Introduction
Symptoms such as bleeding and tissue breakdown due
to progression of locally advanced breast cancer (LABC) and locally
recurrent breast cancer (LRBC) are events that lead to a reduction
in quality of life (QOL), and it is often difficult to deal with
them because the symptoms cannot be alleviated at the terminal
stage. Surgical treatment is difficult for advanced lesions that
cannot be controlled by radiation therapy (RT). If RT enables local
control, the patient's QOL will improve. However, LABC and LRBC are
often situations in which drug therapy is ineffective, and it is
difficult to obtain local control with RT alone, because such
lesions contain numerous hypoxic cancer cells and antioxidant
enzymes that may confer resistance to RT (1-3).
As reported in past studies (1-5),
a novel radiosensitizer, Kochi Oxydol Radiation Therapy for
Unresectable Carcinomas II (KORTUC II), was developed for the
treatment of cancers that contain numerous hypoxic cancer cells and
antioxidant enzymes. Following KORTUC II therapy, hypoxic and
radioresistant cancer cells become hyperoxic and radiosensitive.
The concept of KORTUC II is to transform radioresistant cancer into
radiosensitive cancer (1-6).
KORTUC II was first used in Kochi University in 2006 and is
currently the most widely used radiosensitizer in clinical practice
in Japan. Our institution has used KORTUC (I+II) in over 250
patients to date since the first patient was treated in May 2010
after obtaining approval from the University's Ethics Committee.
(KORTUC I involves application of the sensitizer to neoplastic
surface tissue rather than injecting the sensitizer into tumor
tissue.) KORTUC II has achieved significant local effects with no
notable adverse events. In this article, we report on the cases
where KORTUC II was used to treat patients with LABC or LRBC.
Subjects and methods
Patient selection
At our institution, KORTUC II treatment was
performed after detailed informed consent given in written form was
obtained from patients who were expected to survive at least a year
and met the following additional criteria: i) Local control by
conventional radiotherapy alone was presumed to be difficult; ii)
the dosage of additional irradiation they could receive was
limited; and iii) they refused surgery as a treatment option.
Hormone therapy or systemic chemotherapy was used concomitantly in
some patients according to the judgment of the attending breast
surgeon and the patient's wishes. KORTUC II treatment was approved
by Osaka Medical College Clinical Trials Registry, trial no. 1973,
(May 10, 2010) and UMIN Clinical Trials Registry, trial no.
UMIN000003734, (June 10, 2010).
Of the 37 patients treated with KORTUC II for LABC
or LRBC between February 2011 and January 2020, the 30 patients who
were followed up for at least 3 months after treatment were
included in the study. These subjects consisted of 9 patients with
LABC (1 in stage IIIA, 5 in stage IIIB, and 3 in stage IV) and 21
patients with LRBC.
Radiotherapy
RT was performed for the purpose of local control
with external beam irradiation (X-ray or electron beam) to locally
advanced lesions, recurrent lesions or metastatic lesions of the
breast, chest wall, axillary lymph nodes, and supraclavicular fossa
lymph nodes. The dose and extent, as well as particular tumor sites
to be irradiated, were determined by the attending physicians
considering factors including tumor size and location, concomitant
therapies, presence/absence of metastases outside the irradiation
fields, and general condition of the patient.
In principle, the irradiation fields were to include
all lesions for stage III LABC, and local areas that required local
control for stage IV LABC and LRBC patients.
Dosing method of the sensitizer
This sensitizer is a solution consisting of 0.83%
sodium hyaluronate and 0.5% hydrogen peroxide
(H2O2, also known as ‘oxydol’ in Japan) by
volume. It is prepared aseptically before each use by adding 2.5 ml
of sodium hyaluronate (Adant® Dispo) and 1 ml of 1%
xylocaine to 0.5 ml of oxydol and mixing them to be dispensed as a
total volume of 4 ml from a single vial. Our standard dosing
protocol called for 1 vial for tumors <3 cm in diameter, 2 vials
for tumors 3-<5 cm in diameter, ≥3 vials for tumors ≥5 cm in
diameter, with a maximum dose of 5 vials for giant tumors. However,
the optimal dose is still uncertain.
The sensitizer was injected into the tumor twice
weekly immediately before RT either under direct vision in the case
of tumors close to the skin, or under ultrasound or CT guidance.
Under ultrasound guidance, when the sensitizer is injected into a
tumor, oxygen is generated in the form of micro-bubbles and the
tumor can immediately be recognized as a high echo area. The
sensitizer was injected so that oxygen was distributed in the
entire tumor. Usually, injections of the sensitizer occurred after
the patient had already received approximately 20 Gy at the
beginning of a course of RT. This was to prevent the increased
intra-tumor pressure from the injections causing viable tumor cells
to infiltrate into nearby lymphatic and blood vessels (5). To prevent dissemination along the
injection route, punctures were made on the skin surface in the
irradiation field, whenever possible.
Items examined
To examine the local effects, the tumor size was
measured before treatment and at the estimated time of greatest
regression (smallest volume) within 2 years after treatment. From
this, the maximum tumor shrinkage (MTS) was calculated according to
the percent decrease in tumor volume revealed by CT imaging at the
estimated time of greatest regression. The tumor volume was
measured based on CT images using the Eclipse radiation treatment
planning system (Varian Medical Systems, Inc.). This interval to
greatest regression was determined based upon prior studies using
contrast-enhanced MRI that show, on average, 14 months are required
between KORTUC II therapy and tumor disappearance according to
RESIST criteria. However, there is no pre-determined protocol for
scheduling follow-up imaging tests. In particular, CT scans to
evaluate treatment effects were performed in a timely manner
depending on the situations of individual cases. In addition to the
CT-determined volume measurements, MRI, and PET-CT imaging were
conducted when deemed appropriate to assess the presence and extent
of any residual tumor in the treated area. The duration of
loco-regional control (LC) was determined by the time in months at
which tumor regrowth in the irradiated target lesion was noted by
one of these imaging techniques, and this event indicated local
recurrence. LC and duration of progression free survival (PFS)
after the completion of RT were determined using the Kaplan-Meier
method. In the case of LC and PFS, death of the subject was
regarded a censoring event.
The irradiation dose was calculated as equivalents
of 2 Gy fractions (EQD2) with the α/β ratio of 3.5 (breast cancer
has a low ratio of α/β), and described as Gy3.5 (7,8).
Additionally, the subjects were divided into two groups, less than
60 Gy3.5 (60 Gy<) and 60 Gy3.5 or more (≥60 Gy), and evaluated
according to whether there was a statistically significant
difference in number of sensitizer injections, MTS, duration of LC,
and time to progression (TTP) using Student's t-test. Comparison of
duration of LC and PFS between the two groups (60 Gy<, ≥60 Gy)
was evaluated using the Kaplan-Meier method.
Statistical analysis
Survival periods were measured starting from the day
after end of treatment. The tumor volume was measured by the
radiotherapy planning device Varian (Varian Medical 85 Systems)
Eclipse ver.11.0. Continuous variables are presented as mean ±
standard deviation. Categorical variables are presented as numbers
(percentage). The Kaplan-Meier method was used to calculate
survival analysis and the differences were compared using Wilcoxon
rank sum test. Comparison between the 2 groups, differences in
parameters depending on the irradiation dose filled was performed
using the Wilcoxon rank sum test. All experiments were performed in
duplicate. For analyses, EZR software, version 1.54 and statistical
data analysis in Excel 2016 was used. P<0.05 was considered to
indicate statistically significant differences.
Results
Patients treated with KORTUC II
Table I shows the
baseline patient characteristics. All of the 30 patients were
women, and the mean age was 61 years (43-75 years). RT was
performed at the median dose of 53 Gy/19 Fr (40 Gy/16 Fr-67.5 Gy/25
F). The median irradiation dose was 60.4 Gy3.5
(43.6-76.1 Gy3.5). The median total number of sensitizer
injections was 5 (2-7).
Of the 30 patients, 22 patients were treated concomitantly with
hormone therapy and 18 patients with chemotherapy. Concomitant
treatment status for one patient was unknown. The median follow-up
period was 19 months (3-106 months).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient no. | Age, years | Disease | Stage | Irradiation site | Radiation dose,
Gy | Number of
fraction | EQD2 Gy3.5 | Number of
sensitizer injections | Hormonal
therapy | Chemotherapy |
|---|
| 1 | 45 | LRBC | | CW | 44 | 18 | 48 | 4 | - | - |
| 2 | 75 | LRBC | | CW | 40 | 16 | 44 | 7 | - | + |
| 3 | 70 | LABC | IIIB | CW, Ax | 67 | 25 | 77 | 6 | + | + |
| 4 | 57 | LABC | IV | CW, Ax | 59 | 21 | 69 | 7 | + | + |
| 5 | 67 | LRBC | | CW | 53 | 19 | 61 | 5 | + | + |
| 6 | 54 | LRBC | | Br, Ax | 59 | 21 | 69 | 3 | + | + |
| 7 | 56 | LRBC | | CW | 53 | 21 | 59 | 6 | UK | UK |
| 8 | 60 | LRBC | | Br | 59 | 22 | 68 | 6 | - | - |
| 9 | 67 | LRBC | | CW, Ax | 58 | 25 | 62 | 6 | + | + |
| 10 | 49 | LRBC | | Br | 58 | 29 | 59 | 5 | + | + |
| 11 | 68 | LRBC | | Br, Ax | 54 | 18 | 65 | 5 | + | - |
| 12 | 75 | LRBC | | Br | 59 | 21 | 69 | 5 | + | - |
| 13 | 58 | LRBC | | CW | 59 | 21 | 69 | 5 | + | + |
| 14 | 73 | LRBC | | CW | 59 | 21 | 69 | 5 | + | - |
| 15 | 75 | LRBC | | Br | 59 | 21 | 69 | 4 | + | + |
| 16 | 67 | LRBC | | CW, Ax, SC | 60 | 30 | 60 | 3 | + | - |
| 17 | 59 | LRBC | | Ax, SC | 60 | 30 | 60 | 5 | + | + |
| 18 | 72 | LABC | IV | CW | 44 | 16 | 51 | 3 | + | + |
| 19 | 51 | LRBC | | SC, Neck | 60 | 30 | 60 | 2 | - | + |
| 20 | 74 | LRBC | | Br, Ax | 53 | 19 | 61 | 5 | + | + |
| 21 | 74 | LABC | IIIB | Br, Ax, SC | 53 | 19 | 61 | 5 | + | - |
| 22 | 52 | LRBC | | CW | 40 | 16 | 44 | 3 | + | + |
| 23 | 43 | LRBC | | CW | 53 | 19 | 61 | 5 | - | + |
| 24 | 58 | LRBC | | Br | 53 | 19 | 61 | 5 | + | + |
| 25 | 61 | LRBC | | CW | 44 | 16 | 51 | 3 | + | + |
| 26 | 43 | LABC | IIIA | Br, Ax, SC | 53 | 19 | 61 | 5 | - | + |
| 27 | 48 | LABC | IV | Br, Ax, SC | 53 | 19 | 61 | 5 | + | - |
| 28 | 59 | LABC | IIIB | Br, Ax | 53 | 19 | 61 | 5 | + | - |
| 29 | 52 | LABC | IIIB | Br, Ax, SC | 53 | 19 | 61 | 5 | - | - |
| 30 | 70 | LABC | IIIB | Br, Ax, SC | 53 | 19 | 61 | 5 | + | - |
Tumor shrinkage rate by KORTUC II
Table II shows the
therapeutic effects. The median baseline breast cancer tumor volume
measured using CT, was 53.2 cm3 and the mean volume was
116.5 cm3 (4.2-642.5 cm3). Following KORTUC
II therapy, the median MTS was 97.0% (standard deviation=9.8%) and
the mean was 91.7% (range 77.2-100%). Fifteen patients (50%) were
assessed to have achieved a clinical complete response (cCR) as a
temporary effect. The median evaluation period until MTS was 8
months (2-17 months).
 | Table IITreatment effects. |
Table II
Treatment effects.
| Patient No. | Pre-TTV,
cm3 | MTS, % | Temporary
effect | Time to MTS,
months | Regrowth in
field | LC, months | Regrowth out of
field | TTP, months | Follow up,
months | Prognosis |
|---|
| 1 | 27.3 | 82.1 | cPR | 15 | - | 36 | + | 15 | 36 | AWD |
| 2 | 32.4 | 100.0 | cCR | 6 | - | 88 | + | 21 | 88 | AWD |
| 3 | 220.3 | 100.0 | cCR | 8 | + | 30 | + | 20 | 35 | DOD |
| 4 | 397.8 | 94.1 | cPR | 12 | - | 35 | + | 21 | 45 | AWD |
| 5 | 4.2 | 100.0 | cCR | 3 | + | 36 | + | 20 | 106 | AWD |
| 6 | 240.5 | 100.0 | cCR | 3 | - | 6 | + | 3 | 6 | DOD |
| 7 | 25.9 | 100.0 | cCR | 3 | - | 3 | - | 3 | 3 | NED |
| 8 | 446.0 | 77.0 | cPR | 2 | - | 3 | - | 3 | 3 | AWD |
| 9 | 179.6 | 86.4 | cPR | 12 | + | 37 | + | 37 | 46 | DOD |
| 10 | 26.9 | 88.1 | cPR | 13 | - | 28 | + | 6 | 28 | DOD |
| 11 | 642.5 | 100.0 | cCR | 8 | - | 21 | + | 4 | 21 | AWD |
| 12 | 71.9 | 100.0 | cCR | 17 | - | 28 | - | 28 | 28 | DOAD (pancreatic
cancer) |
| 13 | 176.9 | 78.7 | cCR | 10 | - | 72 | + | 4 | 72 | AWD |
| 14 | 25.3 | 100.0 | cCR | 5 | - | 33 | - | 33 | 33 | NED |
| 15 | 39.0 | 100.0 | cCR | 8 | - | 13 | + | 13 | 13 | DOD |
| 16 | 4.2 | 100.0 | cCR | 17 | - | 62 | - | 62 | 62 | NED |
| 17 | 29.5 | 100.0 | cCR | 9 | - | 59 | + | 11 | 59 | AWD |
| 18 | 260.1 | 84.5 | cPR | 12 | - | 37 | + | 4 | 37 | AWD |
| 19 | 68.2 | 90.8 | cPR | 5 | - | 8 | + | 1 | 8 | DOD |
| 20 | 65.6 | 76.1 | cPR | 12 | - | 25 | + | 5 | 25 | AWD |
| 21 | 23.7 | 100.0 | cCR | 16 | - | 16 | - | 16 | 16 | NED |
| 22 | 116.7 | 74.7 | cPR | 7 | - | 17 | + | 9 | 17 | AWD |
| 23 | 5.4 | 72.2 | cPR | 3 | + | 12 | + | 2 | 14 | DOD |
| 24 | 20.3 | 100.0 | cCR | 9 | - | 9 | - | 9 | 9 | DOD |
| 25 | 113.5 | 73.1 | cPR | 1 | - | 3 | + | 1 | 3 | AWD |
| 26 | 35.7 | 100.0 | cCR | 7 | - | 7 | - | 7 | 7 | NED |
| 27 | 7.2 | 100.0 | cCR | 5 | - | 11 | - | 11 | 11 | NED |
| 28 | 26.2 | 89.3 | cPR | 6 | - | 9 | - | 9 | 9 | AWD |
| 29 | 83.2 | 92.5 | cPR | 4 | - | 7 | - | 7 | 7 | AWD |
| 30 | 78.1 | 91.4 | cPR | 5 | - | 7 | - | 7 | 7 | AWD |
Loco-regional control and progression
free survival
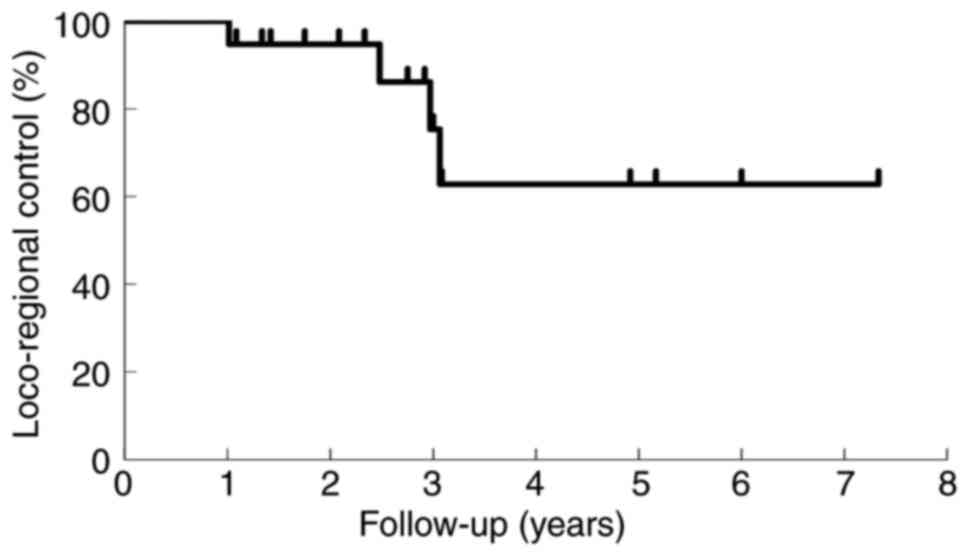
Tumor regrowth in treated lesions occurred in 4
patients (13.3%) at 30, 37, 36, and 12 months after treatment. The
proportion of patients with enduring LC at 1, 2, and 3 years was
100, 94.7, and 75.4%, respectively, as shown in the Kaplan-Meier
curve (Fig. 1). Seventeen patients
(56.7%) presented with tumor exacerbation outside the irradiation
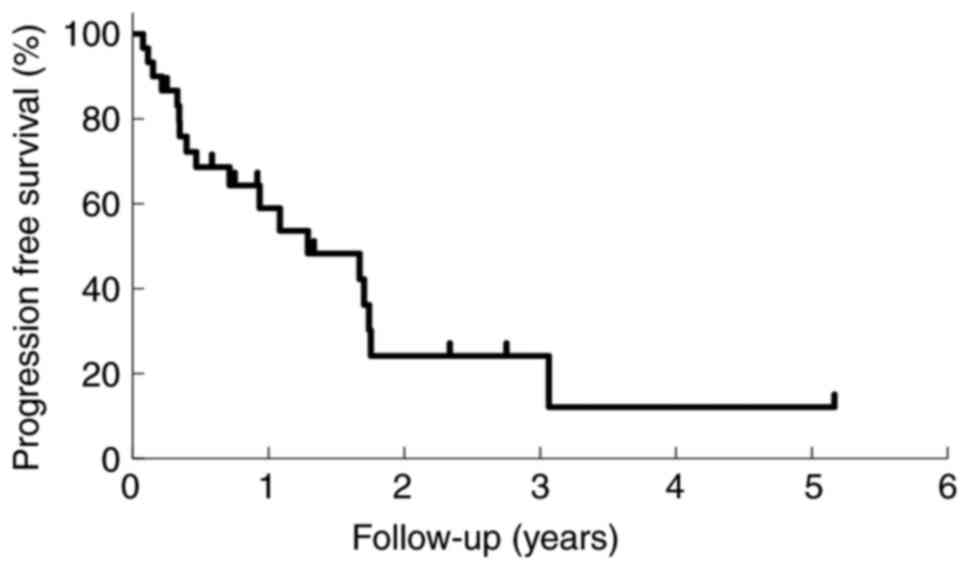
field. The median duration of PFS was 9 months (2-62 months). Nine
patients (30%) died-eight of the primary disease, and one of other
disease (pancreatic cancer). The proportion of patients with PFS
after RT at 1 and 2 years was 59.0, and 24.1%, respectively, as
shown in Fig. 2. Two patients (nos.
6 and 23) developed chest wall necrosis in the irradiated area 2
and 3 months after RT, respectively.
Subgroup analysis between 60 Gy<
and ≥60 Gy
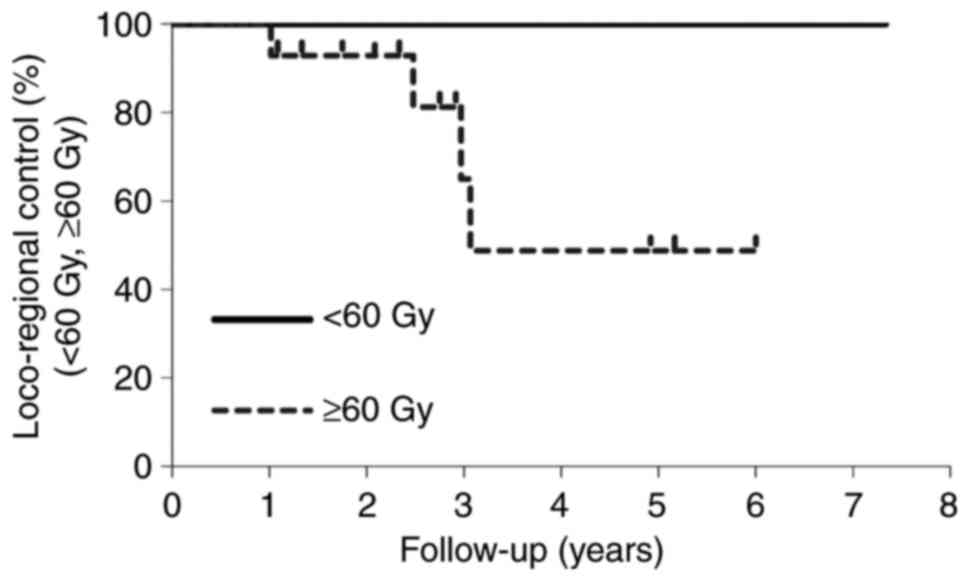
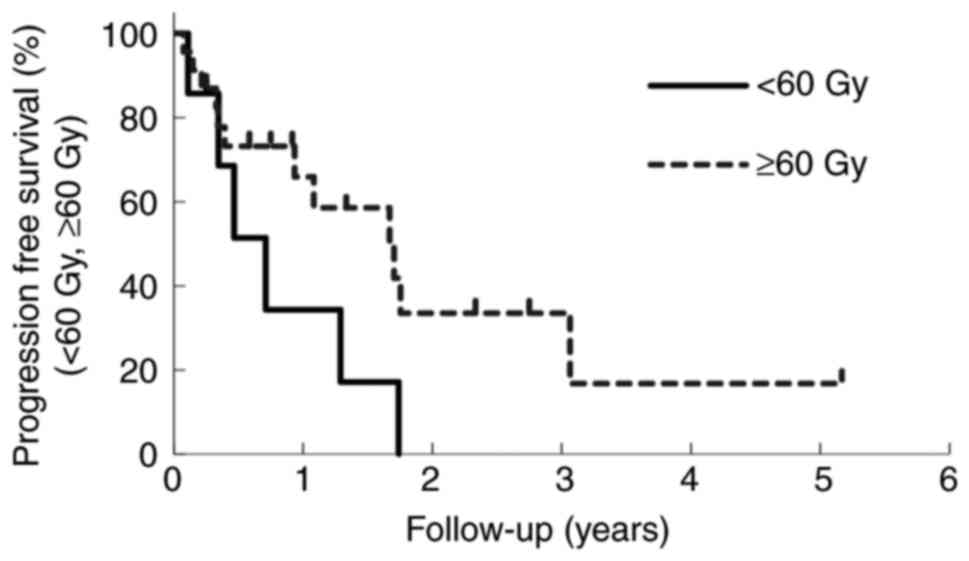
Table III shows
that the difference in the calculated EQD2 between the two groups,
60 Gy< and ≥60 Gy, was statistically significant (P<0.01). On
the other hand, there was no significant difference in number of
sensitizer injections (P=0.40), MTS (P=0.09), duration of LC
(P=0.49), and TTP (P=0.30). There was no difference in duration of
LC (P=0.19) and PFS (P=0.21) between the two groups, as shown in
Figs. 3 and 4.
 | Table IIIDifferences in selected parameters
according to radiation dose (Wilcoxon rank sum test). |
Table III
Differences in selected parameters
according to radiation dose (Wilcoxon rank sum test).
| Outcome | <60 Gy | ≥60 Gy | P-value |
|---|
| Number of patients
(%) | 7 (23%) | 23 (77%) | |
| EQD2(3.5), avg.
Gy | 50.2 | 63.5 | <0.01 |
| Total number of
injections, avg. | 4.4 | 4.9 | 0.40 |
| MTS, avg. % | 86.1 | 93.4 | 0.09 |
| Duration of LC,
avg. months | 30.3 | 23.7 | 0.49 |
| TTP, avg.
months | 8.4 | 14.5 | 0.30 |
Discussion
Radiosensitizers have been widely studied as a
method to enhance the effects of RT. Although a number of
radiosensitizers, including misonidazole, were developed in the
past (9-12),
many of them have not been used in clinical settings due to adverse
reactions such as peripheral neuropathy. In this context, KORTUC
II, a new enzyme-targeting and radiation sensitizer developed at
Kochi University, has gathered attention (1-6).
The sensitizer contains H2O2 and hyaluronic
acid as its main components, which, along with their decomposition
products, are harmless to the human body. This suggests it is a
safe sensitizer if proper dosage is used and attention is paid to
avoid procedural problems such as incorrect administration into
blood vessels (5). The severity of
acute-phase adverse events, such as radio-dermatitis, has been
reported to be comparable with that following adjuvant radiation
for regular breast-conserving therapy (13-15).
Also, no particular delay in acute-phase injuries has been
observed. At our institution, we have used KORTUC II to treat over
250 cases of various solid cancers, including the LABC and LRBC
reported in this article, but have observed no marked adverse
reactions.
In our study, the 30 patients who underwent KORTUC
II treatment experienced median MTS of 97%, the cCR rate was 50%,
and the LC rate was 100, 94.7, and 75.4% at 1, 2, and 3 years,
respectively (median follow-up period 19 months). Generally,
excellent LC was obtained after therapy. Patients with LABC and
LRBC have the potential for long term survival, and treatment that
provides continuous symptom relief and local control is desired.
The standard treatment for LABC is multidisciplinary treatment,
with chemotherapy followed by local therapy such as surgery and RT
(16-18).
Twenty one of the 30 patients who received KORTUC treatment (70%)
experienced tumor re-growth, despite all having received chemo or
hormonal therapy. Even in these cases, tumor shrinkage was also
observed and QOL was improved.
Regarding the radiation dose, it has been reported
that irradiation of 30 Gy or more had a significant effect on
symptom relief, and that patients who received 60 Gy or more at the
primary site had a higher local control rate for 5 years compared
with patients who received less than 60 Gy (19,20).
Sheldon et al (19)
concluded that high-dose RT without mastectomy is an effective
means of local control of LABC. In our case, we administered a high
dose with a median 60.4 Gy3.5, but at this relatively high dose
level there was no statistically significant difference in MTS,
duration of LC and TTP depending on the irradiation dose. Although
the responses of these 30 patients seems favorable considering
their pre-treatment conditions, because individualized
multidisciplinary treatment is used for LABC and LRBC, it would be
difficult to compare the responses with those of patients who did
not receive KORTUC II. Future well controlled cohort or
retrospective case-control studies are necessary to address this
issue.
Takaoka et al (21) reported the in vivo efficacy
of radiotherapy combined with prior intratumoral
H2O2 injection. A dose-modifying factor of
1.3-1.5 would be expected when combined with fractionated
radiotherapy. If 3% H2O2 were injected alone,
it would cause severe pain at the injection site. However, diluting
the sensitizer fivefold with sodium hyaluronate reduces this pain
to a mild level in the experience of our institution. In addition,
mixing the moderately viscous sodium hyaluronate with the
sensitizer retards its enzymatic breakdown and dispersion,
resulting in an elevated oxygen partial pressure inside the tumor
for over 24 h (3,4,6,22).
Therefore, twice-weekly intra-tumor local injection may be the best
regimen, considering the sensitizing effects, need to limit patient
discomfort, and the effort of injection. Another major advantage is
that H2O2 and sodium hyaluronate are
inexpensive agents.
In addition, sodium hyaluronate itself may have the
potential to suppress cancer progression and metastasis (23-26).
It has highly metabolized in the lymphatic system, and migrates
readily via lymphatic capillaries to regional lymph nodes following
injection into breast tumor tissue (27-29).
When accompanied by H2O2, these two compounds
together can sensitize metastatic foci in lymph nodes. In the cases
of KORTUC II treatment for first-episode breast cancer in our
institution, we have experienced many cases of regression of
axillary and supraclavicular fossa lymph node metastases in
patients who received local injections of the sensitizer into the
primary tumor, although none was injected into the metastatic
nodes. CD44, which is highly expressed on the surface of cancer
stem cells, is an adhesion molecule for which hyaluronic acid is a
ligand (26,30,31).
This sensitizer is believed to target even the breast cancer stem
cells (14).
Generally, KORTUC II aimed for local tumor control
and symptom relief in patients with LABC and LRBC. Patients with
unresectable LABC and LRBC often have severely compromised QOL, due
to massive exudation and bleeding from the lesion, odor, and
disfigurement. NCCN guidelines version 5. 2020(32) recommends multidisciplinary treatment
for LABC with a focus on drug therapy supplemented by surgery and
RT. However, in many cases, regular RT fails to achieve
satisfactory results for patients with unresectable tumors and many
of these patients also do not respond to drug therapy. In this
study, we have achieved significant local effects in the treatment
of LABC and LRBC with a diameter of 10 cm or larger and open skin
lesions. KORTUC II has improved greatly QOL and has been
appreciated by the patients who received this therapy (15), suggesting it is a highly
satisfactory treatment option.
Although the KORTUC II is effective in LC, it
requires precautions, as soft tissue necrosis of the chest wall was
observed in two patients after treatment. Their common features
were that the tumor had invaded deep into the chest wall and the
soft tissue necrosis occurred at the same time as the malignant
lesions were expanding. The soft tissue necrosis may have been
caused by inhibition of normal tissue recovery together with tumor
tissue necrosis. Therefore, if imaging shows the tumor is invading
deep into the chest wall and the tumor is growing rapidly, it may
be best to forego or limit KORTUC II therapy to reduce the risk of
soft tissue necrosis. It should be noted that soft tissue necrosis
of the chest wall has also been reported with RT alone (33).
Many fundamental issues remain to be clarified
related to KORTUC II, particularly quantified levels of patient
benefit and how KORTUC II can be combined optimally with radiation,
chemotherapy and immunotherapy to treat various cancers. To date
only a single phase 1 clinical trial has been completed, and this
showed no significant adverse effects in patients with LABC
(34). Nimalasena et al
(34) reported that injection pain
was tolerable, dermatitis was not exacerbated, and the tumor
regression rate was 50-100%. Biomarker tests demonstrated
significant changes in IL-4, MIP-1α, IL-1β, and TRAIL compared with
those of the patient group without sensitizer, suggesting apoptosis
induced by TNF-related apoptosis-inducing ligands associated with
activated T-cell signaling and increased macrophage stimulation
(34). Kariya et al
(35) reported that
H2O2 enhanced lysosome-dependent
X-ray-induced apoptosis in an in vitro experiment. A phase 2
study has been underway in five sites in the United Kingdom since
June 2020-the only phase 2 trial to date. In the future, we need
more clinical trials to promote widespread use of KORTUC II and to
include it within insurance coverage.
In conclusion, KORTUC II demonstrated high rates of
LC for LABC and LRBC. These effects may not be achievable with
regular RT alone. Moreover, this method can play a major role in
alleviating symptoms. KORTUC II is expected to be an inexpensive
and extremely promising mode of RT with an excellent
radiosensitizing effect.
Acknowledgements
The authors would like to thank Dr Yasuhiro Ogawa
(Department of Radiation Oncology, Kochi General Rehabilitation
Hospital, Kochi, Japan), visionary inventor of the KORTUC.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS and KN designed the study and wrote the
manuscript. TS, YU and HA analyzed and interpreted the patient's
clinical data for the manuscript. TS, MN, HY, CS, AH, KK and MI
performed the KORTUC treatment and statistical analysis. KY
contributed to collecting the relevant literature and to data
analysis, and reviewed and critically interpreted the information.
TS and HY confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients. Approval was obtained from the Ethics Committee of Osaka
Medical College (Osaka, Japan), trial no. 1973 (May 10, 2010).
Patient consent for publication
The patients provided written informed consent for
the publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ogawa Y, Kubota K, Ue H, Nishioka A,
Kariya S, Yokota N, Sasaki T, Suzuki K, Nakatani K, Yamanishi T, et
al: Development and clinical application of a new radiosensitizer
containing hydrogen peroxide and hyaluronic acid sodium for topical
tumor injection-A new enzyme-targeting radiosensitization
treatment, KORTUC II (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas, Type II). Strahlenther Onkol. 183:100–101.
2007.
|
|
2
|
Ogawa Y, Ue H, Tsuzuki K, Tadokoro M,
Miyatake K, Sasaki T, Yokota N, Hamada N, Kariya S, Hitomi J, et
al: New radiosensitization treatment (KORTUC I) using hydrogen
peroxide solution soaked gauze bolus for unresectable and
superficially exposed neoplasms. Oncol Rep. 19:1389–1394.
2008.PubMed/NCBI
|
|
3
|
Ogawa Y, Kubota K, Ue H, Kataoka Y,
Tadokoro M, Miyatake K, Tsuzuki K, Yamanishi T, Itoh S, Hitomi J,
et al: Phase I study of a new radiosensitizer containing hydrogen
peroxide and sodium hyaluronate for topical tumor injection: A new
enzyme-targeting radiosensitization treatment, Kochi
Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II
(KORTUC II). Int J Oncol. 34:609–618. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tokuhiro S, Ogawa Y, Tsuzuki K, Akima R,
Ue H, Kariya S and Nishioka A: Development of a novel
enzyme-targeting radiosensitizer (KORTUC) containing hydrogen
peroxide for intratumoral injection for patients with low linear
energy transfer-radioresistant neoplasms. Oncol Lett. 1:1025–1028.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ogawa Y, Kubota K, Ue H, Tadokoro M,
Matsui R, Yamanishi T, Hamada N, Kariya S, Nishioka A, Nakajima H,
et al: Safety and effectiveness of a new enzyme-targeting
radiosensitization treatment (KORTC II) for intratumoral injection
for low-LET radioresistant tumors. Int J Oncol. 39:553–560.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ogawa Y: Paradigm shift in radiation
biology/radiation oncology-exploitation of the
‘H2O2 effect’ for radiotherapy using low-LET
(linear energy transfer) radiation such as X-rays and high-energy
electrons. Cancers (Basel). 8(28)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Holloway CL, Panet-Raymond V and Olivotto
I: Hypofractionation should be the new ‘standard’ for radiation
therapy after breast conserving surgery. Breast. 19:163–167.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qi XS, White J and Li XA: Is α/β for
breast cancer really low? Radiother Oncol. 100:282–288.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Karim AB, Faber DB, Haas RE, Hoekstra FH
and Njo KH: Metronidazole as a radiosensitizer: A preliminary
report on estimation in serum and saliva. Int J Rad Oncol Biol
Phys. 6:1233–1236. 1980.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Carabell SC, Bruno LA, Weinstein AS,
Richter MP, Chang CH, Weiler CB and Goodman RL: Misonidazole and
radiotherapy to treat malignant glioma: A phase II trial of the
radiation therapy oncology group. Int J Radiat Oncol Biol Phys.
7:71–77. 1981.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jette DC, Wiebe LI and Chapman JD:
Synthesis and in vivo studies of the radiosensitizer4-(82Br)
bromo-misonidazole. Int J Nucl Med Biol. 10:205–201.
1983.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Coleman CN: Hypoxic cell radiosensitizers:
Expectations and progress in drug development. Int J Rad Oncol Biol
Phys. 11:323–329. 1985.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aoyama N, Ogawa Y, Yasuoka M, Ohgi K,
Iwasa H, Miyatake K, Yoshimatsu R, Yamanishi T, Hamada N, Tamura T,
et al: Therapeutic results of a novel enzyme-targeting
radiosensitization treatment, Kochi oxydol-radiation therapy for
unresectable carcinomas II, in patients with stage I primary breast
cancer. Oncol Lett. 13:4741–4747. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ogawa Y, Kubota K, Aoyama N, Yamanishi T,
Kariya S, Hamada N, Nogami M, Nishioka A, Onogawa M and Miyamura M:
Non-surgical breast-conserving treatment (KORTUC-BCT) using a new
radiosensitization method (KORTUC II) for patients with stage I or
II breast cancer. Cancers (Basel). 7:2277–2289. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shibamoto Y, Murai T, Suzuki K, Hashizume
C, Ohta K, Yamada Y, Niwa M, Torii A and Shimohira M: Definitive
radiotherapy with SBRT or IMRT boost for breast cancer: Excellent
local control and cosmetic outcome. Technol Cancer Res Treat.
17(1533033818799355)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Costa R, Hansen N and Gradishar WJ:
Locally advanced breast cancer. Breast. e6:819–831. 2018.
|
|
17
|
Gröhn P, Heinonen E, Klefström P and
Tarkkanen J: Adjuvant postoperative radiotherapy, chemotherapy, and
immunotherapy in stage III breast cancer. Cancer. 54:670–674.
1984.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Effects of chemo-therapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sheldon T, Hayes DF, Cady B, Parker L,
Osteen R, Silver B, Recht A, Come S, Henderson IC and Harris JR:
Primary radiation therapy for locally advanced breast cancer.
Cancer. 60:1219–1225. 1987.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vempati P, Knoll MA, Dharmarajan K, Green
S, Tiersten A and Bakst RL: Palliation of ulcerative breast lesions
with radiation. Anticancer Res. 36:4701–4705. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Takaoka T, Shibamoto Y, Matsuo M, Sugie C,
Murai T, Ogawa Y, Miyakawa A, Manabe Y, Kondo T, Nakajima K, et al:
Biological effects of hydrogen peroxide administered intratumorally
with or without irradiation in murine tumors. Cancer Sci.
108:1787–1792. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aoyama N, Ogawa Y, Yasuoka M, Takahashi M,
Iwasa H, Miyatake K, Yamanishi T, Hamada N, Tamura T, Nishioka A
and Yamagami T: Therapeutic response to a novel enzyme-targeting
radiosensitization treatment (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas) in patients with recurrent breast cancer.
Oncol Lett. 12:29–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ooki T, Murata-Kamiya N,
Takahashi-Kanemitsu A, Wu W and Hatakeyama M: High-molecular-weight
hyaluronsan is a hippo pathway ligand directing cell
density-dependent growth inhibition via PAR1b. Dev Cell.
49:590–604.e9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Witschen PM, Chaffee TS, Brady NJ, Huggins
DN, Knutson TP, LaRue RS, Munro SA, Tiegs L, McCarthy JB, Nelson AC
and Schwertfeger KL: Tumor cell associated hyaluronan-CD44
signaling promotes pro-tumor inflammation in breast cancer. Cancers
(Basel). 12(1325)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han W, Song L, Wang Y, Lv Y, Chen X and
Zhao X: Preparation, characterization, and inhibition of hyaluronic
acid oligosaccharides in triple-negative breast cancer.
Biomolecules. 9(436)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bourguignon LY, Shiina M and Li JJ:
Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA
functions, chemoresistance, and radiation resistance in cancer stem
cells leading to tumor progression. Adv Cancer Res. 123:255–275.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pandey MS, Harris EN, Weigel JA and Weigel
PH: The cytoplasmic domain of the hyaluronan receptor for
endocytosis (HARE) contains multiple endocytic motifs targeting
coated pit-mediated internalization. J Biol Chem. 283:21453–21461.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Weigel JA, Raymond RC, McGary C, Singh A
and Weigel PH: A blocking antibody to the hyaluronan (HA) receptor
for endocytosis (HARE) inhibits HA clearance by perfused liver. J
Biol Chem. 278:9808–9812. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhou B, Weigel JA, Saxena A and Weigel PH:
Molecular cloning and functional expression of the rat 175-kDa
hyaluronan receptor for endocytosis. Mol Biol Cell. 13:2853–2868.
2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dick JE: Breast cancer stem cells
revealed. Proc Natl Acad Sci USA. 100:3547–3549. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
NCCN guidelinrs version 5.2020 Breast
cancer. Available from: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf.
|
|
33
|
Wahl AO, Rademaker A, Kiel KD, Jones EL,
Marks LB, Croog V, McCormick BM, Hirsch A, Karkar A, Motwani SB, et
al: Multi-institutional review of repeat irradiation of chest wall
and breast for recurrent breast cancer. Int J Rad Oncol Biol Phys.
70:477–484. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nimalasena S, Gothard L, Anbalagan S,
Allen S, Sinnett V, Mohammed K, Kothari G, Musallam A, Lucy C, Yu
S, et al: Intratumoral hydrogen peroxide with radiation therapy in
locally advanced breast cancer: Results from a phase 1 clinical
trial. Int J Rad Oncol Biol Phys. 108:1019–1029. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kariya S, Sawada K, Kobayashi T, Karashima
T, Shuin T, Nishioka A and Ogawa Y: Combination treatment of
hydrogen peroxide and x-rays induces apoptosis in human prostate
cancer PC-3 cells. Int J Rad Oncol Biol Phys. 75:449–454.
2009.PubMed/NCBI View Article : Google Scholar
|