-antibody (clone 7;
BioLegend) for 20 min at 4˚C in the dark. The samples were then
diluted in PBS/EDTA and transferred into the wells of a 96-well
microtiter plate (Greiner Bio-one). Analysis of fluorescence was
performed using a fluorescence scanning microscope (ScanR;
Olympus). To verify vitality, PI staining of spheres was performed
before the analysis. Finally, only vital CTC spheres with intact
morphology and without PI staining were counted.
Primary culture from tumor tissue
In case of surgery of the primary tumor, a small
piece of tissue from the middle of the tumor (ø depending on the
size of the tumor) was obtained in a sterile falcon in 10 ml
transportation medium (RPMI-1640, 5% FBS, 5 µg/ml insulin, 2.75
µg/ml transferrin, 20 mM sodium selenite, 55 µg/ml sodium pyruvate,
1 µM hydrocortisone, 1,000 U/ml penicillin, 1,000 µg/ml
streptomycin, 250 mg/ml amphotericin B, 15 mM HEPES, 100 µg/ml
gentamycin, 5 µg/ml metronidazole) directly from the operating
theater and kept at 4˚C for transportation. All samples were
processed within 24 h after withdrawal. For further processing, the
tumor tissue was washed 3-5 times in PBS by extensive shaking and
put into a sterile petri dish. Before the tissue was chopped into
small pieces of about 1 mm in diameter by anti-parallel movement of
two scalpels, it was covered with a small amount of sphere culture
medium (RPMI-1640 supplemented with l-glutamine, HEPES,
penicillin/streptomycin and growth factors such as EGF, insulin and
hydrocortisone). After one more washing step with PBS, the tissue
was enzymatically homogenized with Accumax™ solution
(Sigma-Aldrich; Merck KGaA) for 45 min under continuous mixing at
rt. Then the cell suspension was filtered using a cell strainer
(mesh size 0.44 µm; Greiner Bio-one) to eliminate bigger cell
clumps and centrifuged at 240 x g for 10 min at rt. The resulting
cell pellet was resuspended in 1 ml of culture medium and the
number of vital cells was determined by bromophenol blue staining.
Finally, the cells were plated in a concentration of approximately
0.6x106 vital cells/ml in culture medium in 6-well
plates and incubated at standard cell culture conditions (37˚C, 5%
CO2) for several weeks. All cultures were checked for
bacterial infections daily and in the case of a minor infection
isolated and treated with additional antibiotics, or in case of a
major infection, discarded. If primary tumor spheres were
detectable after a few weeks, a small amount of the culture was
harvested and immunostained for further characterization and
documentation.
Statistical analysis
Statistical analysis was performed using SigmaPlot
(version 14.0; Systat Software Inc.) for Windows. Comparisons
between variables were performed using ANOVA (analysis of variance)
followed by a post hoc test for parametric data, or Kruskal-Wallis
test followed by Dunn's test for nonparametric data. The
significance level was set at P<0.05.
Results
General
A total of 22 patients with histologically confirmed
colorectal cancer (16 patients with rectal cancer, 6 patients with
colon cancer) were enrolled in this study. Patients'
characteristics are given in Table
I.
Considering all patients (ICD10: C18 and C20), 1
patient was at stage I (4%), 7 patients were at stage II (32%), 12
patients were at stage III (55%), and 2 patients were at stage IV
(9%). Six patients (27%) suffered from colon cancer and 16 (73%)
from rectal cancer. The age of the patients ranged from 51 to 80
years (median 65.5 years). The median number of CETCs of all 22
patients with colorectal cancer was 55 CETCs per 100 µl cell
suspension (ranging from 0 to 640) from which colon cancer patients
(ICD10: C18) had a median CETC number per 100 µl of 45 (ranging
from 0 to 145), and rectal cancer patients (ICD10: C20) of 65
(range from 0 to 640). No statistically significant differences in
CETC numbers were observed in correlation to tumor size, lymph node
status or distant metastasis (data not shown).
CETC quantification
Using the Maintrac® method we detected
CETCs in 100% of colorectal cancer patients included in this study.
CETC numbers of all patients during the course of therapy are
specified in Table SI. In addition
to epithelial characteristics as assigned by immunostaining, we
could demonstrate proliferative and stemness properties of CETCs
under specific conditions, which were identical to those of cells
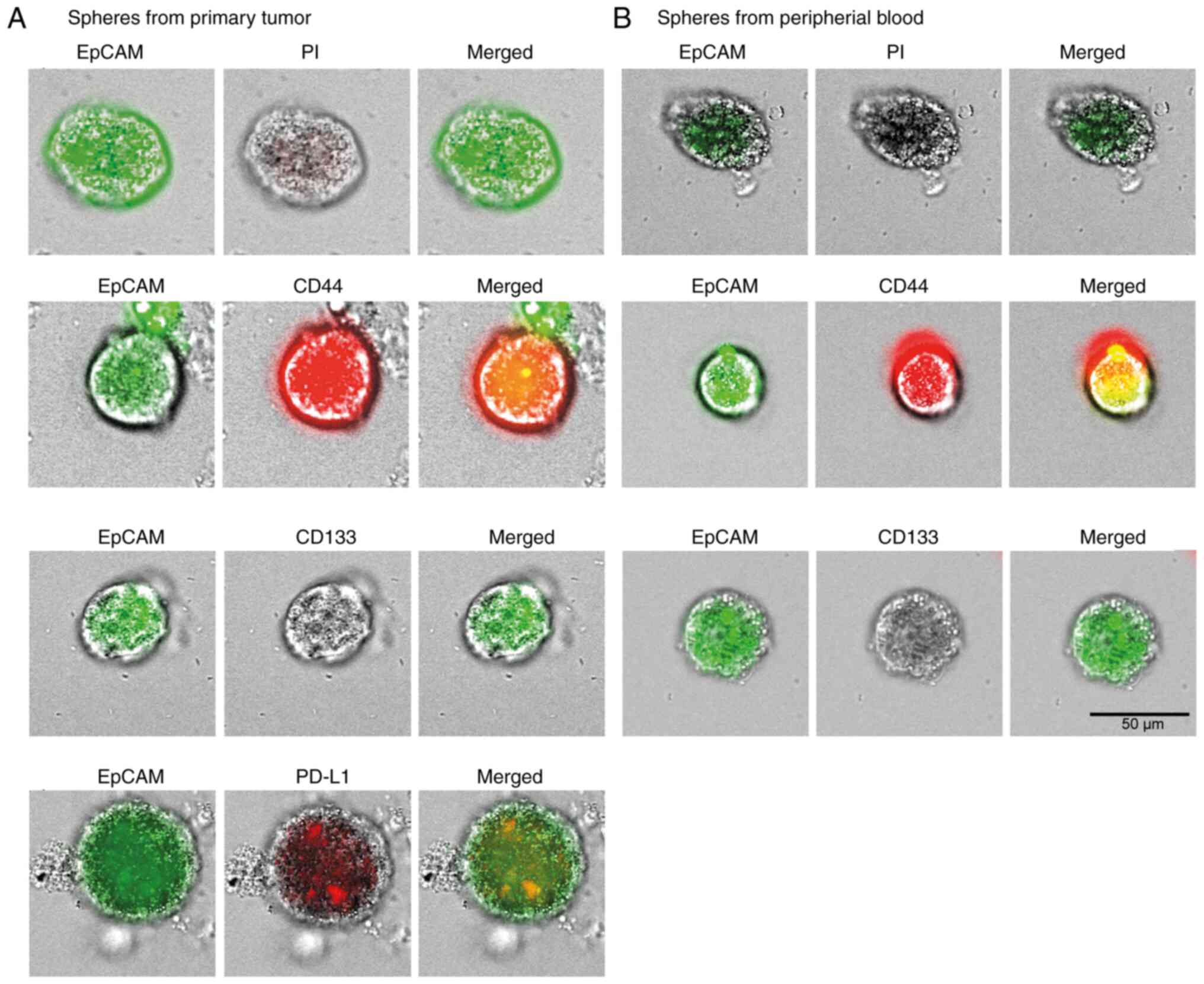
derived from the primary tumor itself (Fig. 1).
CETC characterization
CETCs from patient #1 were investigated for their
proliferative activity by growing non-adhesive suspension cultures.
Formation of EpCAM-positive spheres was observed after the first
cycle of neoadjuvant R/CT (5 spheres/100 µl blood). Interestingly,
CETCs from all other samples did not show any sphere formation.
During surgery of patient 1, a small piece of tumor tissue was set
aside and stored on ice until further processing. After separation
and washing, primary tumor cells were cultured under the same
conditions as CETCs, also resulting in the formation of spherical
structures. Both, spheres from the primary tumor, as well as
spheres from the peripheral blood of patient #1 were further
characterized by immunostaining (Fig.
1). The viability of the spheres was ensured by counterstaining
with PI (propidium iodide), which cannot permeate live cells.
Expression patterns in primary tumor spheres of specific stem cell
markers, which are regularly over-expressed in colorectal tumors
(CD44 and CD133), correlated with those in spheres from peripheral
blood. Moreover, primary tumor spheres expressed high levels of
PD-L1.
Response to neoadjuvant R/CT in rectal
cancer patients
14 patients with rectal cancer received neoadjuvant
R/CT, 7 (50%) of whom showed a good response (Fig. 2) and 7 (50%) did not or only
partially respond to the therapy (Fig.
3). In the group of good responders, the mean CETC number
before R/CT was 105 CETCs/100 µl of cell suspension. After the
first cycle of the R/CT it decreased to 47 per 100 µl. With a
P-value of 0.543, the differences between the three time points did
not reach statistical significance likely due to the small sample
size. Nevertheless, the results show a trend. In detail, the CETC
numbers declined in 5 of 7 patients (71%) and increased in only 2
patients (29%) with good response to neoadjuvant R/CT (Fig. 2). In the group of poor responders (7
patients), the mean CETC number was initially 40 CETCs/100 µl cell
suspension, and increased continuously from 73 after the first
cycle of the R/CT to 210 CETCs/100 µl cell suspension before
surgery (Fig. 3). Again, the small
number of participants (n=7) might be the major cause for the lack
of statistical significance (P=0.428).
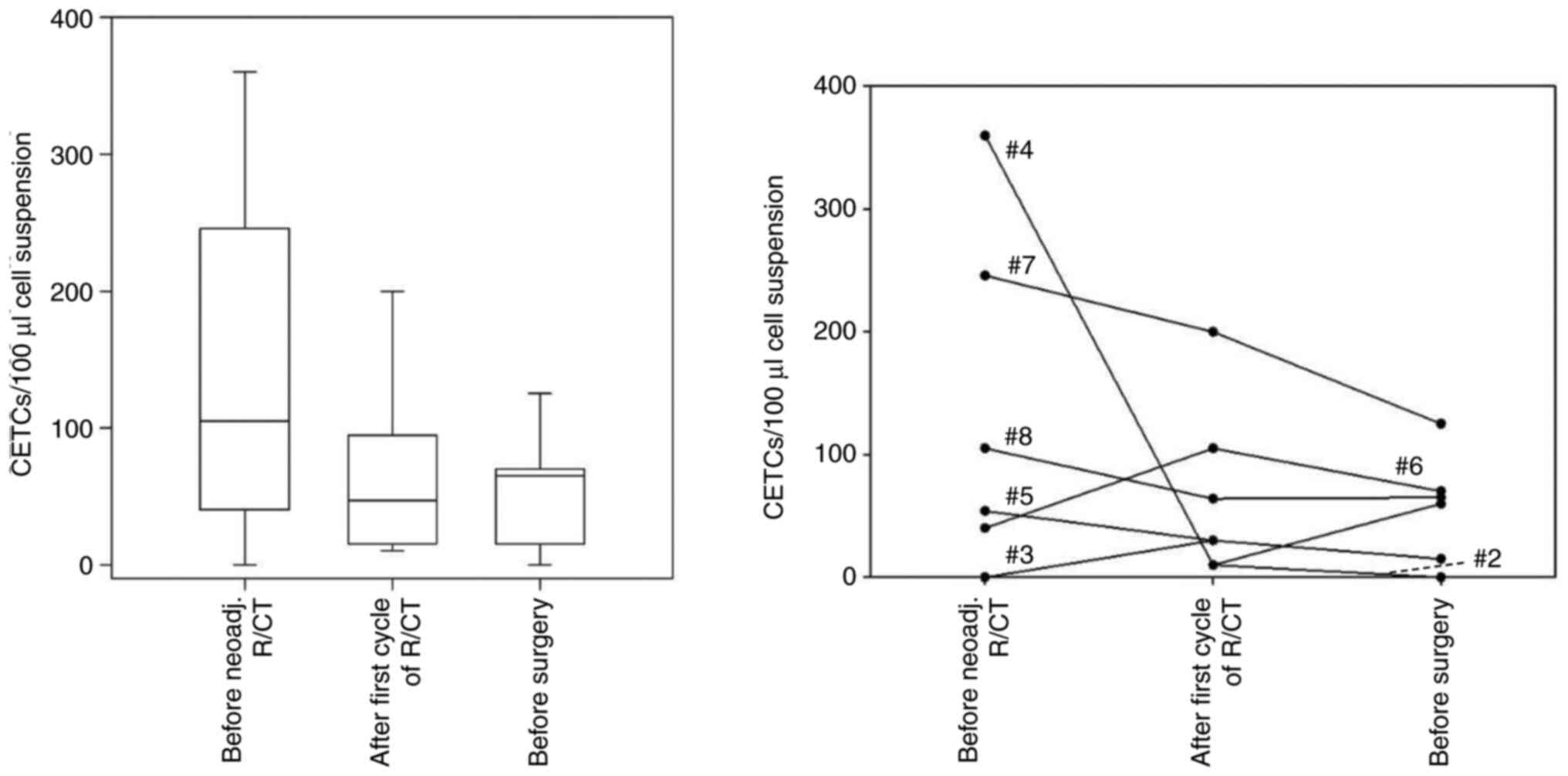 | Figure 2Number of CETCs in the blood of
patients with rectal cancer with good response to neoadj. R/CT.
Blood samples were drawn before R/CT, after the first cycle of R/CT
and after completion of R/CT immediately before surgery. Left,
boxplot with median CETC values, quartiles and variability at each
time point; right, individual CETC numbers at all time points, each
line represents one patient. Patient #4 (360/10/60 CETC/100 µl),
patient #7 (246/200/125 CETC/100 µl), patient #8 (105/64/65
CETC/100 µl), patient #5 (54/30/n.d. CETC/100 µl), patient #6
(40/105/70 CETC/100 µl), patient #3 (0/30/15 CETC/100 µl), patient
#2 (n.d./10/0 CETC/100 µl). Assignment of patients in Tables II and SI. n.d., not defined; CETC, circulating
epithelial tumor cell; neoadj., neoadjuvant; R/CT,
radio/chemotherapy. |
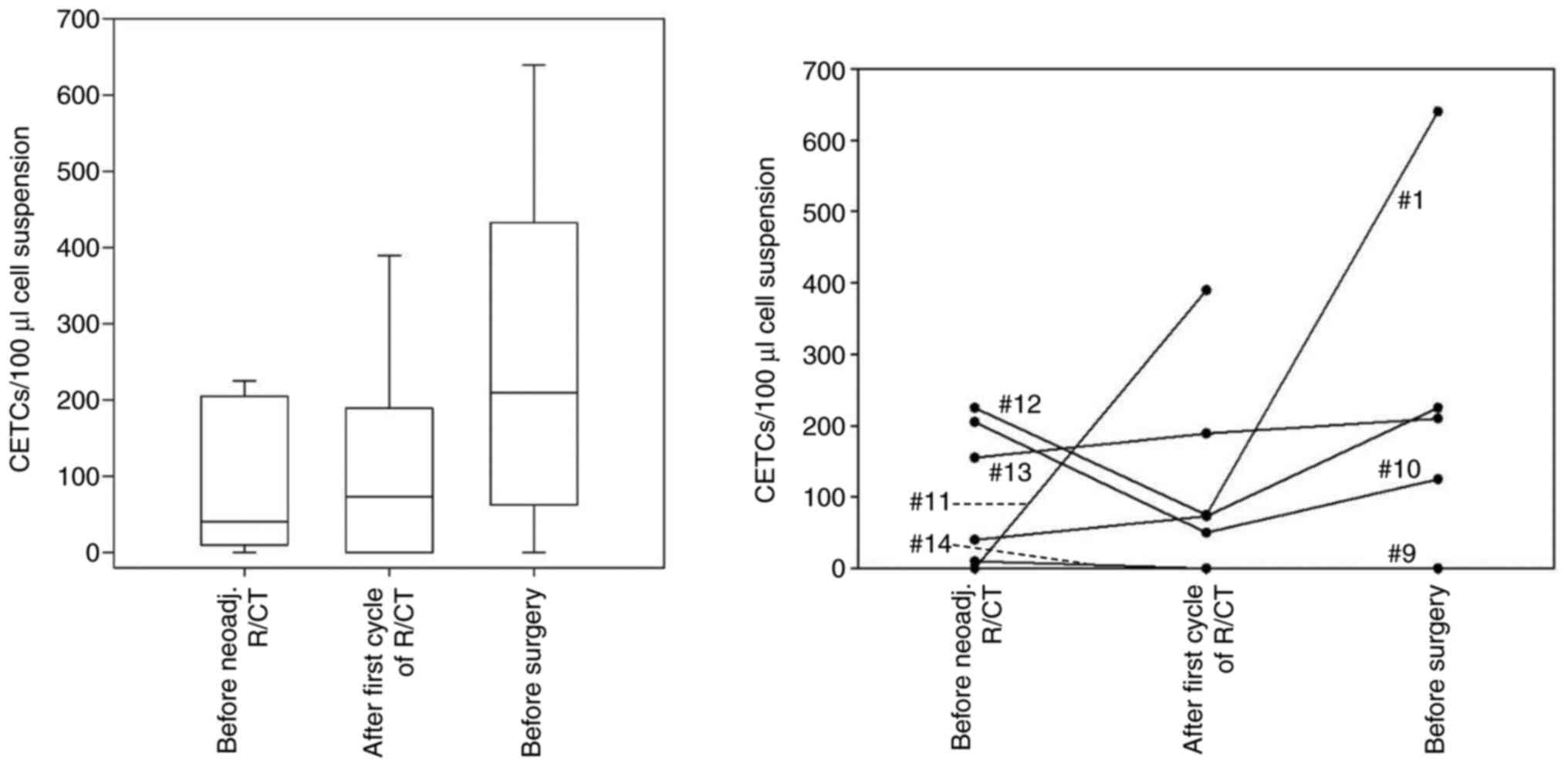 | Figure 3Number of CETCs in the blood of
patients with rectal cancer with poor response to neoadj. R/CT.
Blood samples were drawn before R/CT, after the first cycle of R/CT
and after completion of R/CT immediately before surgery. Left,
boxplot with median CETC values, quartiles and variability at each
time point; right, individual CETC numbers at all time points, each
line represents one patient. Patient #1 (40/73/225 CETC/100 µl),
patient #9 (10/0/0 CETC/100 µl), patient #10 (205/50/125 CETC/100
µl), patient #11 (0/390/n.d. CETC/100 µl), patient #12 (225/75/640
CETC/100 µl), patient #13 (155/189/210 CETC/100 µl), patient #14
(10/0/n.d. CETC/100 µl). Assignment of patients in Tables II and SI. n.d., not defined; CETC, circulating
epithelial tumor cell; neoadj., neoadjuvant; R/CT,
radio/chemotherapy. |
Response to adjuvant therapy in
colorectal cancer patients
9 patients (41%; 6 patients with colon cancer and 3
patients with rectal cancer) received adjuvant chemotherapy and
CETCs were quantified before surgery, before the beginning of
chemotherapy and after the first cycle of adjuvant therapy
(Fig. 4). Before surgery the median
CETC number was 55/100 µl cell suspension, 6-8 weeks after surgery
(before the beginning of the adjuvant CT) the median value was 65,
and after the first cycle of CT the median was 20 CETCs/100 µl cell
suspension. The difference between the mean values of the three
time points was not statistically significant (P=0.114).
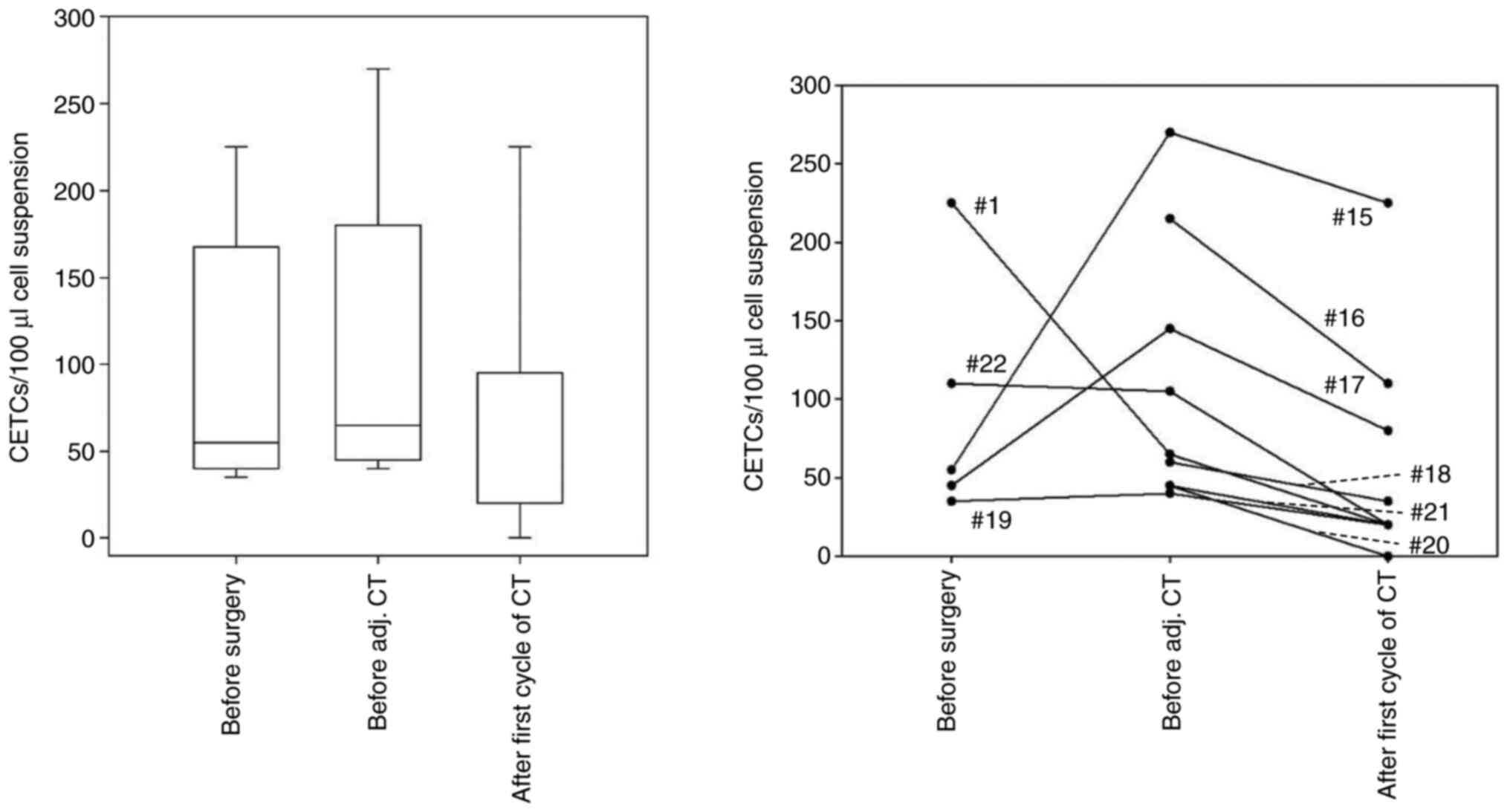 | Figure 4Number of CETCs in the blood of
patients with colorectal cancer with adj. CT. Blood samples were
drawn directly before surgery, 6-8 weeks after surgery and after
the first cycle of adj. CT. Left, boxplot with median CETC values,
quartiles and variability at each time point; right, individual
CETC numbers at all time points, each line represents one patient.
Patient #1 (C20; 225/65/20 CETC/100 µl), patient #15 (C20;
55/270/225 CETC/100 µl), patient #16 (C20; n.d./215/110 CETC/100
µl), patient #17 (C18; 45/145/80 CETC/100 µl), patient #18 (C18;
n.d./60/35 CETC/100 µl), patient #19 (C18; 35/40/20 CETC/100 µl),
patient #20 (C18; n.d./45/0 CETC/100 µl), patient #21 (C18;
n.d./45/20 CETC/100 µl), patient #22 (C18; 110/105/20 CETC/100 µl).
Assignment of patients in Table
SI. n.d., not defined; CETC, circulating epithelial tumor cell;
adj., adjuvant; CT, chemotherapy; C18, colon carcinoma; C20, rectum
carcinoma. |
Interestingly, all patients showed decreasing CETC
numbers under adjuvant chemotherapy.
Expression of the proliferation marker
Ki-67 during therapy
Ki-67-positive CETCs were detected in 20 patients
(91%) and the percentage ranged from 0-100 (median: 25
Ki-67-positive CETCs/100 µl cell suspension). The median of
Ki-67-positive CETCs/100 µl cell suspension in colon cancer
patients was 18 (ranging from 0 to 170), and in rectal cancer
patients 25 (ranging from 0 to 169). Although the differences in
the Ki-67-positive CETCs at the three time points were not
statistically significant neither for patients with neoadjuvant
R/CT (P=0.202), nor in the group of patients with adjuvant CT
(P=0.151), there was a trend in the number of Ki-67-positive CETCs
to decrease under adjuvant CT, and to increase in patients
receiving neoadjuvant R/CT (Fig.
5).
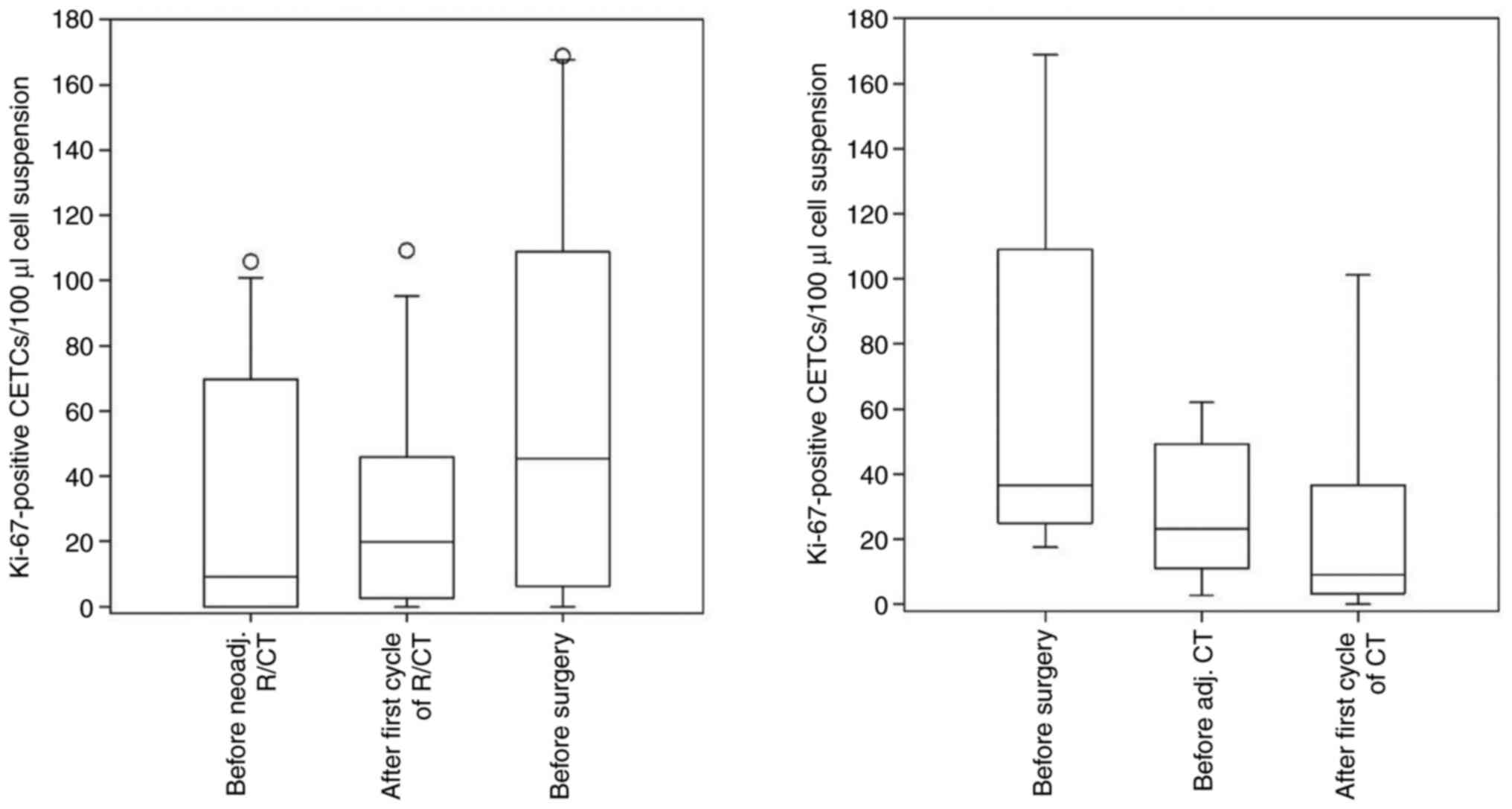 | Figure 5Boxplots with median values,
quartiles and variabilities of Ki-67-positive CETCs in the blood of
patients with colorectal cancer. Left, patients with neoadj. R/CT;
blood samples were drawn before the beginning of the neoadj. R/CT,
after the first cycle of R/CT and after completion of R/CT (before
surgery). Right, patients with adj. CT; blood samples were drawn
directly before surgery, 6-8 weeks after surgery and after the
first cycle of CT. CETC, circulating epithelial tumor cell;
neoadj., neoadjuvant; adj., adjuvant; CT, chemotherapy; R/CT,
radio/chemotherapy. |
Case report
Fig. 6 shows an
example of a serial analysis of the CETC numbers during the therapy
of a 63-year-old patient with stage III (T3, N1, M0; G2) rectal
cancer. The patient was treated with neoadjuvant R/CT (Dworak 1,
poor response), followed by surgery (R0-resection) and additional
adjuvant chemotherapy. During neoadjuvant therapy the CETC numbers
increased significantly and reached their maximum (225 CETCs/100 µl
cell suspension) before surgical removal of the primary tumor.
Eight weeks after surgery the CETC number had fallen to a level
similar to that at the beginning of the R/CT. It continued to
decrease until there were no residual CETCs detectable at the last
day of the adjuvant CT. Until 9 months after completion of the
adjuvant therapy, this patient has remained free of relapse.
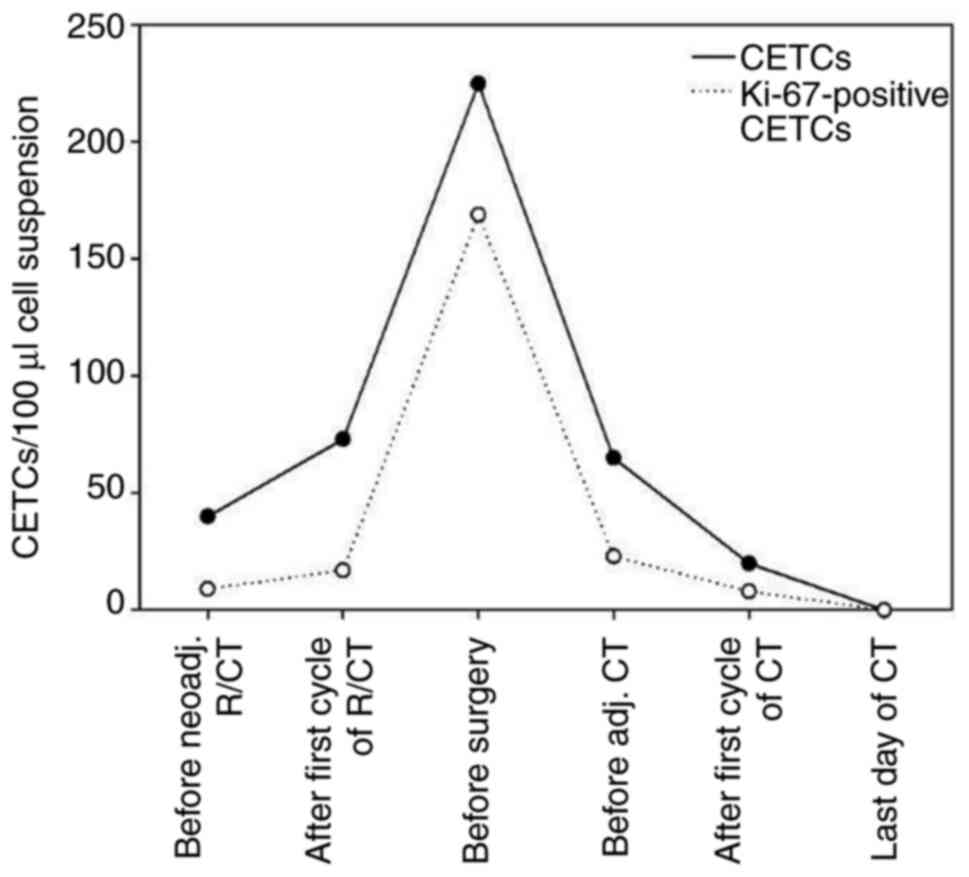 | Figure 6Number of CETCs and Ki-67-positive
CETCs in the blood of patient #1 with rectal cancer receiving
neoadj. R/CT, as well as adj. CT after surgical removal of the
primary tumor (R0-resection). Blood samples were drawn prior to the
neoadj. R/CT (40 CETCs/100 µl), after the first cycle of R/CT (73
CETCs/100 µl), immediately before surgery (225 CETCs/100 µl), 6-8
weeks after surgery/before the beginning of adj. CT (65 CETCs/100
µl), after the first cycle of adj. CT (20 CETCs/100 µl) and on the
last day of adj. CT (0 CETCs/100 µl). CETC, circulating epithelial
tumor cell; neoadj., neoadjuvant; adj., adjuvant; CT, chemotherapy;
R/CT, radio/chemotherapy. |
Discussion
Although disseminated tumor cells play a major role
in the metastatic process of tumors, their detection and monitoring
does not play a decisive role in standard clinical procedures.
Monitoring of circulating tumor cells in the blood of cancer
patients during therapy has already been shown to be a powerful
prognostic tool for tumors of different entities including
colorectal tumors (15,24-26).
From a clinical perspective, assessment of patients' response to
antitumoral therapy by detection of circulating tumor cells in the
peripheral blood appears comfortable, both for the physician (time-
and cost-efficient), as well as for the patients (non-invasive,
neither toxic nor painful), and may be easily repeated as a
monitoring tool without great efforts.
In recent years, different techniques have been
described for the detection of circulating tumor cells (27-32).
Various studies demonstrated that their detection via CellSearch
system could be used to predict treatment responses and long-term
prognosis for stage IV colorectal cancer patients (33,34).
But in the case of non-metastatic patients, the detection rate via
CellSearch system is too low (11-25%) to further analyze the
correlation between circulating tumor cells and patients'
characteristics and treatment responses (35). In the present
proof-of-principle study with a small group of colorectal
cancer patients of all stages using the Maintrac® method
for CETC identification, we detected CETCs in the peripheral blood
of 100% of the patients. Moreover, we were able to show that some
of the cells, which have been quantified, possess proliferative and
stemness characteristics matching those found in the primary
tumor.
Up until now, only a few studies investigated the
role of circulating tumor cells for evaluating the response to
neoadjuvant R/CT for patients with rectal cancer. In a study by
Zitt et al the circulating tumor cells were investigated
during neoadjuvant R/CT in 26 patients with locally advanced rectal
cancer using a non-quantitative RT-PCR-based method (36). Sun et al used a
size-dependent detection method to analyze changes in circulating
tumor cell numbers during neoadjuvant R/CT within a collective of
115 rectal cancer patients (37).
Keeping in mind the respective drawbacks of each method, both
studies agreed, that responders had an obvious decrease of the
numbers of circulating tumor cells after neoadjuvant R/CT, while
there was no noticeable alteration after treatment in
non-responders. These results were confirmed by other studies
(38,39). In our study, applying the
Maintrac® approach for CETC detection, we allocated 14
rectal cancer patients either to the group of good or poor
responders to neoadjuvant R/CT, based upon alterations in TNM
staging before and after R/CT and on Dworak regression grades of
their surgical specimens. For both groups we confirmed that a
decrease in CETC numbers correlates with, and thus indicates, a
good response, whereas an increase of CETC numbers is rather
indicative of a poor response to neoadjuvant R/CT using the
Maintrac® approach for CETC detection. Whether this
observation is of general relevance, and thus a potential
prognostic tool for the clinician, must be clarified by extended
studies with larger collectives of patients. As all patients in our
study experienced a decline of CETCs under adjuvant treatment, it
would be interesting to know if and when individual rectal cancer
patients benefit from an adjuvant chemotherapy after neoadjuvant
R/CT. Moreover, it would also be interesting to see, if CETC
monitoring may also be able to identify individual rectal cancer
patients, which benefit from TNT. The latest results of the PRODIGE
23 and RAPIDO phase III clinical studies have shown that TNT is
able to extent disease free survival, as well as to improve the
pathological complete remission (pCR) rate, organ preservation and
local control in patients with locally advanced rectal cancer in
comparison to conventional, neoadjuvant/adjuvant therapy regimes
(9,10).
As discussed above, we observed a heterogenic
reaction of the CETC profile for patients receiving neoadjuvant
R/CT. In contrast, a constant decrease in CETC numbers during
adjuvant therapy was found. A potential explanation for this
discrepancy may be the tumor burden in the adjuvant versus the
neoadjuvant situation. While the neoadjuvant therapy targets the
whole, intact tumor, in the adjuvant situation the tumor burden is
low, because only microscopic tumor residues remain in the patient
after surgery which may be more sensitive to chemotherapy and
radiation. In addition, because of the reduced number of tumor
cells, the development of resistance is less likely when compared
to the neoadjuvant situation (40,41).
The proliferation marker Ki-67 is widely utilized in
routine clinical diagnostic of breast cancer patients (42). Lumachi et al suggested Ki-67
as a predictive parameter for colorectal cancer, as they found an
inverse correlation between Ki-67 expression and overall survival
in a small retrospective study (43). However, its prognostic value for
colorectal tumors remains controversial. While some studies
completely failed to demonstrate its prognostic significance in the
case of colorectal tumors (44),
others found Ki-67 overexpression indicating a good clinical
outcome for colorectal cancer patients (45). In our study, we observed that the
Ki-67 index, and thus the proliferative activity, of CETCs from the
blood of colorectal cancer patients increased during neoadjuvant
R/CT, and decreased during adjuvant CT. Considering the above
mentioned findings from literature, these results cannot be
interpreted regarding a good or poor prognosis for the patients. On
a cellular level, one possible explanation for the rise in Ki-67
expression under neoadjuvant R/CT may be the radiotherapy-induced
inflammation, which in turn induces an increase of the
proliferating activity of tumor cells, and consequently of
circulating tumor cells (46,47).
Finally, the general trends reported in this study
could be exemplified by a case report of a rectal cancer patient
(#1) receiving neoadjuvant R/CT, as well as adjuvant CT. This
patient seemed to benefit from the surgery and from the additional
adjuvant CT as CETC numbers decreased continuously after surgery to
reach zero level on the last day of adjuvant therapy. This patient
has remained free of relapse until nine months after the completion
of therapy.
Supplementary Material
Number of CETCs of all patients during
the treatment course.
Acknowledgements
Not applicable.
Funding
The present study was supported by a PhD fellowship from the
Bayerische Eliteförderungsgesetz (BayEFG).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS, AK, KP and MG contributed to the design of the
present study and developed the methodology. MG collected the
bioinformatics data, performed the experiments, analyzed the
results and wrote the manuscript. AK contributed to the collection
of patient data. RS, AK and KP critically revised the manuscript
and approved the final version to be published. All authors agreed
to be accountable for all aspects of the study. All authors have
read and approved the final manuscript. MG and RS confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the University of Bayreuth (approval no. O 1305/1-GB;
Bayreuth, Germany). Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
Katharina Pachmann holds a patent protecting the
Maintrac® method used in the present study (patent no.
EP 3128325 B1; dated February 8th, 2017). The other authors declare
that they have no competing interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kerr J, Anderson C and Lippman SM:
Physical activity, sedentary behaviour, diet, and cancer: An update
and emerging new evidence. Lancet Oncol. 18:e457–e471.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Argilés G, Tabernero J, Labianca R,
Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P,
Yoshino T, Taieb J, et al: Localised colon cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 31:1291–1305. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Glynne-Jones R, Wyrwicz L, Tiret E, Brown
G, Rödel C, Cervantes A and Arnold D: ESMO Guidelines Committee.
Rectal cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28 (Suppl 4):iv22–iv40.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
van Cutsem E, Nordlinger B and Cervantes
A: ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO
clinical practice guidelines for treatment. Ann Oncol. 21 (Suppl
5):v93–v97. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Boussios S, Ozturk MA, Moschetta M,
Karathanasi A, Zakynthinakis-Kyriakou N, Katsanos KH, Christodoulou
DK and Pavlidis N: The developing story of predictive biomarkers in
colorectal cancer. J Pers Med. 9(12)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moertel CG, Fleming TR, Macdonald JS,
Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA,
Tormey DC and Glick JH: Levamisole and fluorouracil for adjuvant
therapy of resected colon carcinoma. N Engl J Med. 322:352–358.
1990.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Punt CJA, Koopman M and Vermeulen L: From
tumour heterogeneity to advances in precision treatment of
colorectal cancer. Nat Rev Clin Oncol. 14:235–246. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bahadoer RR, Dijkstra EA, van Etten B,
Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID,
Beets-Dan RGH, Blomqvist L, et al: Short-course radiotherapy
followed by chemotherapy before total mesorectal excision (TME)
versus preoperative chemoradiotherapy, TME, and optional adjuvant
chemotherapy in locally advanced rectal cancer (RAPIDO): A
randomised, open-label, phase 3 trial. Lancet Oncol. 22:29–42.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Conroy T, Lamficheck N, Etienne PL, Rio E,
Francois E, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O,
Gargot D, et al: Total neoadjuvant therapy with mFOLFIRINOX versus
preoperative chemoradiation in patients with locally advanced
rectal cancer: Final results of PRODIGE 23 phase III trial, a
UNICANCER GI trial. J Clin Oncol. 38(S4007)2020.
|
|
11
|
Ebert MPA, Tänzer M, Balluff B,
Burgermeister E, Kretzschmar AK, Hughes DJ, Tetzner R, Lofton-Day
C, Rosenberg R, Reinacher-Schick AC, et al: TFAP2E-DKK4 and
chemoresistance in colorectal cancer. N Engl J Med. 366:44–53.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Friedman AA, Letai A, Fisher DE and
Flaherty KT: Precision medicine for cancer with next-generation
functional diagnostics. Nat Rev Cancer. 15:747–756. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Garnett MJ, Edelman EJ, Heidorn SJ,
Greenman CD, Dastur A, Lau KW, Greninger P, Thompson R, Luo X,
Soares J, et al: Systematic identification of genomic markers of
drug sensitivity in cancer cells. Nature. 483:570–575.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang L, Zhou S, Zhang W, Wang J, Wang M,
Hu X, Liu F, Zhang Y, Jiang B and Yuan H: Circulating tumor cells
as an independent prognostic factor in advanced colorectal cancer:
A retrospective study in 121 patients. Int J Colorectal Dis.
34:589–597. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang C, Chen F, Wang S and Xiong B:
Circulating tumor cells in gastrointestinal cancers: Current status
and future perspectives. Front Oncol. 9(1427)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kapeleris J, Kulasinghe A, Warkiani ME,
Vela I, Kenny L, O'Byrne K and Punyadeera C: The prognostic role of
circulating tumor cells (CTCs) in lung cancer. Front Oncol.
8(311)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Banys-Paluchowski M, Krawczyk N and Fehm
T: Potential role of circulating tumor cell detection and
monitoring in breast cancer: A review of current evidence. Front
Oncol. 6(255)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xun Y, Cao Q, Zhang J, Guan B and Wang M:
Clinicopathological and prognostic significance of circulating
tumor cells in head and neck squamous cell carcinoma: A systematic
review and meta-analysis. Oral Oncol. 104(104638)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schlüter C, Duchrow M, Wohlenberg C,
Becker MH, Key G, Flad HD and Gerdes J: The cell
proliferation-associated antigen of antibody Ki-67: A very large,
ubiquitous nuclear protein with numerous repeated elements,
representing a new kind of cell cycle-maintaining proteins. J Cell
Biol. 123:513–522. 1993.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pizon M, Schott DS, Pachmann U and
Pachmann K: B7-H3 on circulating epithelial tumor cells correlates
with the proliferation marker, Ki-67, and may be associated with
the aggressiveness of tumors in breast cancer patients. Int J
Oncol. 53:2289–2299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Union for International Cancer Control
(UICC): Colon and Rectum. In: TNM classification of malignant
tumours. Brierley J, Gospodarowicz MK and Wittekind C (eds) John
Wiley & Sons Ltd., Chichester, pp73-76, 2017.
|
|
22
|
Pox C: Update der S3-leitlinie zum
kolorektalen karzinom. Best Pract Onkol. 13:254–262. 2018.(In
German).
|
|
23
|
Dworak O, Keilholz L and Hoffmann A:
Pathological features of rectal cancer after preoperative
radiochemotherapy. Int J Colorectal Dis. 12:19–23. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pachmann K, Willecke-Hochmuth R, Schneider
K and Kaatz M: Circulating epithelial tumor cells as a prognostic
tool for malignant melanoma. Melanoma Res. 28:37–43.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Krebs MG, Sloane R, Priest L, Lancashire
L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G,
et al: Evaluation and prognostic significance of circulating tumor
cells in patients with non-small-cell lung cancer. J Clin Oncol.
29:1556–1563. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rahbari NN, Aigner M, Thorlund K, Mollberg
N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M and Weitz
J: Meta-analysis shows that detection of circulating tumor cells
indicates poor prognosis in patients with colorectal cancer.
Gastroenterology. 138:1714–1726. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239.
2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lara O, Tong X, Zborowski M and Chalmers
JJ: Enrichment of rare cancer cells through depletion of normal
cells using density and flow-through, immunomagnetic cell
separation. Exp Hematol. 32:891–904. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Park JM, Lee JY, Lee JG, Jeong H, Oh JM,
Kim YJ, Park D, Kim MS, Lee HJ, Oh JH, et al: Highly efficient
assay of circulating tumor cells by selective sedimentation with a
density gradient medium and microfiltration from whole blood. Anal
Chem. 84:7400–7407. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pachmann K: Current and potential use of
MAINTRAC method for cancer diagnosis and prediction of metastasis.
Expert Rev Mol Diagn. 15:597–605. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Desitter I, Guerrouahen BS, Benali-Furet
N, Wechsler J, Jänne PA, Kuang Y, Yanagita M, Wang L, Berkowitz JA,
Distel RJ and Cayre YE: A new device for rapid isolation by size
and characterization of rare circulating tumor cells. Anticancer
Res. 31:427–441. 2011.PubMed/NCBI
|
|
32
|
Wang L, Balasubramanian P, Chen AP, Kummar
S, Evrard YA and Kinders RJ: Promise and limits of the cellSearch
platform for evaluating pharmacodynamics in circulating tumor
cells. Semin Oncol. 43:464–475. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cohen SJ, Punt CJA, Iannotti N, Saidman
BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller
MC, et al: Relationship of circulating tumor cells to tumor
response, progression-free survival, and overall survival in
patients with metastatic colorectal cancer. J Clin Oncol.
26:3213–3221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tol J, Koopman M, Miller MC, Tibbe A, Cats
A, Creemers GJM, Vos AH, Nagtegaal ID, Terstappen LWMM and Punt
CJA: Circulating tumour cells early predict progression-free and
overall survival in advanced colorectal cancer patients treated
with chemotherapy and targeted agents. Ann Oncol. 21:1006–1012.
2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sastre J, Maestro ML, Puente J, Veganzones
S, Alfonso R, Rafael S, Gracía-Saenz JA, Vidaurreta M, Martín M,
Arroyo M, et al: Circulating tumor cells in colorectal cancer:
Correlation with clinical and pathological variables. Ann Oncol.
19:935–938. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zitt M, Zitt M, Müller HM, Dinnewitzer AJ,
Schwendinger V, Goebel G, De Vries A, Amberger A, Weiss H,
Margreiter R, et al: Disseminated tumor cells in peripheral blood:
A novel marker for therapy response in locally advanced rectal
cancer patients undergoing preoperative chemoradiation. Dis Colon
Rectum. 49:1484–1491. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sun W, Li G, Wan J, Zhu J, Shen W and
Zhang Z: Circulating tumor cells: A promising marker of predicting
tumor response in rectal cancer patients receiving neoadjuvant
chemo-radiation therapy. Oncotarget. 7:69507–69517. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Magni E, Botteri E, Ravenda PS, Cassatella
MC, Bertani E, Chiappa A, Luca F, Zorzino L, Bianchi PP, Adamoli L,
et al: Detection of circulating tumor cells in patients with
locally advanced rectal cancer undergoing neoadjuvant therapy
followed by curative surgery. Int J Colorectal Dis. 29:1053–1059.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hinz S, Röder C, Tepel J, Hendricks A,
Schafmayer C, Becker T and Kalthoff H: Cytokeratin 20 positive
circulating tumor cells are a marker for response after neoadjuvant
chemoradiation but not for prognosis in patients with rectal
cancer. BMC Cancer. 15(953)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Imyanitov EN and Yanus GA: Neoadjuvant
therapy: Theoretical, biological and medical consideration. Chin
Clin Oncol. 7(55)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Leary A, Cowan R, Chi D, Kehoe S and
Nankivell M: Primary surgery or neoadjuvant chemotherapy in
advanced ovarian cancer: The debate continue. Am Soc Clin Oncol
Educ Book. 35:153–162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Inwald EC, Klinkhammer-Schalke M,
Hofstädter F, Zeman F, Koller M, Gerstenhauer M and Ortmann O:
Ki-67 is a prognostic parameter in breast cancer patients: Results
of a large population-based cohort of a cancer registry. Breast
Cancer Res Treat. 139:539–552. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lumachi F, Orlando R, Marino F, Chiara GB
and Basso SMM: Expression of p53 and Ki-67 as prognostic factors
for survival of men with colorectal cancer. Anticancer Res.
32:3965–3967. 2012.PubMed/NCBI
|
|
44
|
Ghiţă C, Vîlcea ID, Dumitrescu M, Vîlcea
AM, Mirea CS, Aşchie M and Vasilescu F: The prognostic value of the
immunohistochemical aspects of tumor suppressor genes p53, bcl-2,
PTEN and nuclear proliferative antigen Ki-67 in resected colorectal
carcinoma. Rom J Morphol Embryol. 53:549–556. 2012.PubMed/NCBI
|
|
45
|
Melling N, Kowitz CM, Simon R, Bokemeyer
C, Terracciano L, Sauter G, Izbicki JR and Marx AH: High Ki67
expression is an independent good prognostic marker in colorectal
cancer. J Clin Pathol. 69:209–214. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kiraly O, Gong G, Olipitz W, Muthupalani S
and Engelward BP: Inflammation-induced cell proliferation
potentiates DNA damage-induced mutations in vivo. PLOS Genet.
11(e1004901)2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Di Maggio FM, Minafra L, Forte GI,
Cammarata FP, Lio D, Messa C, Gilardi MC and Bravatà V: Portrait of
inflammatory response to ionizing radiation treatment. J Inflamm.
12(14)2015.PubMed/NCBI View Article : Google Scholar
|