Introduction
Stromal tumors, which originate from outside the gastrointestinal tract and share similar pathological characteristics are known as extra-gastrointestinal stromal tumors (EGISTs), account for <5% of GISTs and was more common in men ≥50 years old worldwide between 2010 and 2012 (1,2). Dissimilar to the common origin of EGISTs, prostatic EGISTs have only been reported in a few cases and lack treatment options, particularly for the high-risk cases, according to the risk classification for EGISTs from the National Institutes of Health in 2008(3).
Most cases of EGIST are considered to be malignant; however, there are a few studies with respect to the incidence rate, pathogenesis, prognosis and tumor-specific biomarkers. The histological and immunohistochemical staining of EGISTs have been described (4). However, little is known on the molecular basis of these tumors, and parameters, such as tumor size, mitotic rate, and the presence of tumor necrosis are commonly used in the evaluation of EGIST (4). Due to the advance in sequencing technology, whole-exome sequencing (WES) allows for the detection of mutations in a small percentage of tumor cells (5), which could provide a vast amount of information to understand the pathogenesis and precise treatment options (6).
A male patient with a primary high-risk prostatic EGIST was admitted to The Sixth Affiliated Hospital of Sun Yat-Sen University (Guangdong, China) in September 2017, presenting differently from previous cases with prostatic EGIST. This rare case harbored several high-risk factors, such as a tumor size ≥10 cm and a positive surgical margin; thus, WES analysis was performed and the patient was prescribed personalized adjuvant therapy. The present case study reported this rare case and discussed the clinicopathological characteristics, differential diagnosis, and therapeutic strategies for primary prostatic EGISTs.
Case report
A 65-year-old male presented with intermittent hematuria and lower urinary tract symptoms (LUTS; hesitancy, prolong voiding, difficulty, and dribbling) for ~3 months, without other digestive system-associated complaints (such as constipation and abdominal pain). The patient previously underwent surgery, specifically, transurethral resection of the prostate (TURP) two years ago, and the pathological diagnosis was a benign prostatic hyperplasia.
A MRI scan showed a 104x86x80 mm solid mass, with isointense on the T1 and slightly hypointense T2 weighted images, and indicated that its positioning was on the posterior surface of the bladder neck and part of the rectum; however, there was no clear signs of communication between them (Fig. 1A-D). A digital rectal examination revealed a large immobile solid pelvic mass of usual consistency. The laboratory examination reported total PSA levels of 1.41 ng/ml, and other related serum cancer biomarkers (CEA, AFP and CA125) were normal. The colonoscopy results showed no gastrointestinal lesions, while a core biopsy was used to diagnose an EGIST of unknown origin.
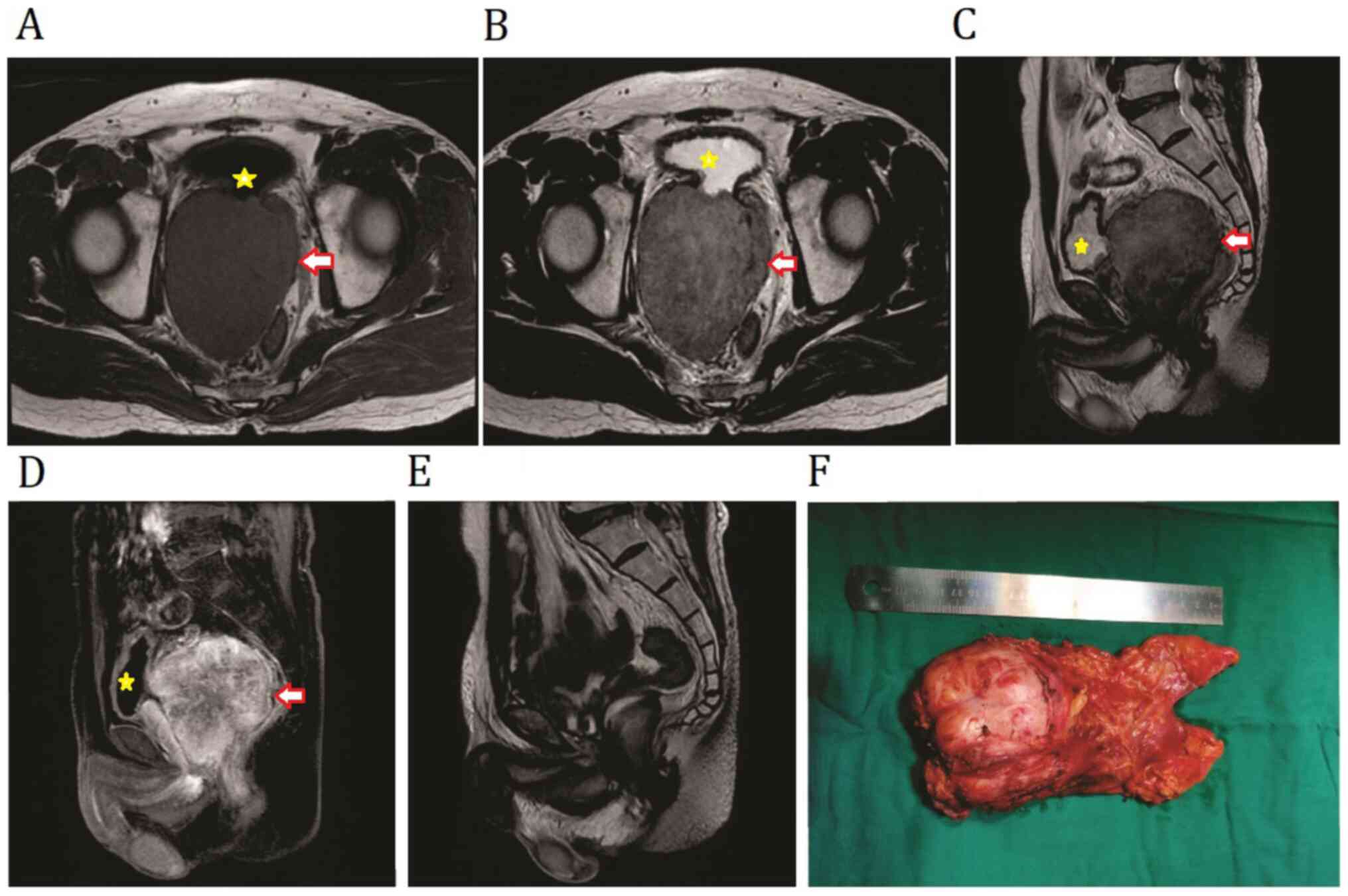 |
Figure 1
Preoperative MRI imaging of primary prostatic extra-gastrointestinal stromal tumor. Non-contrast (A) T1W1 and (B) T2W1 transverse images showing a mass between the bladder and the rectum. (C) T2WI and (D) T1WI contrast-enhanced sagittal plane of the tumor was predominantly confined to the prostate. (E) Sagittal view of T2WI MRI revealed no sign of recurrence at 1 year following surgery. (F) Image of the resected sample in the present case. The tumor is indicated by the arrows and the compressed bladder is indicated by the asterisks. WI, weighted image.
|
Differential diagnosis
The case presented with three diagnostic considerations: Benign prostatic hyperplasia (BPH) recurrence, prostate cancer (PCa) or a GIST of the rectum or EGIST of the rectal mesentery. With the history of TURP and the diagnosis of BPH, prostate recurrence was the primary concern, which does not occur in all men with a history of surgery.
Diagnosis assessment and management
The preoperative pathology results indicated an EGIST of unknown primary origin, thus, a radical prostatocystotomy and ileal conduit were performed.
The size of the tumor was 125x84x80 mm (Fig. 1F), and the final pathological results confirmed the diagnosis of a prostatic EGIST. The tumor cells were spindle cells and spiral-shaped, and found to be positive for CD34, DOG1, PSA and CD117, slightly positive for AR but negative for cytokeratin, SMA, S-100 and desmin using immunohistology; in addition, the Ki-67 index was ~5% (Fig. 2). As expected with a positive surgical margin, there were no signs of tumor involvement in the pelvic lymph nodes, bladder, seminal vesicle, or the ureterovesical junction.
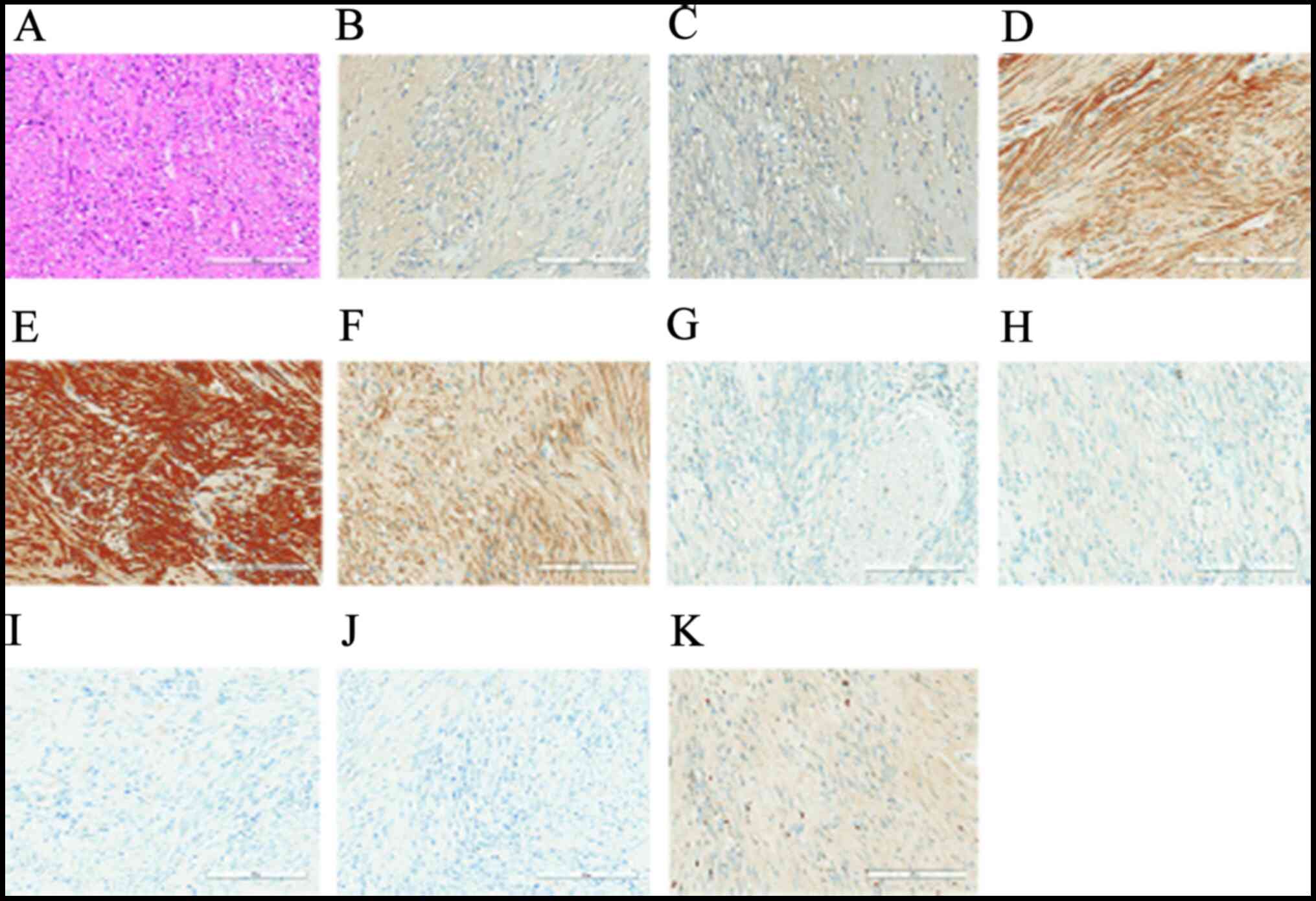 |
Figure 2
Representative staining results in the primary prostatic extra-gastrointestinal stromal tumor. (A) Hematoxylin and eosin staining showed that the tumor cells predominantly consisted of spindle cells growing in fascicles. Immunohistochemistry staining revealed positive staining for (B) prostate-specific antigen, (C) androgen receptor, (D) DOG1, (E) CD34, (F) CD117; negative staining for (G) cytokeratin, (H) SMA, (J) S-100 and (J) Desmin, and (K) Ki-67 (at 5%).
|
WES results
WES was performed by 3D-Medicines Corp.. Total DNA was extracted from the postoperative tissue and the peripheral blood. WES results identified a high number of mutations, including 110 single nucleotide variants (SNVs) from 124 genes and 793 copy number variations (CNVs). A total of 866 (95.9%) genes were mapped to distinct proteins using the ConsensusPathDB database (P<0.01) (http://cpdb.molgen.mpg.de/). Following further pathway analysis, 68 genes (64.2%) with SNVs were found to be present in >1 of the 12 pathways and 449 candidates (59.1%) with CNVs were associated with 35 pathways (Table SI).
However, the functions of the majority of the genes mutated in the primary prostatic EGIST remain unknown. A database of Food and Drug Administration-approved drug candidates for personalized adjuvant therapy was generated using a drug-gene interaction database (DGIdb; www.dgidb.org). Drug candidates, which scored >10, according to a previous study (7) are summarized in Table SII. Imatinib (400 mg per day) and bicalutamide (50 mg per day) were prescribed for adjuvant therapy, and the treatment was stopped when the PSA levels remained stable, at <0.01 ng/ml for 3 months. No signs of recurrence (Fig. 1E) and elevated PSA level were observed within 19 months during follow-up.
Discussion
GIST account for only 0.1-3% of all gastrointestinal malignancies, and ~5% cases originate in the rectum (8). Tumors that rarely arise outside of the GI tract (5%) are known as EGISTs (9), and the other common sites include the omentum, mesentery, retroperitoneum, liver, pancreas and prostate (10). A small portion of GISTs originate from the pelvis, which are misdiagnosed as prostatic EGIST. The most common problems, which can occur from an enlarged prostate are BPH and PCa; however, a giant pelvic mass is rare in PCa, although it has been reported to occur when the cancer invades the bladder or rectum (11).
Nearly twenty years have passed since the initial diagnosis of EGISTs (12); however, to the best of our knowledge, there is still no consensus or guidelines for differentiation and prognosis. EGISTs share histological and immunohistochemical features with GISTs, demonstrating similar features, such as the presence of c-KIT and PDGFR gene mutations (4,13); however, a previous report, has suggested that patients with EGISTs and GISTs have different prognoses (14). Therefore, it has been hypothesized that EGISTs should be considered as a separate type of tumor (15). EGISTs are considered to be an aggressive tumor, with a high risk of recurrence, and surgery remains the standard method of management, whereas radical resection is required for specific cases when it is suspected that the tumor has disseminated to surrounding tissues or organs (16). The 5-year survival rate for EGISTs ranges from 38% in Turkey (17) to 60.9% in Singapore (15).
The high-risk factors in the present case were a tumor size >10 cm (16), a positive surgical margin, and the large number of mutated genes identified, for which the effect and mechanism involved is currently unknown. As there are limited data available on prognosis and tailored therapy of the primary prostatic EGISTs, the present study used next-generation sequencing (NGS) on the exome to target treatment and prognosis.
Since the development and improvement of NGS, it has been widely used in cancer genomics research (18), prognosis (5), molecule-guided drug selection, and drug metabolism studies (19). With WES, all exons are sequenced at a lower cost, which enables the efficient and accurate detection, and the subsequent development of directed target therapies in rare types of tumors (20,21). Due to the limited number of cases or lack of interest, rare tumors have not been investigated in great detail. Furthermore, there is a current debate on whether WES provides valuable information for rare tumors, However, it has been indicated that studying rare types of tumors, such as prostatic EGISTs may provide novel strategies for the treatment of related diseases, such as sarcoma (22). In a ‘genome first, histology second’ method, NGS has shown potential for targeted therapy in rare tumors (23). Several studies have reported precision medicine strategies based on WES, which were compiled in a meta-analysis, proving that WES was an independent prognosis factor for rare tumor cases (23,24), such as sarcoma, Erdheim-Chester disease, castleman's disease and high-grade serous ovarian cancer.
The WES results showed the presence of a c-KIT exon 11 deletion and AR mutation. Previous studies reported that the exon 11 deletion of c-KIT was associated with a poor prognosis and malignancy of GISTs (12,25), but it demonstrated an improved response rate to treatment with imatinib compared with KIT exon 17 or PDGFR-1 D842V mutations, and a favorable progression-free survival was observed with imatinib with a standard dose of 400 mg daily (26). However, no related studies have been performed in EGISTs.
The size of the tumor has been suggested to be an independent risk factor for the survival of patients with EGISTs. For example, a previous study reported that the majority of the cases with EGISTs had a mean or median tumor size of >10 cm, while only a few cases reported a tumor <5 cm, and no EGISTs cases presented with a tumor of ≤2 cm (4). Unlike a previous report on primary EGIST and the treatment strategies used (27), the positive expression of PCa-related markers, such as PSA, AR, and P504s, have not been reported in primary prostatic EGIST. The related mechanism of these markers is mostly unknown; however, it was important to investigate the PCa-related markers for the specific case of primary prostatic EGIST, to compare the results with cases of PCa, which could then be used to determine prognosis, and develop a treatment plan using adjuvant therapy. In the present case, mutational analysis also identified a CNV in AR, and an approved drug was found from the database of FDA-approved drug candidates. By compiling the information from the DGIdb database, primary literature and expert opinions, bicalutamide was used for adjuvant therapy with close follow-up, which is also used in patients with PCa. With the 18 months follow-up, no signs of recurrence of the positive surgical margin site was found, which indicated that the personalized drugs were effective for this case, and there was no requirement for radiology, transurethral resection of the prostate or waiting.
In conclusion, primary prostatic EGIST is a rare tumor and physicians should be aware of the diagnosis and differential diagnoses using a combination of imaging, pathological and immunohistochemistry detection. There is currently a considerable gap in the knowledge in the understanding the mechanisms of primary prostatic EGIST. However, WES has the potential to determine the tumorigenesis, identify target-drug therapeutic strategies and provide prognosis for primary prostatic EGIST.
Supplementary Material
Pathways identified from the mutated genes/proteins analyzed using the ConsensusPathDB database.
Therapeutic candidate drugs predicted using the mutated genes.
Acknowledgements
The authors would like to thank Wenwen Zhong and Lei Ye (Department of Urology, The Sixth Affiliated Hospital of Sun Yat-Sen University, Guangdong, China) for providing the statistical analysis and assistance in writing the manuscript.
Funding
The present study was supported by a grant from the Natural Science Foundation of Guangdong Province (grant no. 2017A030310208).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the regulation of the People's Republic of China on the administration of human genetic resources but are available from the corresponding author on reasonable request.
Authors' contributions
LL, HQ, ZYW and DLR conceived/designed the present study. LL, ZYW and DLR wrote the manuscript. LL, ZYW and DLR authenticated the primary data. LL, HQ, DJW, BY, BM and JGQ analyzed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures were performed with the approval of The Sixth Affiliated Hospital of Sun Yat-Sen University (Guangdong, China).
Patient consent for publication
Written informed consent for publication was provided by the patient.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Kim KH, Nelson SD, Kim DH, Choi KU, Kim SJ, Min KW, Jang KS, Paik SS, Oh YH, Wan S, et al: Diagnostic relevance of overexpressions of PKC-theta and DOG-1 and KIT/PDGFRA gene mutations in extragastrointestinal stromal tumors: A Korean six-centers study of 28 cases. Anticancer Res. 32:923–937. 2012.PubMed/NCBI
|
|
2
|
Liegl-Atzwanger B, Fletcher JA and Fletcher CD: Gastrointestinal stromal tumors. Virchows Arch. 456:111–127. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Joensuu H: Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 39:1411–1419. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Reith JD, Goldblum JR, Lyles RH and Weiss SW: Extragastrointestinal (soft tissue) stromal tumors: An analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol. 13:577–585. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK and Zhang W: Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 306:1557–1565. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nakagawa H and Fujita M: Whole genome sequencing analysis for cancer genomics and precision medicine. Cancer Sci. 109:513–522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wagner AH, Coffman AC, Ainscough BJ, Spies NC, Skidmore ZL, Campbell KM, Krysiak K, Pan D, McMichael JF, Eldred JM, et al: DGIdb 2.0: Mining clinically relevant drug-gene interactions. Nucleic Acids Res. 44 (D1):D1036–1044. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miettinen M and Lasota J: Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 42:399–415. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Monabati A, Safavi M and Solhjoo F: Extragastrointestinal stromal tumor presenting as omental cyst. J Gastrointest Surg. 20:1275–1277. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Iqbal N, Sharma A and Iqbal N: Clinicopathological and treatment analysis of 13 extragastrointestinal stromal tumors of mesentery and retroperitoneum. Ann Gastroenterol. 28:105–108. 2015.PubMed/NCBI
|
|
11
|
Miah S and Catto J: BPH and prostate cancer risk. Indian J Urol. 30:214–218. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS and Sobin LH: Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: Clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 23:1109–1118. 1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yamamoto H, Oda Y, Kawaguchi K, Nakamura N, Takahira T, Tamiya S, Saito T, Oshiro Y, Ohta M, Yao T and Tsuneyoshi M: c-kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue). Am J Surg Pathol. 28:479–488. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goh BK, Chow PK, Kesavan S, Yap WM and Wong WK: Outcome after surgical treatment of suspected gastrointestinal stromal tumors involving the duodenum: Is limited resection appropriate? J Surg Oncol. 97:388–391. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hatipoglu E: Extragastrointestinal stromal tumor (EGIST): A 16-year experience of 13 cases diagnosed at a single center. Med Sci Monit. 24:3301–3306. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al: Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 33:459–465. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zheng S, Huang KE, Tao DY and Pan YL: Gene mutations and prognostic factors analysis in extragastrointestinal stromal tumor of a Chinese three-center study. J Gastrointest Surg. 15:675–681. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hui P: Next generation sequencing: chemistry, technology and applications. Top Curr Chem. 336:1–18. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Harris TJ and McCormick F: The molecular pathology of cancer. Nat Rev Clin Oncol. 7:251–265. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Groisberg R, Roszik J, Conley A, Patel SR and Subbiah V: The role of next-generation sequencing in sarcomas: Evolution from light microscope to molecular microscope. Curr Oncol Rep. 19(78)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kato S, Kurasaki K, Ikeda S and Kurzrock R: Rare tumor clinic: The university of California San Diego Moores cancer center experience with a precision therapy approach. Oncologist. 23:171–178. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Groisberg R, Hong DS, Roszik J, Janku F, Tsimberidou AM, Javle M, Meric-Bernstam F and Subbiah V: Clinical next-generation sequencing for precision oncology in rare cancers. Mol Cancer Ther. 17:1595–1601. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schwaederle M, Zhao M, Lee JJ, Eggermont AM, Schilsky RL, Mendelsohn J, Lazar V and Kurzrock R: Impact of precision medicine in diverse cancers: A meta-analysis of phase II clinical trials. J Clin Oncol. 33:3817–3825. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Subbiah V and Kurzrock R: Universal genomic testing needed to win the war against cancer: Genomics is the diagnosis. JAMA Oncol. 2:719–720. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Andersson J, Bumming P, Meis-Kindblom JM, Sihto H, Nupponen N, Joensuu H, Odén A, Gustavsson B, Kindblom LG and Nilsson B: Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology. 130:1573–1581. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: A meta-analysis of 1,640 patients. J Clin Oncol. 28:1247–1253. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu S, Yu Q, Han W, Qi L, Zu X, Zeng F, Xie Y and Liu J: Primary gastrointestinal stromal tumor of the prostate: A case report and literature review. Oncol Lett. 7:1925–1929. 2014.PubMed/NCBI View Article : Google Scholar
|
















