Introduction
Lung cancer has a high mortality rate for both males
and females (1). At the time of
diagnosis, ~50% of the patients with lung cancer have reached a
state of distant metastases (2).
Approximately 30% of the metastatic lung cancers are determined to
be oligometastatic (3,4). The overall survival for
oligometastatic lung cancer was reported to exceed the generally
expected 5-year survival of the widespread metastatic disease (30
vs. 4-6%) (5,6).
In patients with non-small cell lung cancer,
isolated metastasis to the adrenal glands, a retroperitoneal organ,
occurs in up to 20% of patients with metastases, among potential
sites of dissemination (4,7). However, there is a paucity of such
reports in the literature and most of them are small case series
(5,6,8).
Patients with isolated synchronous or metachronous adrenal
metastasis may be offered surgery as part of multimodality
treatment for lung cancer. In general, urologists are in charge of
the surgical treatment of metastatic adrenal tumors.
Carcinoembryonic antigen (CEA) is one of the most
commonly used prognostic markers for lung cancer. Recent studies in
the field of colorectal cancer suggested that the post/preoperative
serum CEA ratio is able to predict the prognosis of cancer patients
(9,10). A retrospective study of 207
patients who underwent resection for colorectal cancer in a single
center indicated that the post/preoperative serum CEA ratio was
beneficial in predicting prognosis. The overall survival rates were
65.1 and 86.3% in patients with high (≥0.5) vs. low (<0.5)
post/preoperative CEA ratios, respectively (P=0.045, log-rank test)
(10). The relevance of the
post/preoperative serum CEA ratio has been investigated in the
field of primary treatment of lung cancer (11,12),
but it has not been previously investigated in the field of lung
cancer with solitary metastasis to the adrenal gland, to the best
of our knowledge. In the present study, it was hypothesized that
the post/preoperative serum CEA ratio is beneficial in predicting
the postoperative prognosis of solitary adrenal metastasis in lung
cancer.
Thus, the present study aimed to investigate the
prognostic value of the post/preoperative serum CEA ratio in
patients with lung cancer with adrenal metastasis who underwent
surgery on the adrenal gland.
Materials and methods
Study population
The medical records of patients with lung cancer
along with synchronous (metastases existing at diagnosis of primary
cancer) or metachronous isolated adrenal metastasis who underwent
adrenalectomy at the National Cancer Center Hospital East (Kashiwa,
Japan) during the period from January 2013 to December 2020, were
retrospectively reviewed.
The retrospective study was ethically approved by
the National Cancer Center Institutional Review Board (approval no.
2018-159).
Indications for adrenalectomy
At our institute, the indications for adrenalectomy
in lung cancer with adrenal metastasis were based on the following
criteria: i) The primary lung cancer had been resected or expected
to be cured by radical chemotherapy; ii) metastasis was restricted
to the adrenal gland; iii) the patient was sufficiently physically
fit to tolerate the double-site (lung and adrenal gland) local
treatments; iv) the operative risk was considered low based on
respiratory reserve and cardiac assessment; v) the patient clearly
expressed volition to adhere to heavy multidisciplinary treatment.
With the above criteria for adrenalectomy, the final decision was
to be made through discussion between the urologist and the
pulmonologist.
Collection of serum CEA levels and
baseline clinicopathological characteristics
Circulating levels of serum CEA were analyzed with
an electrochemiluminescence immunoassay using the Cobas 8000 e602
modular analyzer series (Hoffmann-La Roche AG) as part of the
routine care for patients both preoperatively and postoperatively.
With the date of measurement determined by the pulmonologist in
charge, the CEA ratio as postoperative serum CEA level/preoperative
serum CEA level was calculated.
The clinicopathological characteristics, such as
age, sex, Charlson co-morbidity index, location of the tumor,
metastasis, timing of metastases, tumor staging, treatment of the
primary tumor, surgical procedure, perioperative data of
adrenalectomy [including operative time, estimated blood loss (EBL)
and postoperative complications, which were scored according to the
Clavien-Dindo classification (13)], were recorded.
Survival follow-up
The routine follow-up examinations included blood
tests such as serum tumor biomarkers such as CEA, as well as
imaging diagnostics, including X-rays, positron emission
tomography, magnetic resonance imaging and computed tomography.
Based on these results, the presence or absence of cancer
recurrence was determined.
Disease-free survival (DFS) was calculated from the
date of adrenalectomy to the date of recurrence, metastasis or
death from any cause. Cancer-specific survival (CSS) was calculated
from the date of adrenalectomy to the date of death from lung
cancer.
Statistical analysis
The time-dependent receiver operating characteristic
(ROC) curve analysis and the Youden index were used to determine
the optimal cutoff value for the CEA ratio to predict DFS (14). This was followed by stratification
of the eligible patients into the high CEA ratio group and low CEA
ratio group.
The survival curve was estimated by the Kaplan-Meier
method and statistically significant differences between the groups
were determined using a log-rank test.
Mean values with standard deviations were used to
express continuous variables with a normal distribution and median
values with interquartile ranges (IQR) were used for those with a
non-normal distribution. An unpaired t-test or Mann-Whitney U test
(continuous variables) and Fisher's exact test (categorical
variables) were used to assess the association between the CEA
ratio and clinical factors.
All P-values were two-sided and P<0.05 was
considered to indicate a statistically significant difference. The
statistical analyses utilized in the present study were performed
using Microsoft Excel 2016 (Microsoft Corporation) and JMP 13
software (SAS Institute Inc.).
Results
Patient inclusion
During the study period, 20 adrenalectomies for
adrenal metastasis were performed at our institute. A total of 5
(25%) patients were excluded from the analysis due to having
metastases of cancer other than lung cancer; furthermore, 1 (5%)
patient was excluded as the intraoperative findings suggested
cancer invasion to the head of the pancreas, which resulted in
cancelation of the surgery.
Thus, a total of 14 patients with a median age of 68
years (range, 50-75 years), of which 9 (64%) were males, were
included in the study (Table
I).
 | Table IComparison of baseline demographic,
clinical and pathological characteristics according to CEA
ratio. |
Table I
Comparison of baseline demographic,
clinical and pathological characteristics according to CEA
ratio.
| | CEA
ratioa | |
|---|
| Variable | Total (n=14) | High (n=6) | Low (n=8) | P-value |
|---|
| Age, years | 68 (60, 69) | 68 (62, 71) | 67 (56, 70) | 0.46 |
| Sex | | | | 0.33 |
|
Male | 9(64) | 3(50) | 6(75) | |
|
Female | 5(36) | 3(50) | 2(25) | |
| Charlson co-morbidity
index | 0 (0, 0) | 0 (0, 1) | 0 (0, 0) | 0.17 |
| Primary lung cancer
treatment | | | | 0.33 |
|
Surgery | 9(64) | 3(50) | 6(75) | |
|
Other | 5(36) | 3(50) | 2(25) | |
| Histologic type of
lung cancer | | | | 0.83 |
|
Adenocarcinoma | 12(86) | 5(83) | 7(88) | |
|
Small
cell | 2(14) | 1(17) | 1(13) | |
| Metachronous | 10(71) | 4(67) | 6(75) | 0.73 |
| cT stage | | | | 0.07 |
|
T1 | 7(50) | 4(67) | 3(38) | |
|
T2 | 3(21) | 0 (0) | 3(38) | |
|
T3 | 2(14) | 2(33) | 0 (0) | |
|
T4 | 2(14) | 0 (0) | 2(25) | |
| cN stage | | | | 0.25 |
|
N0 | 7(50) | 2(33) | 5(63) | |
|
N1 | 4(29) | 2(33) | 2(25) | |
|
N2 | 1(7) | 0 (0) | 1(13) | |
|
N3 | 2(14) | 2(33) | 0 (0) | |
| Stage | | | | 0.30 |
|
Ⅰ (ⅠA,
ⅠB) | 3(21) | 0 (0) | 3(38) | |
|
II (IIA,
IIB) | 3(21) | 1(17) | 2(25) | |
|
III (IIIA,
IIIB) | 3(21) | 2(33) | 1(13) | |
|
IV | 5(36) | 3(50) | 2(25) | |
Optimal cutoff value for the CEA ratio
to predict DFS
The median preoperative serum CEA level was 10.3
(IQR, 4.8-15.1) ng/ml and the measurement date of CEA was a median
of 13 (IQR, 3-48) days prior to the adrenalectomy. The median
postoperative serum CEA level was 4.6 (IQR, 2.4-6.6) ng/ml and the
measurement date of CEA was a median of 33 (IQR, 22-63) days after
the adrenalectomy (data not shown).
The median CEA ratio was 0.46 (IQR, 0.32-0.73) ng/ml
(data on individual CEA levels for all included patients are not
shown). To determine the optimal CEA ratio cutoff value for
predicting DFS, a time-dependent ROC curve analysis and the Youden
index were used. The optimal cutoff point for the CEA ratio was
0.60 with 60% sensitivity and 100% specificity (data not shown).
Thus, the patients were categorized into a high CEA ratio (≥0.60)
group and low CEA ratio (<0.60) group (data not shown).
Clinicopathological patient
characteristics
During the follow-up of the 14 eligible patients, 10
(71%) patients had experienced recurrence of lung cancer with a
median time to recurrence of 4.1 months (range, 2.1-43.9 months).
Cancer recurrence was identified based on CEA, as well as image
diagnostics including X-rays, positron emission tomography,
magnetic resonance imaging and computed tomography (data not
shown).
A total of 5 (36%) patients died of lung cancer and
the median time from adrenalectomy to death was 29.0 months (range,
6.9-36.1 months). Furthermore, 1 (7%) patient died 24.9 months
after adrenalectomy from a cause other than lung cancer. The
five-year survival rate for the eligible patients in the present
study stood at 41.0% with a median survival time of 31.0
months.
Demographic, clinical and pathological
characteristics of the patients are presented in Table I. Out of the 14 patients included
in the study, 10 patients (71%) were observed to have metachronous
disease and 4 (29%) had synchronous metastatic disease. In 9
patients (64%), the primary tumor was treated with surgery, 4
patients (29%) were treated by thoracic chemo-radiotherapy and 1
patient (7%) was treated by chemotherapy alone. No significant
differences were observed between patients in the high CEA ratio
group and low CEA ratio group for the above parameters, age, sex,
Charlson co-morbidity Index and histological type of lung cancer.
Similarly, there were no significant differences in the
distribution of clinical tumor stage and clinical node stage
between the high CEA ratio group and low CEA ratio group.
Peri-operative parameters of
adrenalectomy
Peri-operative parameters are presented in Table II. The median operating time for
adrenalectomy was 145 min (IQR, 100-175 min), while the median EBL
was 19 ml (IQR, 4-56 ml).
 | Table IIComparison of peri-operative
parameters according to CEA ratio. |
Table II
Comparison of peri-operative
parameters according to CEA ratio.
| | CEA
ratioa | |
|---|
| Variable | Total (n=14) | High (n=6) | Low (n=8) | P-value |
|---|
| Tumor location | | | | 0.12 |
|
Left | 8(57) | 4(67) | 2(25) | |
|
Right | 6(43) | 2(33) | 6(75) | |
| ASA-PS | 2 (1, 2) | 2 (2, 2) | 2 (1, 2) | 0.21 |
| Preoperative CEA,
ng/ml | 10.3 (4.8,
15.1) | 4.5 (2.2, 9.8) | 13.4 (10.0,
17.8) | 0.01 |
| Postoperative CEA,
ng/ml | 4.6 (2.4, 6.6) | 4.9 (1.9, 9.6) | 4.5 (2.7, 6.2) | 0.51 |
| Operative time,
minutes | 145 (100, 175) | 163 (113, 190) | 130 (97, 168) | 0.80 |
| EBL, ml | 19 (4, 56) | 35 (10, 117) | 12 (1, 59) | 0.51 |
| Type of surgical
procedure | | | | 0.23 |
|
Transabdominal | 1(7) | 1(17) | 0 (0) | |
|
Retroperitoneal | 3(21) | 2(33) | 1(13) | |
|
Transabdominal
(laparoscopic) | 7(50) | 3(50) | 4(50) | |
|
Retroperitoneal
(laparoscopic) | 3(21) | 0 (0) | 3(38) | |
| Post-operative
complications, highest Clavien-Dindo grade | | | | 0.37 |
|
0 | 13(93) | 6(100) | 7(88) | |
|
Ⅰ | 0 (0) | 0 (0) | 0 (0) | |
|
II | 1(7) | 0 (0) | 1(13) | |
|
≥III | 0 (0) | 0 (0) | 0 (0) | |
Of the 14 patients, 4 patients (29%) underwent open
surgery (3 by retroperitoneal approach) and 10 patients (71%)
underwent laparoscopic adrenalectomy (3 by retroperitoneal
approach). Among the 10 patients who underwent laparoscopic
adrenalectomy, 1 patient (10%) required open conversion due to
bleeding from the inferior vena cava.
There were no cases of postoperative complications,
except for 1 case (7%) of Clavien-Dindo grade 2 complications
(post-operative blood transfusion).
Preoperative CEA levels were observed to be
significantly higher in the low CEA ratio group as compared to the
high CEA ratio group (P=0.01).
Prognostic significance of CEA
ratio
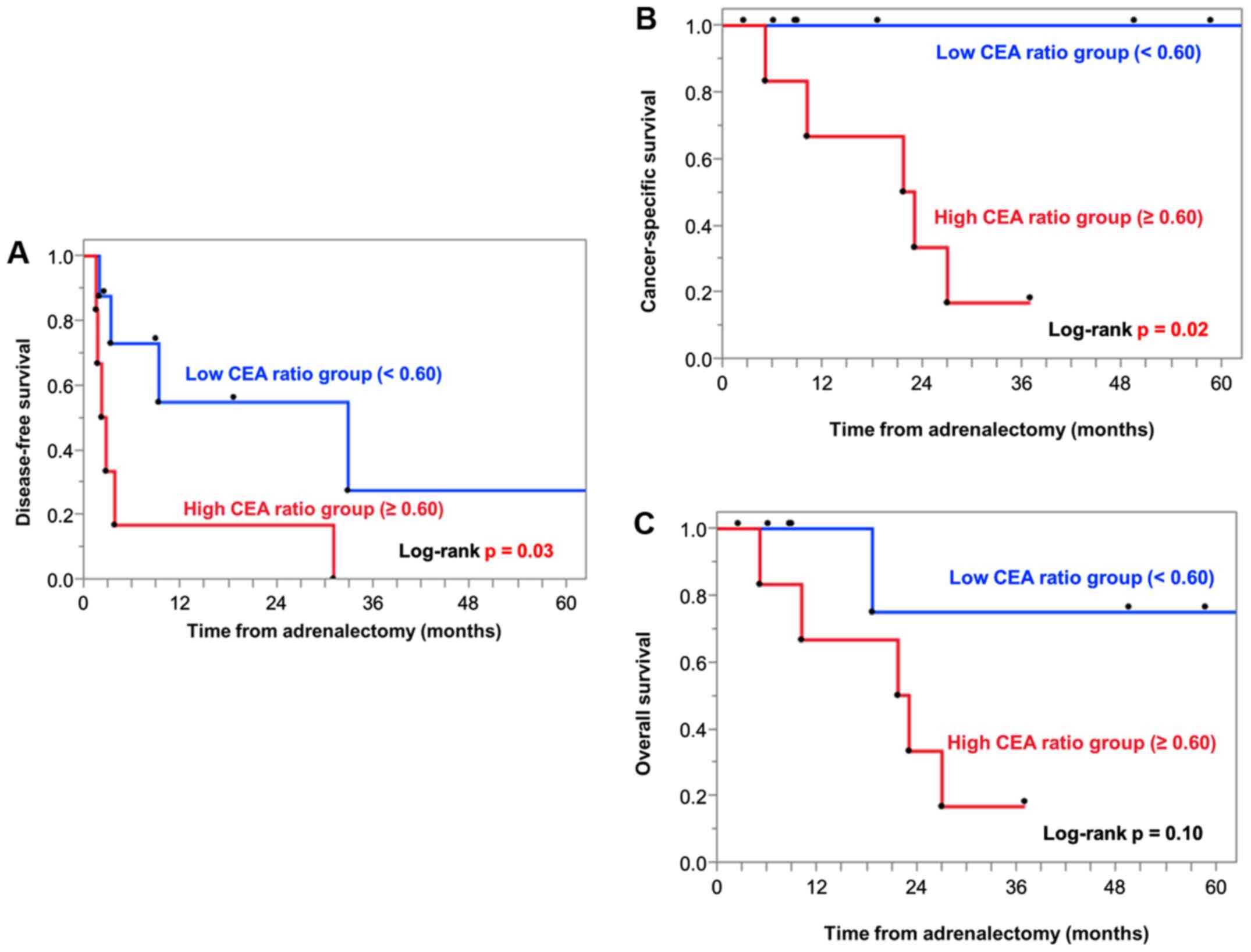
As presented in Fig.
1, patients with a high CEA ratio had significantly poorer DFS
(P=0.03; Fig. 1A) and CSS (P=0.02;
Fig. 1B) as compared to patients
with a low CEA ratio. Similarly, for OS, patients with a high CEA
ratio tended to have poorer OS than those with a low CEA ratio;
however, the difference was not statistically significant (P=0.10;
Fig. 1C).
Discussion
The prognosis of lung cancer associated with adrenal
metastasis is generally poor. However, it has been reported that
surgical resection of adrenal metastasis may increase survival when
metastasis of the disease is limited to the adrenal gland (2,5,6,8,15).
In the present study, adrenalectomy was performed
for lung cancer with solitary metastasis to the adrenal gland. A
5-year survival rate of 41.0% and a median survival of 31.0 months
were determined. This was a relatively high survival rate for
metastatic lung cancers. Similar results have been obtained by
previous studies of lung cancers with adrenal solitary metastasis,
wherein patients underwent adrenalectomy (6,16).
The serum CEA level has been commonly used to
evaluate the prognosis of patients with lung cancer. However, serum
CEA is not specific to the diagnosis of lung cancer, as CEA levels
have been reported to be frequently elevated in patients with
colorectal cancer, long-term smoking habit, cardiovascular disease,
gynecological disease and other diseases (9). In the case of colorectal cancer, it
has been reported that clinical prognosis may be predicted by
evaluating not only the preoperative CEA level but also the
post/preoperative serum CEA ratio (9,10). A
retrospective single-center study including 187 patients with
colorectal cancer suggested that the CEA ratio was not able to
improve the predictive efficiency for prognosis as compared to
postoperative CEA levels in terms of sensitivity and specificity.
Furthermore, the CEA ratio was indicated to be useful as a
prognostic indicator for patients with colorectal cancer and normal
postoperative CEA by Kaplan-Meier survival curve and subgroup
multivariate analysis (9).
The present study indicated for the first time, to
the best of our knowledge, DFS of patients with a high CEA ratio
(≥0.60) was significantly poorer compared to those with a low CEA
ratio (<0.60) after adrenalectomy for solitary adrenal
metastasis from lung cancer. In addition, the CSS was determined to
be significantly poorer in the group with a high CEA ratio (≥0.60).
There was no significant difference in OS, although there was a
trend toward unfavorable OS in the group with a higher CEA ratio
(≥0.60); this may be worth examining to observe what the results
would be if the sample size were larger. The group with a lower CEA
ratio (<0.60) exhibited higher preoperative CEA levels than
their counterpart. On the other hand, there was no significant
difference in postoperative CEA levels between the two groups.
These results suggested the relevance of not only evaluating
preoperative or postoperative CEA values but also evaluating the
CEA ratio in predicting prognosis after adrenalectomy for lung
cancer with solitary adrenal metastasis. Furthermore, there was no
statistically significant difference in the distribution of the
clinical T or N stage of lung cancer between the high CEA ratio
group and the low CEA group. In other words, taking into account
the factor of the CEA ratio as well as cancer staging may be a
useful adjunct tool in predicting prognosis.
There were no significant differences in
postoperative CEA levels between the high CEA ratio and the low CEA
ratio groups. Of note, the group with a lower CEA ratio (<0.60)
had higher preoperative CEA levels. A similar trend was observed in
a previous study that examined the prognostic value of the CEA
ratio in colorectal cancer (9).
Cases of lung cancer with high preoperative CEA levels may be
considered as a high-CEA secretion type and the changes in CEA
levels may reflect the degree of residual tumor cells in the body.
The fact that no experiments were performed to demonstrate this
hypothesis is a limitation of the present study.
The present study had several other limitations.
First, this retrospective study was a single cohort study that was
based on the limited data available. Owing to the small sample
size, no definitive independent variable to predict prognosis was
determined. Furthermore, the study did not have any fixed protocol
for the date of measurement of the serum CEA levels, and therefore,
there was variation in the measurement dates, as measurements were
performed at a median of 13 (IQR, 3-48) days prior to surgery and a
median of 33 (IQR, 22-63) days after surgery. In general, the
half-life of CEA values is 3-7 days (12,17-19)
and therefore, the different measurement dates from the
postoperative period may have resulted in different CEA levels even
in the same patient. This may therefore raise the concern about the
reproducibility of the analysis results regarding that the CEA
ratio is able to predict patient prognosis. Finally, the
interpretation of the present findings is limited by losses to
follow-up (two patients were lost to follow-up; the observation
periods were 247 days and 2,351 days, respectively).
In conclusion, the present study suggested that the
perioperative CEA ratio may be a simple and important emerging
prognostic factor for patients with lung cancer with solitary
adrenal gland metastasis. However, this was a retrospective study
with a small sample size and validation in a future prospective
study with an appropriate sample size is essential for the
generalization of this finding and assessment of applicability in
clinical practice.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SY, YN and HM conceived the present study. SY, KT
and MT designed the study. KT and SM acquired the data. SY, KT and
SM analyzed and interpreted the data and drafted the manuscript. YN
performed critical revision of the manuscript. MT and HM supervised
the study and revised the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript. YN and HM checked and approved the authenticity of the
raw data. All authors agree to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved (according to the ICMJE).
Ethics approval and consent to
participate
This study is retrospective and informed consent
from the patients was not required. The present study was approved
by the National Cancer Center Institutional Review Board (Kashiwa,
Japan; approval no. 2018-159).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Walters S, Maringe C, Coleman MP, Peake
MD, Butler J, Young N, Bergström S, Hanna L, Jakob-sen E, Kölbeck
K, et al: ICBP Module 1 Working Group: Lung cancer survival and
stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden
and the UK: A population-based study, 2004-2007. Thorax.
68:551–564. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Albain KS, Crowley JJ, LeBlanc M and
Livingston RB: Survival determinants in extensive-stage
non-small-cell lung cancer: The Southwest Oncology Group
experience. J Clin Oncol. 9:1618–1626. 1991.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eberhardt WEE, Mitchell A, Crowley J,
Kondo H, Kim YT, Turrisi A, Goldstraw P and Rami-Porta R: The IASLC
Lung Cancer Staging Project: Proposals for the revision of the M
descriptors in the forthcoming eighth edition of the TNM
classification of lung cancer. J Thorac Oncol. 10:1515–1522.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Raz DJ, Lanuti M, Gaissert HC, Wright CD,
Mathisen DJ and Wain JC: Outcomes of patients with isolated adrenal
metastasis from non-small cell lung carcinoma. Ann Thorac Surg.
92:1788–1793. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao XL, Zhang KW, Tang MB, Zhang KJ, Fang
L-N and Liu W: Pooled analysis for surgical treatment for isolated
adrenal metastasis and non-small cell lung cancer. Interact
Cardiovasc Thorac Surg. 24:1–7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Higashiyama M, Doi O, Kodama K, Yokouchi
H, Imaoka S and Koyama H: Surgical treatment of adrenal metastasis
following pulmonary resection for lung cancer: Comparison of
adrenalectomy with palliative therapy. Int Surg. 79:124–129.
1994.PubMed/NCBI
|
|
8
|
Luketich JD and Burt ME: Does resection of
adrenal metastases from non-small cell lung cancer improve
survival? Ann Thorac Surg. 62:1614–1616. 1996.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xie HL, Gong YZ, Kuang JA, Gao F, Tang SY
and Gan JL: The prognostic value of the postop-erative serum CEA
levels/preoperative serum CEA levels ratio in colorectal cancer
patients with high preoperative serum CEA levels. Cancer Manag Res.
11:7499–7511. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Odeny TA, Farha N, Hildebrandand H, Allen
J, Vazquez W, Martinez M, Paluri RK and Kasi A: Association between
primary perioperative CEA ratio, tumor site, and overall survival
in patients with colorectal cancer. J Clin Med.
9(3848)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nonaka M, Kataoka D, Yamamoto S, Bito A,
Matsuoka J, Kawada T and Takaba T: Pre- and post-operative serum
carcinoembryonic antigen in primary lung adenocarcinoma. Ann Thorac
Cardiovasc Surg. 10:281–284. 2004.PubMed/NCBI
|
|
12
|
Ito K, Hibi K, Ando H, Hidemura K,
Yamazaki T, Akiyama S and Nakao A: Usefulness of analyti-cal CEA
doubling time and half-life time for overlooked synchronous
metastases in colorectal carci-noma. Jpn J Clin Oncol. 32:54–58.
2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Clavien PA, Barkun J, de Oliveira ML,
Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J,
Slankamenac K, Bassi C, et al: The Clavien-Dindo classification of
surgical complications: Five-year experience. Ann Surg.
250:187–196. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Akobeng AK: Understanding diagnostic tests
3: Receiver operating characteristic curves. Acta Pae-diatr.
96:644–647. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Howell GM, Carty SE, Armstrong MJ, Stang
MT, McCoy KL, Bartlett DL and Yip L: Outcome and prognostic factors
after adrenalectomy for patients with distant adrenal metastasis.
Ann Surg Oncol. 20:3491–3496. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mazzella A, Loi M, Mansuet-Lupo A, Bobbio
A, Blons H, Damotte D and Alifano M: Clinical characteristics,
molecular phenotyping, and management of isolated adrenal
metastases from lung cancer. Clin Lung Cancer. 20:405–411.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Park YA, Lee KY, Kim NK, Baik SH, Sohn SK
and Cho CW: Prognostic effect of perioperative change of serum
carcinoembryonic antigen level: A useful tool for detection of
systemic recurrence in rectal cancer. Ann Surg Oncol. 13:645–650.
2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Choi JS and Min JS: Significance of
postoperative serum level of carcinoembryonic antigen (CEA) and
actual half life of CEA in colorectal cancer patients. Yonsei Med
J. 38:1–7. 1997.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yakabe T, Nakafusa Y, Sumi K, Miyoshi A,
Kitajima Y, Sato S, Noshiro H and Miyazaki K: Clini-cal
significance of CEA and CA19-9 in postoperative follow-up of
colorectal cancer. Ann Surg Oncol. 17:2349–2356. 2010.PubMed/NCBI View Article : Google Scholar
|