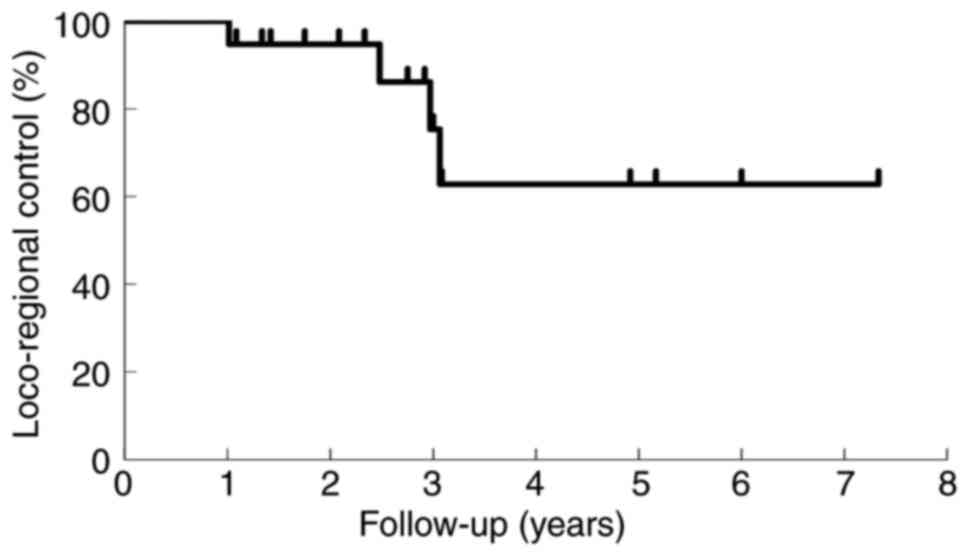
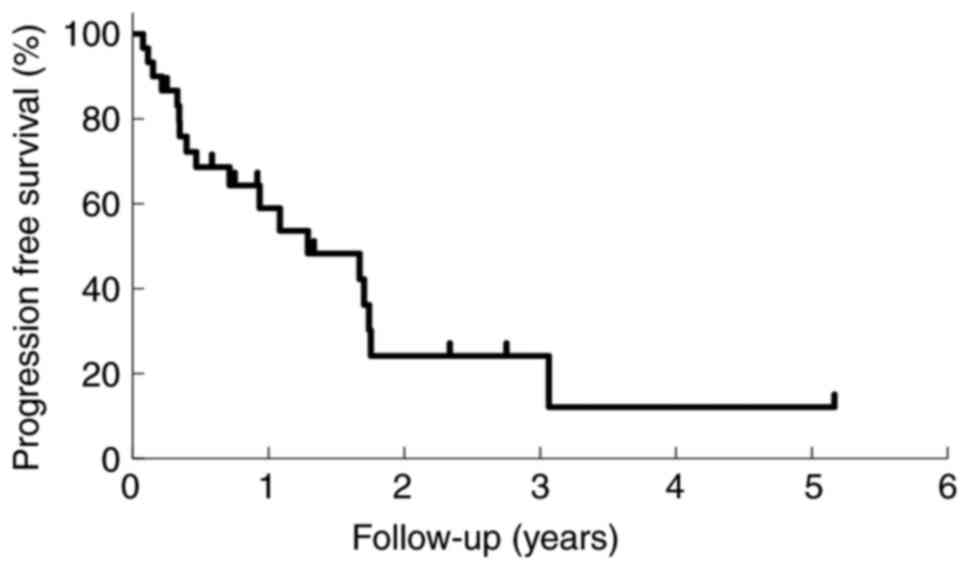
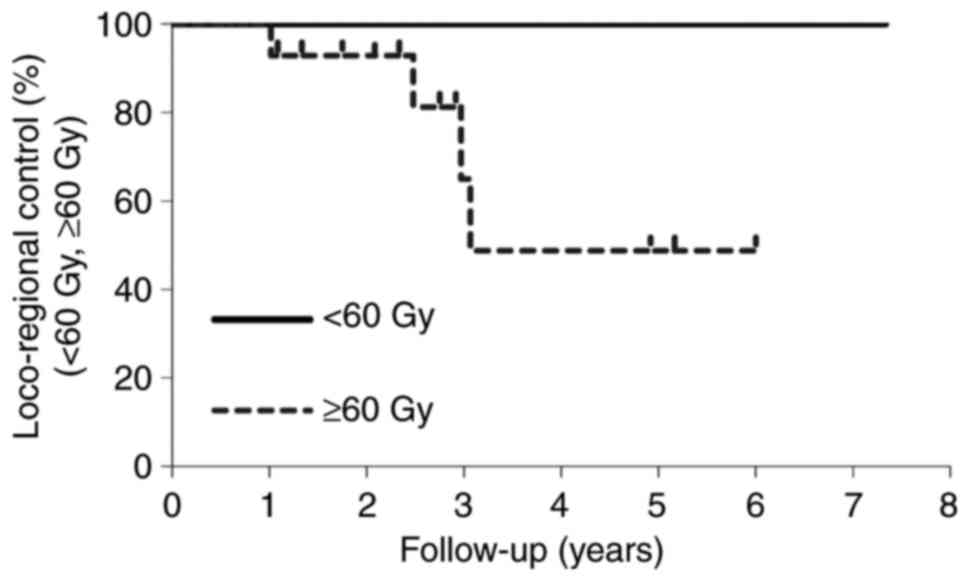
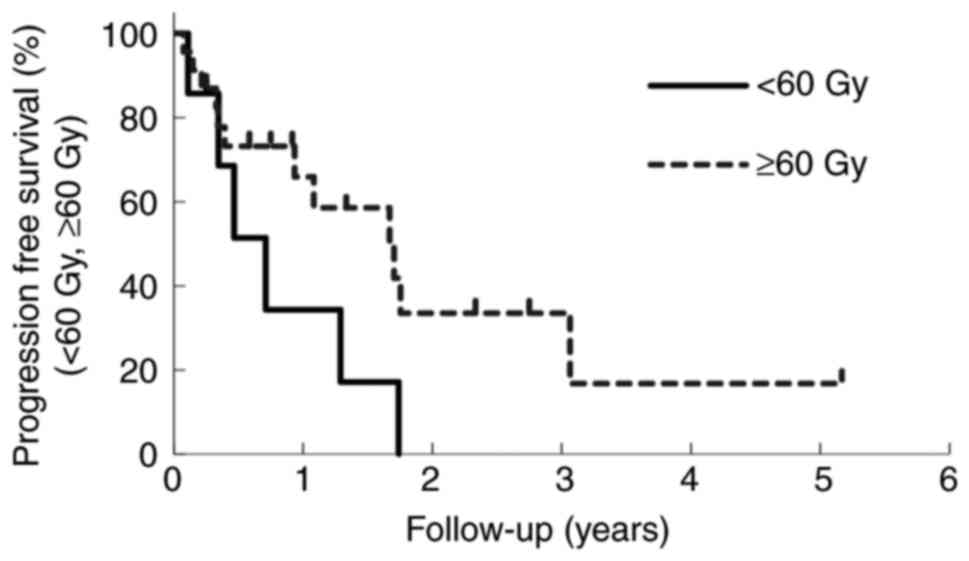
|
1
|
Ogawa Y, Kubota K, Ue H, Nishioka A,
Kariya S, Yokota N, Sasaki T, Suzuki K, Nakatani K, Yamanishi T, et
al: Development and clinical application of a new radiosensitizer
containing hydrogen peroxide and hyaluronic acid sodium for topical
tumor injection-A new enzyme-targeting radiosensitization
treatment, KORTUC II (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas, Type II). Strahlenther Onkol. 183:100–101.
2007.
|
|
2
|
Ogawa Y, Ue H, Tsuzuki K, Tadokoro M,
Miyatake K, Sasaki T, Yokota N, Hamada N, Kariya S, Hitomi J, et
al: New radiosensitization treatment (KORTUC I) using hydrogen
peroxide solution soaked gauze bolus for unresectable and
superficially exposed neoplasms. Oncol Rep. 19:1389–1394.
2008.PubMed/NCBI
|
|
3
|
Ogawa Y, Kubota K, Ue H, Kataoka Y,
Tadokoro M, Miyatake K, Tsuzuki K, Yamanishi T, Itoh S, Hitomi J,
et al: Phase I study of a new radiosensitizer containing hydrogen
peroxide and sodium hyaluronate for topical tumor injection: A new
enzyme-targeting radiosensitization treatment, Kochi
Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II
(KORTUC II). Int J Oncol. 34:609–618. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tokuhiro S, Ogawa Y, Tsuzuki K, Akima R,
Ue H, Kariya S and Nishioka A: Development of a novel
enzyme-targeting radiosensitizer (KORTUC) containing hydrogen
peroxide for intratumoral injection for patients with low linear
energy transfer-radioresistant neoplasms. Oncol Lett. 1:1025–1028.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ogawa Y, Kubota K, Ue H, Tadokoro M,
Matsui R, Yamanishi T, Hamada N, Kariya S, Nishioka A, Nakajima H,
et al: Safety and effectiveness of a new enzyme-targeting
radiosensitization treatment (KORTC II) for intratumoral injection
for low-LET radioresistant tumors. Int J Oncol. 39:553–560.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ogawa Y: Paradigm shift in radiation
biology/radiation oncology-exploitation of the
‘H2O2 effect’ for radiotherapy using low-LET
(linear energy transfer) radiation such as X-rays and high-energy
electrons. Cancers (Basel). 8(28)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Holloway CL, Panet-Raymond V and Olivotto
I: Hypofractionation should be the new ‘standard’ for radiation
therapy after breast conserving surgery. Breast. 19:163–167.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qi XS, White J and Li XA: Is α/β for
breast cancer really low? Radiother Oncol. 100:282–288.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Karim AB, Faber DB, Haas RE, Hoekstra FH
and Njo KH: Metronidazole as a radiosensitizer: A preliminary
report on estimation in serum and saliva. Int J Rad Oncol Biol
Phys. 6:1233–1236. 1980.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Carabell SC, Bruno LA, Weinstein AS,
Richter MP, Chang CH, Weiler CB and Goodman RL: Misonidazole and
radiotherapy to treat malignant glioma: A phase II trial of the
radiation therapy oncology group. Int J Radiat Oncol Biol Phys.
7:71–77. 1981.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jette DC, Wiebe LI and Chapman JD:
Synthesis and in vivo studies of the radiosensitizer4-(82Br)
bromo-misonidazole. Int J Nucl Med Biol. 10:205–201.
1983.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Coleman CN: Hypoxic cell radiosensitizers:
Expectations and progress in drug development. Int J Rad Oncol Biol
Phys. 11:323–329. 1985.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aoyama N, Ogawa Y, Yasuoka M, Ohgi K,
Iwasa H, Miyatake K, Yoshimatsu R, Yamanishi T, Hamada N, Tamura T,
et al: Therapeutic results of a novel enzyme-targeting
radiosensitization treatment, Kochi oxydol-radiation therapy for
unresectable carcinomas II, in patients with stage I primary breast
cancer. Oncol Lett. 13:4741–4747. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ogawa Y, Kubota K, Aoyama N, Yamanishi T,
Kariya S, Hamada N, Nogami M, Nishioka A, Onogawa M and Miyamura M:
Non-surgical breast-conserving treatment (KORTUC-BCT) using a new
radiosensitization method (KORTUC II) for patients with stage I or
II breast cancer. Cancers (Basel). 7:2277–2289. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shibamoto Y, Murai T, Suzuki K, Hashizume
C, Ohta K, Yamada Y, Niwa M, Torii A and Shimohira M: Definitive
radiotherapy with SBRT or IMRT boost for breast cancer: Excellent
local control and cosmetic outcome. Technol Cancer Res Treat.
17(1533033818799355)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Costa R, Hansen N and Gradishar WJ:
Locally advanced breast cancer. Breast. e6:819–831. 2018.
|
|
17
|
Gröhn P, Heinonen E, Klefström P and
Tarkkanen J: Adjuvant postoperative radiotherapy, chemotherapy, and
immunotherapy in stage III breast cancer. Cancer. 54:670–674.
1984.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Effects of chemo-therapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sheldon T, Hayes DF, Cady B, Parker L,
Osteen R, Silver B, Recht A, Come S, Henderson IC and Harris JR:
Primary radiation therapy for locally advanced breast cancer.
Cancer. 60:1219–1225. 1987.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vempati P, Knoll MA, Dharmarajan K, Green
S, Tiersten A and Bakst RL: Palliation of ulcerative breast lesions
with radiation. Anticancer Res. 36:4701–4705. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Takaoka T, Shibamoto Y, Matsuo M, Sugie C,
Murai T, Ogawa Y, Miyakawa A, Manabe Y, Kondo T, Nakajima K, et al:
Biological effects of hydrogen peroxide administered intratumorally
with or without irradiation in murine tumors. Cancer Sci.
108:1787–1792. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aoyama N, Ogawa Y, Yasuoka M, Takahashi M,
Iwasa H, Miyatake K, Yamanishi T, Hamada N, Tamura T, Nishioka A
and Yamagami T: Therapeutic response to a novel enzyme-targeting
radiosensitization treatment (Kochi Oxydol-Radiation Therapy for
Unresectable Carcinomas) in patients with recurrent breast cancer.
Oncol Lett. 12:29–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ooki T, Murata-Kamiya N,
Takahashi-Kanemitsu A, Wu W and Hatakeyama M: High-molecular-weight
hyaluronsan is a hippo pathway ligand directing cell
density-dependent growth inhibition via PAR1b. Dev Cell.
49:590–604.e9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Witschen PM, Chaffee TS, Brady NJ, Huggins
DN, Knutson TP, LaRue RS, Munro SA, Tiegs L, McCarthy JB, Nelson AC
and Schwertfeger KL: Tumor cell associated hyaluronan-CD44
signaling promotes pro-tumor inflammation in breast cancer. Cancers
(Basel). 12(1325)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han W, Song L, Wang Y, Lv Y, Chen X and
Zhao X: Preparation, characterization, and inhibition of hyaluronic
acid oligosaccharides in triple-negative breast cancer.
Biomolecules. 9(436)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bourguignon LY, Shiina M and Li JJ:
Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA
functions, chemoresistance, and radiation resistance in cancer stem
cells leading to tumor progression. Adv Cancer Res. 123:255–275.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pandey MS, Harris EN, Weigel JA and Weigel
PH: The cytoplasmic domain of the hyaluronan receptor for
endocytosis (HARE) contains multiple endocytic motifs targeting
coated pit-mediated internalization. J Biol Chem. 283:21453–21461.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Weigel JA, Raymond RC, McGary C, Singh A
and Weigel PH: A blocking antibody to the hyaluronan (HA) receptor
for endocytosis (HARE) inhibits HA clearance by perfused liver. J
Biol Chem. 278:9808–9812. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhou B, Weigel JA, Saxena A and Weigel PH:
Molecular cloning and functional expression of the rat 175-kDa
hyaluronan receptor for endocytosis. Mol Biol Cell. 13:2853–2868.
2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dick JE: Breast cancer stem cells
revealed. Proc Natl Acad Sci USA. 100:3547–3549. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
NCCN guidelinrs version 5.2020 Breast
cancer. Available from: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf.
|
|
33
|
Wahl AO, Rademaker A, Kiel KD, Jones EL,
Marks LB, Croog V, McCormick BM, Hirsch A, Karkar A, Motwani SB, et
al: Multi-institutional review of repeat irradiation of chest wall
and breast for recurrent breast cancer. Int J Rad Oncol Biol Phys.
70:477–484. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nimalasena S, Gothard L, Anbalagan S,
Allen S, Sinnett V, Mohammed K, Kothari G, Musallam A, Lucy C, Yu
S, et al: Intratumoral hydrogen peroxide with radiation therapy in
locally advanced breast cancer: Results from a phase 1 clinical
trial. Int J Rad Oncol Biol Phys. 108:1019–1029. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kariya S, Sawada K, Kobayashi T, Karashima
T, Shuin T, Nishioka A and Ogawa Y: Combination treatment of
hydrogen peroxide and x-rays induces apoptosis in human prostate
cancer PC-3 cells. Int J Rad Oncol Biol Phys. 75:449–454.
2009.PubMed/NCBI View Article : Google Scholar
|