Introduction
Head and neck squamous cell carcinomas (HNSCCs)
comprise a group of cancers that evolve in the mucosal lining of
the upper aerodigestive tract. HNSCCs are heterogeneous in nature
(1). Nasopharyngeal carcinoma
(NPC) and cancer of the larynx are the most prevalent cancers
amongst HNSCC, followed by lip and oral cavity carcinomas (2). As per global cancer statistics, it
was reported that 177,422 new laryngeal cancers and 129,079 new NPC
cases were diagnosed globally in 2018, and they ranked the 23rd and
25th most commonly occurring cancers, respectively, with an
incidence of 1.7% of all cancers (2).
In 1964, the Epstein-Barr virus (EBV) was the first
human tumor virus discovered in Burkitt lymphoma tumor cells
(3). Later studies found
antibodies in Burkitt lymphoma patients and postnasal space
carcinomas (4). De Schryver et
al (5) demonstrated EBV
antigen detection by immunofluorescence assay, which was followed
by zur Hausen et al (6) who
used the hybridization method in NPC. EBV infection is very common
amongst individuals, and infects >90% of populations globally
(7,8). Usually, an EBV infection is
asymptomatic; however, the involvement of EBV in cancer
pathogenesis was established in several epithelial and lymphoid
malignancies (9).
The global burden of EBV-associated malignancies
increased by 14.6% between 1990 and 2010, and East Asia accounted
for almost 50% of EBV infected cancer cases during this time period
(10). The incidence of
EBV-associated cancers is ~1.5% of all cancers globally and
represents 1.8% of all cancer-associated deaths (11-13).
Amongst EBV associated cancer deaths, 92% are attributable to NPC
and gastric cancer (10). The
involvement of EBV in the pathogenesis of NPC may be through
modification of epigenetic profiles, inducing genomic instability,
evading host immunity, promoting cell survival, or contributing
stem cell like properties in NPC cells and somatic mutations in
apoptotic and tumor suppressor genes (14).
The tumor suppressor p53 and the anti-apoptotic
protein B cell lymphoma-2 (Bcl-2) are hypothesized to play a key
role in carcinogenesis, and upregulated expression of these
proteins has been observed in other types of HNSCC (15). TP53 is a critical tumor suppressor
that responds to various stress signals by regulating an
anti-proliferative transcriptional program, including transient
cell cycle arrest, cellular senescence and apoptosis (16). Despite the wide variety of TP53
network regulators and various TP53 regulated biological pathways,
a clear comprehension is lacking about how and in what context TP53
exerts its diverse effects (17).
Amongst EBV-associated cancers, upregulated
expression of wildtype TP53 has been observed, and this was shown
to be associated with the latent EBV membrane protein 1 (LMP1). The
mutual regulation between TP53 and LMP1 may play a vital role in
EBV infection and latency (18).
Similarly, Bcl-2, an antiapoptotic protein, is a key inhibitor of
programmed cell death or apoptosis in cancer (19,20).
In the pathogenesis of NPC, the upregulated expression of Bcl-2
plays an important role by regulating apoptosis (21). The present study evaluated the
protein expression pattern of TP53 and Bcl-2 in association with
EBV infection in a Saudi cohort with nasopharyngeal and laryngeal
cancer to determine the correlation and interplay between these two
proteins with EBV infection.
Materials and methods
Study cohort
Formalin fixed paraffin embedded (FFPE) tissue
samples from confirmed nasopharyngeal (n=22) and laryngeal (n=11)
carcinoma patients were collected from the Pathology Department,
King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal
University (IAU), Dammam, Saudi Arabia. The median age of the
patients was 51 years (age range, 18-90 years) with 22 males and 11
females forming the cohort. This study was approved by IAU's
Institutional Review Board (approval no. IRB-2017-01-059). A signed
informed consent form was obtained from each patient included in
the study. The procedures in the present study adhered to the
principles of the Declaration of Helsinki (22). All the samples were biopsy
specimens. Sample size was determined based on the cancer incidence
report, Saudi Arabia, 2014 using the ‘sample sizeʼ module from the
Open-Source Project in Epidemiologic Computing program (openepi.com/SampleSize/SSPropor.htm).
The tissue samples were collected from the patients who attended
the oncology clinic at the King Fahd Hospital of the University
between May 2000 and February 2015. Inclusion criteria for the
collection of samples was based on the confirmation of a carcinoma
of the larynx and/or nasopharynx by histopathology analysis. FFPE
samples with <50% tumor tissue content were excluded. The
present study included only tumor tissue obtained from the
patients.
Immunohistochemistry
The FFPE blocks were reassessed for tumor content
and diagnosis using Hematoxylin (cat. no. 6765001; Thermo Fisher
Scientific, Inc.) and Eosin (cat. no. 6766008; Thermo Fisher
Scientific, Inc.) staining. The tissue sections were prepared at
room temperature and the total duration of Hematoxylin and Eosin
staining procedure was 45 min. Immunohistochemistry was performed
to detect TP53 and Bcl-2 protein expression, and EBV infection on
4-µM FFPE sections. The primary antibody for TP53 (cat. no.
760-2542; Ventana Medical Systems, Inc.; Roche Diagnostics) used in
this study detected the wild and mutant form of the TP53 protein.
The anti-Bcl-2 antibody (cat. no. 790-4604; Ventana Medical
Systems, Inc.; Roche Diagnostics) binds to the N terminal region of
human Bcl-2 protein. EBV primary antibodies bind to latent membrane
protein (LMP-1) of EBV (cat. no. 760-2640; Cell Marque;
Sigma-Aldrich; Merck KGaA). All of the above antibodies were used
without dilution, as they were obtained in a ready to use form. The
incubation temperature was 37˚C for all three antibodies and the
timing of the incubation for TP53, Bcl-2 and EBV was 16, 20 and 26
min, respectively. Ultra-View Universal DAB Detection kit (Ventana
Medical Systems, Inc.; Roche Diagnostics) was used to visualize the
primary antibodies bound to target antigens with hydrogen peroxide
substrate and 3,3'-diaminobenzidine tetrahydrochloride chromogen,
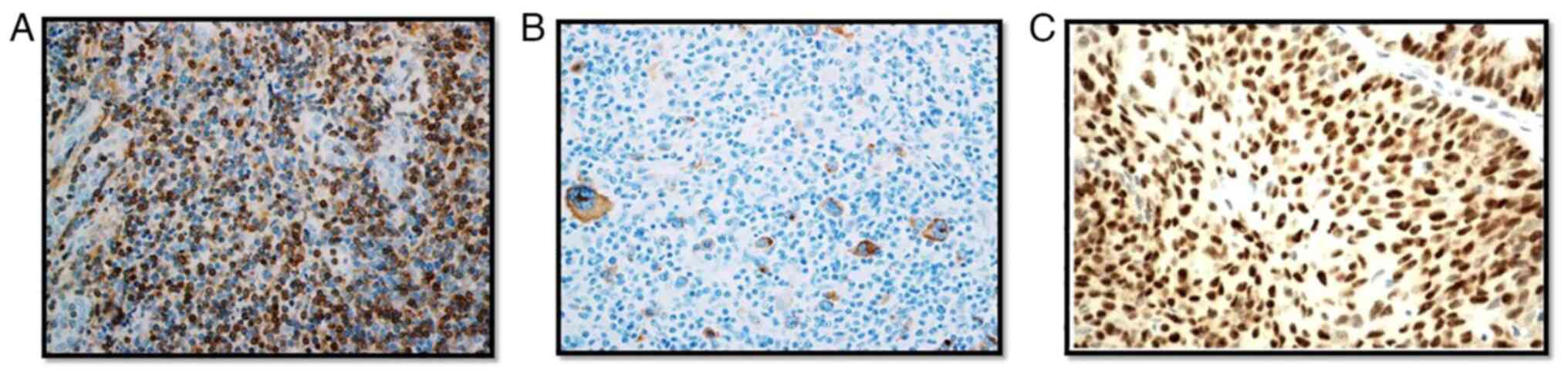
which produces a brown colored precipitate (Fig. 1). BenchMark ULTRA staining
instrument (Roche Diagnostics) was used for staining, and the
results were interpreted qualitatively by two independent
pathologists using a light microscope (magnification, x40; Leica
Microsystems, Inc.). A tumor cell was considered TP53 positive if
they showed nuclear staining and Bcl-2 positivity was confirmed if
neoplastic cells showed cytoplasmic and/or nuclear staining.
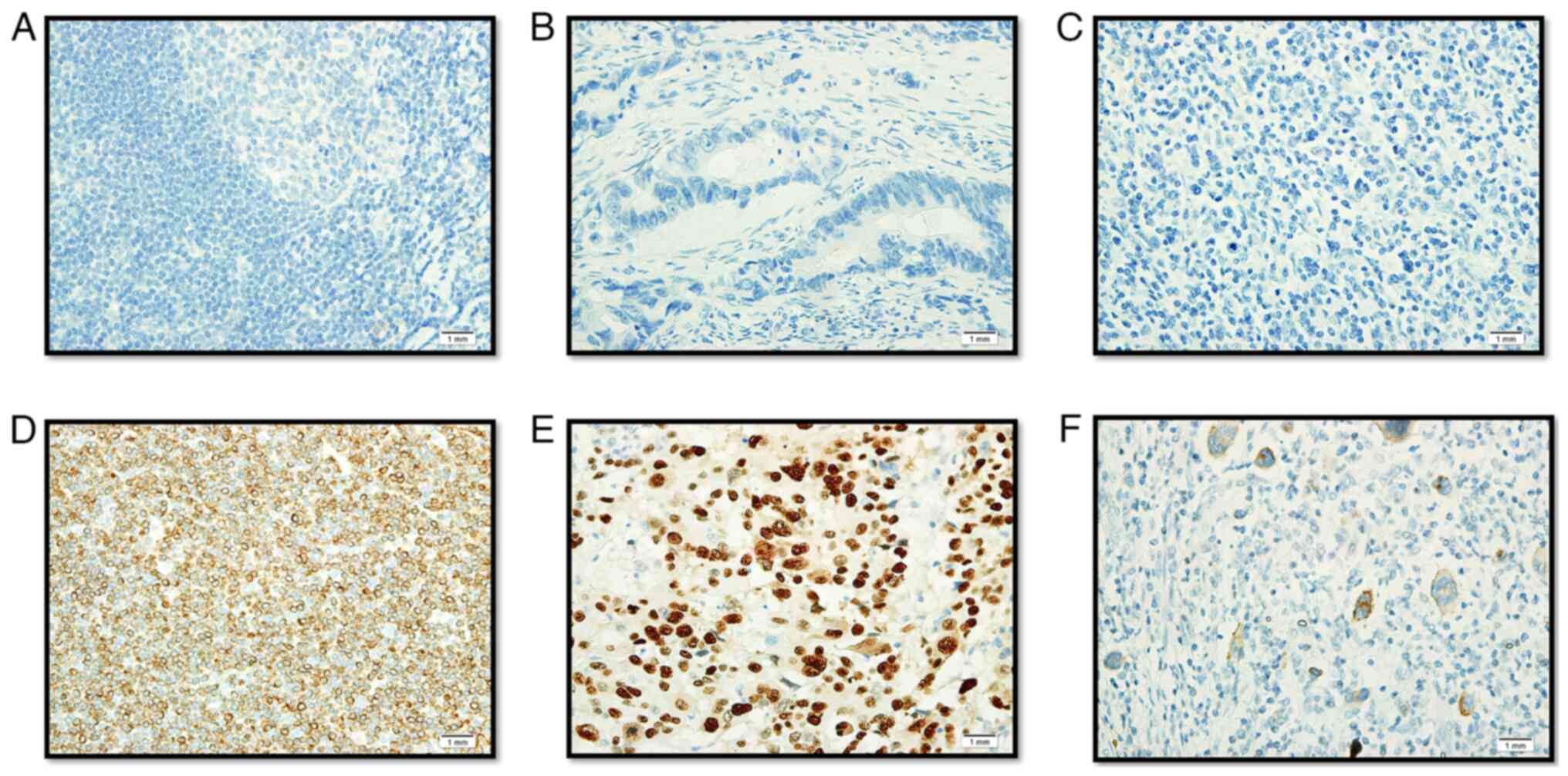
Positive and negative tissue control was used with every staining
procedure (Fig. 2). The origin of
the control samples for Bcl-2 and TP53 immunohistochemistry were
from the tonsils and colon cancer, respectively. The patients were
both male, aged 51 years old (tonsil cancer) and 48 years old
(colon cancer). These samples were collected from the patients who
attended the oncology clinic at the King Fahd Hospital of the
University after obtaining consent. An EBV positive control slide
was commercially procured (cat. no. CSE0125P; StatLab).
Statistical analysis
The clinical and histopathological data along with
studied protein expression (qualitative) results were analyzed
using SPSS version 20 (IBM Corp.). Distribution of proportions
across category variables were analyzed using a Fisher's exact test
(Table I), and for continuous
variables an unpaired Student's t-test was used (Table I). The bivariate relationships
between the variables were determined using a Spearman's Rank
Correlation test (Tables II and
III). P<0.05 was considered
to indicate a statistically significant difference.
 | Table IClinical, pathological and protein
expression parameters amongst the nasopharynx and larynx group of
patients. |
Table I
Clinical, pathological and protein
expression parameters amongst the nasopharynx and larynx group of
patients.
| Parameter | Nasopharynx | Larynx | P-value |
|---|
| Sample size | 22 | 11 | |
| Age, years, mean ±
standard deviation | 48.8±15.4 | 63.3±16.2 | 0.008b |
| Sex, n | | | 0.258 |
|
Male | 13 | 9 | |
|
Female | 9 | 2 | |
| Grade, n | | | 0.0004b |
|
1 | 0 | 3 | |
|
2 | 3 | 7 | |
|
3 | 19 | 1 | |
| Stage, n | | | 0.265 |
|
I and
II | 10 | 8 | |
|
III and
IV | 12 | 3 | |
| Smoking status,
n | | | 0.07 |
|
Smokers | 8 | 8 | |
|
Non-smokers | 14 | 3 | |
| Prognosis, n | | | 0.027a |
|
Good | 11 | 1 | |
|
Bad | 11 | 10 | |
| Epstein Barr virus,
n | | | 0.143 |
|
Positive | 5 | 0 | |
|
Negative | 17 | 11 | |
| B cell lymphoma-2,
n | | | 0.027a |
|
Positive | 11 | 1 | |
|
Negative | 11 | 10 | |
| TP53, n | | | 0.712 |
|
Positive | 11 | 7 | |
|
Negative | 11 | 4 | |
| Chemotherapy,
n | | | 0.067 |
|
Yes | 7 | 0 | |
|
No | 15 | 11 | |
 | Table IICorrelation between EBV, Bcl-2 or
TP53 expression with clinical and histopathological parameters in
the total cohort (nasopharynx and larynx groups). |
Table II
Correlation between EBV, Bcl-2 or
TP53 expression with clinical and histopathological parameters in
the total cohort (nasopharynx and larynx groups).
| Parameter | Smoking status,
r-value | Tumor site,
r-value | Tumor grade,
r-value | Tumor stage,
r-value | Prognosis,
r-value | EBV, r-value | Bcl-2, r-value | TP53, r-value |
|---|
| Smoking status | 1.000 | -0.320 | 0.156 | -0.97 | 0.099 | 0.078 | -0.170 | 0.182 |
| Tumor site | -0.032 | 1.000 | -0.614a | -0.291 | 0.480a | -0.178 | -0.213 | 0.190 |
| Tumor grade | 0.156 | -0.614a | 1.000 | 0.386a | -0.281 | 0.244 | 0.263 | -0.117 |
| Tumor stage | -0.097 | -0.291 | 0.386a | 1.000 | -0.110 | 0.298 | 0.097 | -0.054 |
| Prognosis | 0.099 | 0.480a | -0.281 | -0.110 | 1.000 | -0.031 | -0.056 | 0.210 |
| EBV | 0.078 | -0.178 | 0.244 | 0.298 | -0.031 | 1.000 | -0.036 | 0.361a |
| Bcl-2 | -0.170 | -0.213 | 0.263 | 0.097 | -0.056 | -0.036 | 1.000 | -0.065 |
| TP53 | 0.182 | 0.190 | -0.117 | -0.054 | 0.210 | 0.361a | -0.065 | 1.000 |
 | Table IIICorrelation between EBV, Bcl-2 or
TP53 expression with clinical and histopathological parameters in
the Nasopharynx cohort. |
Table III
Correlation between EBV, Bcl-2 or
TP53 expression with clinical and histopathological parameters in
the Nasopharynx cohort.
| Parameter | Smoking status,
r-value | Tumor grade,
r-value | Tumor stage,
r-value | Prognosis
r-value | EBV r-value | Bcl-2, r-value | TP53, r-value |
|---|
| Smoking status | 1.000 | -0.025 | -0.394 | 0.378 | 0.184 | -0.378 | 0.378 |
| Tumor grade | -0.025 | 1.000 | -0.099 | 0.132 | 0.215 | 0.132 | 0.132 |
| Tumor stage | -0.394 | -0.099 | 1.000 | 0.076 | 0.262 | -0.038 | -0.356 |
| Prognosis | 0.378 | 0.132 | 0.076 | 1.000 | 0.108 | 0.091 | 0.091 |
| EBV | 0.184 | 0.215 | 0.262 | 0.108 | 1.000 | -0.325 | 0.542a |
| Bcl-2 | -0.378 | 0.132 | -0.038 | 0.091 | -0.325 | 1.000 | -0.091 |
| TP53 | 0.378 | 0.132 | -0.356 | 0.091 | 0.542a | -0.091 | 1.000 |
Results
Sex distribution analysis revealed that in the
nasopharynx and larynx groups, 59 and 81% of the patients,
respectively, were males. The median age of patients with
nasopharyngeal carcinoma is 49.5 years compared to 65 years in the
larynx group (P=0.008). In the nasopharynx group, 68.2% had
undifferentiated tumors, poorly differentiated tumors accounted for
22.7 and 9.1% were moderately differentiated tumors. In the larynx
group 27.3% tumors were well differentiated, 63.6% tumors were
moderately differentiated and 9.1% were poorly differentiated. The
majority of the patients with NPC presented with grade 3 (86.4%)
disease, whereas in laryngeal cancer cases, grade 1 and 2 tumors
(91%) were predominant (P=0.0004). Similarly, 54.5% of the patients
with NPC were at an advanced stage of the disease and in the larynx
cancer group, the advanced stage was observed only in 27.3%. With
regards to smoking, the percentage was higher in the larynx cancer
group of patients (72.7%) compared to the patients with NPC
(36.4%).
Prognosis was calculated based on the follow-up of
the patient for 5 years. Recurrence or death due to disease was
considered a poor prognostic outcome. A poor prognosis was observed
in 91% of the larynx cancer patient group compared to 50% in the
patients with NPC (P=0.027). EBV infection was completely absent in
the larynx carcinoma patients, whereas 22.7% of patients were
infected with EBV in the NPC group. A high proportion of Bcl-2
expression was seen in the patients with NPC (P=0.027) compared to
the laryngeal carcinoma group. TP53 expression did not yield any
significant difference between both patient groups (Table I). Bivariate association between
clinical, histopathological and protein expression variables
overall, also confirmed the poor prognosis in laryngeal carcinoma
patients (P=0.002) and advanced tumor grade in NPC cases (P≤0.005;
Table II). These results showed
that EBV infection was associated with TP53 expression in the NPC
cohort (P=0.009; Table III).
Discussion
EBV is currently a well-established carcinogen and
an etiological factor implicated in several cancers, including
cancer of epithelial and lymphoid origins, gastric cancer, NPC, and
Hodgkin's and non-Hodgkin's lymphoma (10). There was a 14.6% increase in EBV
associated cancers between 1990-2010, and 50% of these cases were
observed in patients in East Asia (10). Approximately 1.5% of cancer cases
are associated with EBV infection amongst all cancer cases globally
(11-13).
NPC is primarily associated with EBV, and the EBV infection
frequency in NPC is varied based on ethnic and geographical
differences (23). Almost all the
NPC cases from the endemic regions presented with EBV infection and
in non-endemic regions, NPC was negative for EBV. The EBV
associated NPC prevalence is highest in Southeast Asia (24); data on the frequency of EBV in NPC
is scarce in the Saudi population. The present study revealed that
22.7% of NPC cases had EBV infection. Similarly, the frequency of
EBV in NPC was found to be 28% in an Iranian cohort (8), 44% from a Brazilian study (25) and 53.84% in a study from Oman
(26). The relatively lower
percentage of the EBV infection-associated NPC in the present study
may be due to the region of study being non-endemic for EBV
(27).
Identifying a single biomarker to calculate the risk
and prognosis of a cancer is difficult due to tumor heterogeneity,
and distinct clinical and histopathological conditions. However, a
single biomarker interaction with other biomarkers in pathways such
as cell signaling, apoptosis and cell differentiation may reveal
the mechanism of oncogenesis. Hence, Bcl-2 and TP53 protein
expression was assessed in all the cases to determine the
correlation between EBV infections. The Bcl-2 family of proteins
are key regulators in the apoptotic pathway (19). Amongst Bcl-2 family proteins, there
were pro and anti-apoptotic regulators; the Bcl-2 protein is an
anti-apoptotic member. Bcl-2 protein upregulation was observed more
frequently in NPC cases compared to other types of HNSCC (28), and a similar pattern was observed
in the present study; there was a lower frequency of upregulated
Bcl-2 expression in patients with larynx cancer compared with
patients with NPC. Bcl-2 upregulation was seen in 74.3% of NPC
cases (29), similarly the present
study showed that Bcl-2 upregulation was observed in 50% of NPC
tumors. Bcl-2 upregulation may contribute towards tumor cell
survival by inhibiting apoptosis in NPC. Thus, Bcl-2 upregulation
may highlight a vital mechanism involved in NPC pathogenesis,
although the exact molecular mechanism is unclear (30).
TP53 plays a vital role in cell cycle arrest and
apoptosis through different mechanisms (31). The positive rate of TP53 expression
in NPC tumors was varied in different studies, with reported values
of 34.7% (32), 65.6% (33) and 52.2% (34). The results of the present study are
in line with these previous studies with 50% of the NPC tumors
exhibiting TP53 upregulation. The present data revealed that TP53
upregulation is associated with EBV infection in NPC. Similarly,
several studies reported wildtype TP53 is upregulated in EBV
associated cancers (18,35-37).
Apoptosis is induced by TP53 upregulation. However, EBV transformed
cells are sensitive to TP53-mediated apoptosis (38). This shows that the EBV positive and
EBV negative tumors are distinct groups. The present study detected
EBV infection by immunohistochemistry targeting the 60 kDa LMP-1 of
EBV, and the results suggested that the mutual regulation between
TP53 and LMP1 may play an important role in EBV associated NPC.
Additional mechanistic studies need to be performed to determine
TP53-mediated LMP1 stimulation. The limitation of this study is the
absence of quantitative data for the immunohistochemistry
experiments.
HNSCCs exhibit a variable prognosis with varying
responses to standard treatment modalities, and this may be due to
the significant etiological and molecular heterogeneity (39). The present data indicates that
laryngeal carcinomas have a poor prognosis compared to NPC. The
majority of the patients with NPC tumors presented with a higher
grade tumor with a good prognosis. It has been reported that a
combination of adjuvant chemotherapy and radiotherapy treatment
improves prognosis and survival (40). To the best of our knowledge, this
is the first pilot study to reveal the EBV associated NPC frequency
using immunohistochemistry in the ethnic population. This study
also attempted to divulge the poorly understood cellular mechanisms
of EBV associated NPC pathogenesis by determining the correlation
between EBV infection and the major tumor suppressor, TP53.
Additional mechanistic studies are required to determine the
TP53-mediated LMP1 stimulation of EBV in NPC. An improved
understanding of the EBV carcinogenic process may aid in the
development of novel therapeutics in NPC.
In conclusion, EBV infection was correlated with
TP53 upregulation in patients with NPC, which suggests mutual
regulation between TP53 and EBV.
Acknowledgements
The authors would like to thank Mr. Shakir Ahmed
(Department of Pathology, King Fahd Hospital of the University,
Imam Abdulrahman Bin Faisal University, Kingdom of Saudi Arabia)
for his technical support.
Funding
This work was supported by the Deanship of Scientific Research,
Imam Abdulrahman Bin Faisal University (grant nos. 2016-090-IRMC
and 2016-111-Med).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CV, CC, SC and AAA conceived and designed the
current study. AMA, AAL and AAH collected samples and the clinical
data. CV and AAS performed the experiments. CV wrote the
manuscript. AAA revised the manuscript. All authors read and
approved the final manuscript. CV, CC, AMA, AAL, AAA confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Imam Abdulrahman Bin Faisal University, Dammam, Saudi
Arabia (approval no. IRB-2017-01-059). The procedures in the
present study adhere to the principles of the Declaration of
Helsinki. Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leemans CR, Snijders PJF and Brakenhoff
RH: The molecular landscape of head and neck cancer. Nat Rev
Cancer. 18:269–282. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Epstein MA and Barr YM: Cultivation in
vitro of human lymphoblasts from burkitt's malignant lymphoma.
Lancet. 1:252–253. 1964.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Old LJ, Boyse EA, Oettgen HF, Harven ED,
Geering G, Williamson B and Clifford P: Precipitating antibody in
human serum to an antigen present in cultured burkitt's lymphoma
cells. Proc Natl Acad Sci USA. 56:1699–1704. 1966.PubMed/NCBI View Article : Google Scholar
|
|
5
|
de Schryver A, Friberg S Jr, Klein G,
Henle W, Henle G, De-Thé G, Clifford P and Ho HC: Epstein-Barr
virus-associated antibody patterns in carcinoma of the post-nasal
space. Clin Exp Immunol. 5:443–459. 1969.PubMed/NCBI
|
|
6
|
zur Hausen H, Schulte-Holthausen H, Klein
G, Henle W, Henle G, Clifford P and Santesson L: EBV DNA in
biopsies of Burkitt tumours and anaplastic carcinomas of the
nasopharynx. Nature. 228:1056–1058. 1970.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cao Y: EBV based cancer prevention and
therapy in nasopharyngeal carcinoma. NPJ Precis Oncol.
1(10)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shahani T, Makvandi M, Samarbafzadeh A,
Teimoori A, Ranjbar N, saki N, Nikakhlagh S, Neisi N, Hosseini Z,
Pourrezaei S, et al: Frequency of Epstein Barr virus type 1 among
nasopharyngeal carcinomas in Iranian patients. Asian Pac J Cancer
Prev. 18:327–331. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Young LS and Dawson CW: Epstein-Barr virus
and nasopharyngeal carcinoma. Chin J Cancer. 33:581–590. 2014.
|
|
10
|
Khan G and Hashim MJ: Global burden of
deaths from Epstein-Barr virus attributable malignancies 1990-2010.
Infect Agent Cancer. 9(38)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Varmus H and Kumar HS: Addressing the
growing international challenge of cancer: A multinational
perspective. Sci Transl Med. 5(175cm2)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Holmes D: The cancer-virus cures. Nat Med.
20:571–574. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lieberman PM: Virology. Epstein-Barr virus
turns 50. Science. 343:1323–1325. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tsao SW, Tsang CM and Lo KW: Epstein-Barr
virus infection and nasopharyngeal carcinoma. Philos Trans R Soc
Lond B Biol Sci. 372(20160270)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sahoo R, Chittibabu V, Patil G, Rao S,
Thakur S, Dhondalay G, Kulkarni AJ, Banerjee A, Ajaikumar BS,
Korlimarla A, et al: Relationship between molecular markers and
treatment response in a retrospective cohort of Indian patients
with primary carcinoma of the larynx. Oral Oncol. 45:e216–e221.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bieging KT, Mello SS and Attardi LD:
Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev
Cancer. 14:359–370. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Kastenhuber ER and Lowe SW: Putting p53 in
Context. Cell. 170:1062–1078. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Q, Lingel A, Geiser V, Kwapnoski Z
and Zhang L: Tumor suppressor p53 stimulates the expression of
epstein-barr virus latent membrane protein 1. J Virol.
91:e00312–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wenmei S, Yanming L, Fenping W, Hongsheng
G, Lixia L, Suwen Z, Zhennan L, Rong L and Zhixiong Y: Bcl-2
regulation by miR-429 in human nasopharyngeal carcinoma in vivo.
Int J Clin Exp Pathol. 9:5998–6006. 2016.
|
|
22
|
World Medical Association. World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kanno M, Narita N, Fujimoto Y, Wakisaka N,
Yoshizaki T, Kodaira T, Makita C, Sato Y, Yamazaki K, Wakaoka T, et
al: Third epidemiological analysis of nasopharyngeal carcinoma in
the central region of Japan from 2006 to 2015. Cancers (Basel).
11(1180)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ngan HL, Wang L, Lo KW and Lui VWY:
Genomic landscapes of EBV-associated nasopharyngeal carcinoma vs.
HPV-associated head and neck cancer. Cancers (Basel).
10(210)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Breda E, Catarino RJ, Azevedo I, Lobão M,
Monteiro E and Medeiros R: Epstein-Barr virus detection in
nasopharyngeal carcinoma: Implications in a low-risk area. Braz J
Otorhinolaryngol. 76:310–315. 2010.PubMed/NCBI
|
|
26
|
Al-Shidhani SNS, Al-Sinawi S, Al-Bahri M,
Al-Kindi M and Mabruk M: Determination of the expression of latent
Epstein Barr virus in Omani nasopharyngeal carcinoma patients.
Biomed Pharmacol J. 14:257–265. 2021.
|
|
27
|
Wu L, Li C and Pan L: Nasopharyngeal
carcinoma: A review of current updates. Exp Ther Med. 15:3687–3692.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang HJ, Cho YJ, Kim HS, Chang MS, Sung MW
and Kim WH: Association of p53 and BCL-2 expression with
Epstein-Barr virus infection in the cancers of head and neck. Head
Neck. 23:629–636. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Sarac S, Akyol MU, Kanbur B, Poyraz A,
Akyol G, Yilmaz T and Sungur A: Bcl-2 and LMP1 expression in
nasopharyngeal carcinomas. Am J Otolaryngol. 22:377–382.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tulalamba W and Janvilisri T:
Nasopharyngeal carcinoma signaling pathway: An update on molecular
biomarkers. Int J Cell Biol. 2012(594681)2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Tabyaoui I, Serhier Z, Sahraoui S, Sayd S,
Cadi R, Bennani OM, Benider A, Zamiati S and Tahiri JN:
Immunohistochemical expression of latent membrane protein 1 (LMP1)
and p53 in nasopharyngeal carcinoma: Moroccan experience. Afr
Health Sci. 13:710–717. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang P, Wu SK, Wang Y, Fan ZX, Li CR,
Feng M, Xu P, Wang WD and Lang JY: p53, MDM2, eIF4E and EGFR
expression in nasopharyngeal carcinoma and their correlation with
clinicopathological characteristics and prognosis: A retrospective
study. Oncol Lett. 9:113–118. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu J, Liu Y, Zhang Z, Sun H, Ji X, Li B,
Zhou X and Gai P: Prognostic value of the Epstein-Barr virus and
tumor suppressor gene p53 gene in nasopharyngeal squamous cell
carcinoma. J Cancer Res Ther. 15:426–436. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Menke DM, Griesser H, Moder KG, Tefferi A,
Luthra HS, Cohen MD, Colon-Otero G and Lloyd RV: Lymphomas in
patients with connective tissue disease. Comparison of p53 protein
expression and latent EBV infection in patients immunosuppressed
and not immunosuppressed with methotrexate. Am J Clin Pathol.
113:212–218. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Niemhom S, Kitazawa S, Murao S, Kunachak S
and Maeda S: Co-expression of p53 and bcl-2 may correlate to the
presence of epstein-barr virus genome and the expression of
proliferating cell nuclear antigen in nasopharyngeal carcinoma.
Cancer Lett. 160:199–208. 2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chou J, Lin YC, Kim J, You L, Xu Z, He B
and Jablons DM: Nasopharyngeal carcinoma-review of the molecular
mechanisms of tumorigenesis. Head Neck. 30:946–963. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Allday MJ, Sinclair A, Parker G, Crawford
DH and Farrell PJ: Epstein-Barr virus efficiently immortalizes
human B cells without neutralizing the function of p53. EMBO J.
14:1382–1391. 1995.PubMed/NCBI
|
|
39
|
Belcher R, Hayes K, Fedewa S and Chen AY:
Current treatment of head and neck squamous cell cancer. J Surg
Oncol. 110:551–574. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Perri F, Della Vittoria Scarpati G,
Buonerba C, Di Lorenzo G, Longo F, Muto P, Schiavone C, Sandomenico
F and Caponigro F: Combined chemo-radiotherapy in locally advanced
nasopharyngeal carcinomas. World J Clin Oncol. 4:47–51.
2013.PubMed/NCBI View Article : Google Scholar
|