Introduction
Several oncogenic alterations, such as mutations in
the epidermal growth factor receptor (EGFR) gene, occur in
non-small cell lung cancer (NSCLC) (1). The results from several large phase
III trials demonstrated that, relative to chemotherapy, EGFR
tyrosine kinase inhibitors (EGFR-TKIs) significantly improved the
survival rates of patients with NSCLC who had EGFR-activating
mutations (2-4).
Consequently, EGFR-TKIs, such as gefitinib (first-generation) and
afatinib (second-generation), are now approved worldwide, and are
currently used as first-line treatments for patients with NSCLC
harboring EGFR mutations.
Although EGFR-TKIs initially achieve a notable
response, almost all patients eventually acquire drug resistance.
Previous studies have reported that the EGFR T790M mutation
accounted for 50-60% of all cases of acquired resistance to
EGFR-TKIs. Amplification of the MNNG/HOS transforming gene (MET),
histological transformation to small cell lung cancer and mutation
of the Kirsten rat sarcoma viral oncogene homolog may also lead to
resistance (1,5,6). The
development and application of next-generation sequencing (NGS)
technologies have enabled the identification of rare mutations. For
example, Gallant et al (7)
identified a mutation in the kinase domain duplication (KDD) of the
EGFR gene (EGFR-KDD) acting as an oncogene in NSCLC.
We herein report a rare case of an EGFR-KDD mutation
that conferred resistance to gefitinib in a patient with NSCLC, who
subsequently responded well to afatinib treatment.
Case report
In September 2015, a 56-year-old male smoker who
presented with complaints of mild hemoptysis for 2 months was
admitted to The First Affiliated Hospital of the University of
Science and Technology of China (Hefei, China). A chest CT scan
revealed a lesion in the lower lobe of the right lung, and a
positron emission tomography/CT scan showed a marked increase of
fluorodeoxyglucose (FDG) uptake in this lesion. No FDG accumulation
was identified in other parts of the body and, therefore, a
right-lower lobectomy was performed. The postoperative
histopathological examination indicated an invasive non-mucinous
adenocarcinoma. According to the 8th edition of the American Joint
Committee on Cancer TNM staging system for NSCLC (8), the cancer was classified as stage IIb
(T1bN1M0). The patient subsequently received four cycles of
gemcitabine (1,000 mg/m2 i.v. on d1 and d8) plus
cisplatin (75 mg/m2 i.v. on d1) as postoperative
adjuvant chemotherapy, and was followed up every 3 months
thereafter.
In August 2016, a contrast-enhanced chest CT scan
revealed mediastinal lymphadenopathy and multiple pleural nodules
with heterogeneous enhancement, indicating recurrence of the lung
cancer. A mutation in EGFR exon 21 (L858R) was detected in the
patient's surgically resected tissue using the
amplification-refractory mutation system (ARMS; Amoy Diagnostics
Co., Ltd.). The patient was treated with gefitinib (250 mg p.o. qd)
and achieved stable disease over the following 21 months.
In March 2018, the patient visited our hospital
again for routine examination. A chest CT scan revealed right-sided
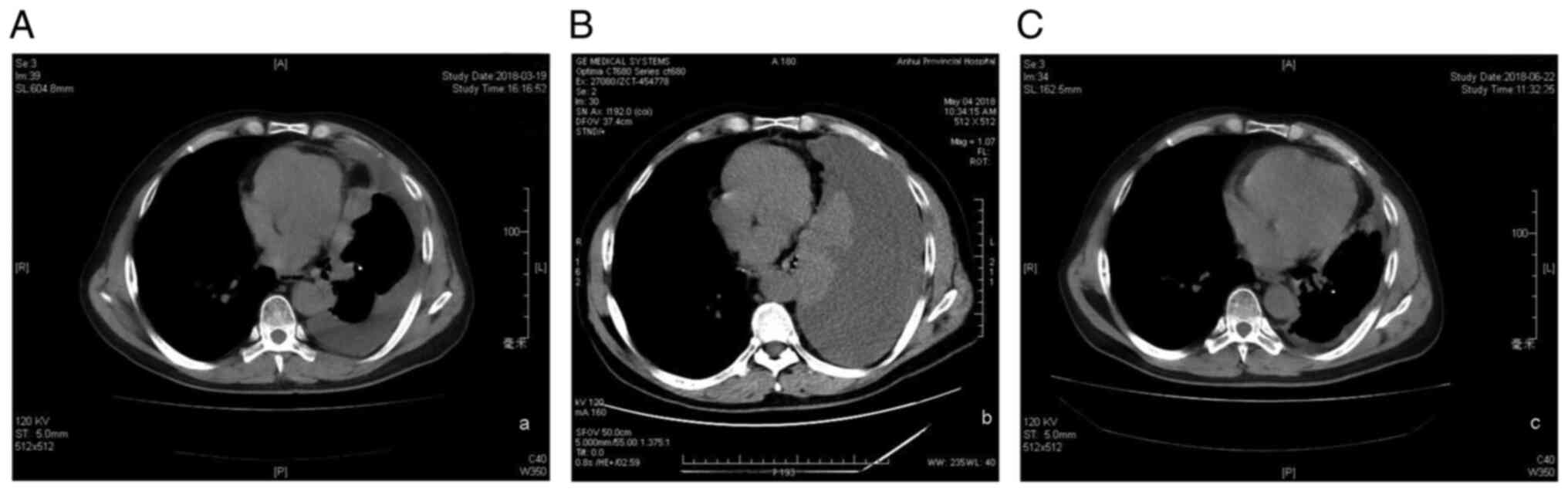
pleural effusion and multiple pleural nodules scattered within the
entire right pleura, which ranged in size from 5 to 15 mm (Fig. 1A). However, as the patient felt
well, he refused to change the treatment regimen at that time.
After 2 months, the patient revisited our hospital
complaining of dyspnea. Another CT scan revealed a massive
right-sided pleural effusion (Fig.
1B), which necessitated thoracentesis using a central
intrathoracic venous catheter to drain the malignant pleural fluid.
Pleural fluid cytological examination revealed the presence of
adenocarcinoma cells. Only a few adenocarcinoma cells were
detected; therefore, genetic testing could not be performed. We
therefore used NGS (HiSeq/MiSeqDx, Illumina, Inc.; performed by
Geneseeq Technology Inc.) of a blood sample to detect the possible
mechanism of resistance. The sequencing depth of the target area
was 5,000x and the coverage of the target area was 99.8%. The
results revealed that the mutation in EGFR exon 21 (L858R) had
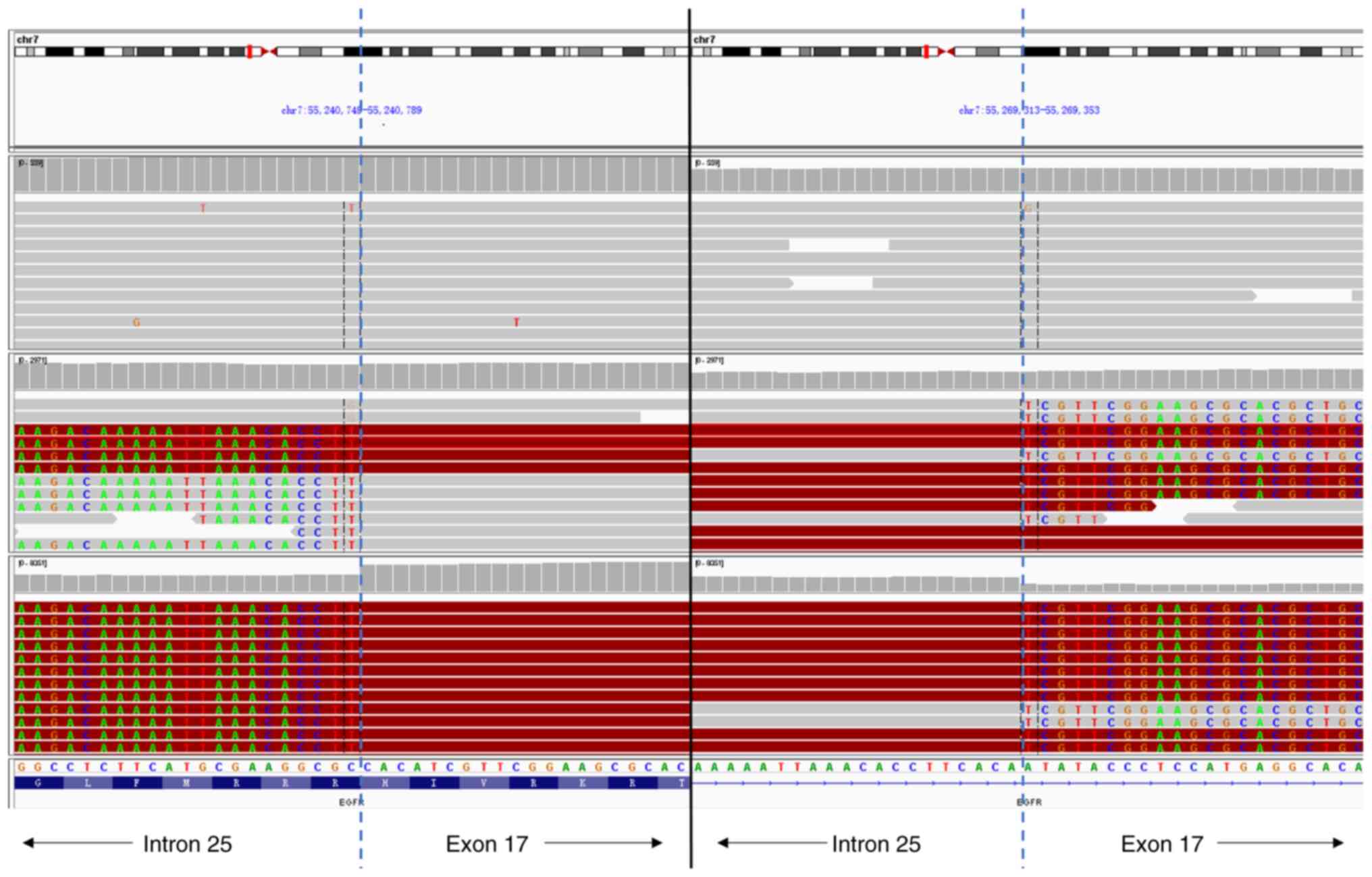
disappeared, and a new EGFR-KDD mutation was identified (Fig. 2). Amplification of the
cyclin-dependent kinase (CDK)4 gene and a mutation in the SMARCA4
gene, which encodes a protein in the SWitch/Sucrose Non-Fermentable
family and functions in DNA remodeling, were also identified.
Therefore, afatinib treatment (40 mg p.o. qd) was
initiated. One month later, a chest CT scan indicated no pleural
effusion and a reduction in the size of the pleural nodules
(Fig. 1C). At the 9-month
follow-up, there was no evidence of recurrence. In March 2019, the
patient returned to our hospital and reported experiencing a
feeling of tiredness. A chest CT scan revealed a ~2-fold increase
in the size of the pleural nodules, and NGS-based liquid biopsy of
blood samples revealed that the EGFR-KDD mutation was still
present. There was also a decreased abundance of EGFR-KDD and
increased amplification of CDK4 (Table
I). Therefore, treatment with palbociclib (125 mg once daily
taken with food for 21 days followed by 7 days off treatment) was
attempted; palbociclib is an oral pyridopyrimidine-derived CDK
inhibitor that was previously proposed as a therapy for overcoming
afatinib resistance in patients with NSCLC (9). However, 1 month later, another chest
CT scan revealed a marked increase in the size of multiple tumor
nodules. Two more cycles of pemetrexed (500 mg/m2 i.v.
on d1) plus bevacizumab (7.5 mg/kg i.v. on d1) were administered;
however, the patient succumbed to the disease in September
2019.
 | Table IDynamic alteration of CNV or mutation
during this case of adenocarcinoma. |
Table I
Dynamic alteration of CNV or mutation
during this case of adenocarcinoma.
| CNV or mutation | August 2016 | Date May 2018 | March 2019 |
|---|
| Abundance of EGFR
p.L858R mutation | 19.9% | - | - |
| Abundance of TP53
mutation | 20.4% | - | - |
| Abundance of EGFR-KDD
mutation | - | 5.1% | 1.3% |
| Abundance of SMARCA4
mutation | - | 11.8% | 9.8% |
| Copy number of EGFR
amplification | - | - | 4 |
| Copy number of CDK4
amplification | - | - | 5 |
Discussion
A mutation in EGFR-KDD, which was first described as
an oncogenic driver of NSCLC in 2015, consists of an in-tandem
duplication of exons 18-25(7).
Functionally, the tandem connection of tyrosine kinase domains may
form an intramolecular dimer that confers constitutive activation
of EGFR (10). To date, there are
only few case reports of this rare mutation in patients with NSCLC
(11,12). A recent multicenter study of 10,579
patients with NSCLC reported that only 0.12% of the cases harbored
the EGFR-KDD mutation (13).
Notably, all those studies described this as the primary mutation
in NSCLC. To the best of our knowledge, there are no previous
reports on the role of the EGFR-KDD mutation in acquired resistance
to EGFR-TKIs.
In the present study, ARMS was used to detect the
L858R point mutation in EGFR exon 21 in a patient with lung
adenocarcinoma who developed postoperative recurrence. Gefitinib
was initially administered; however, after disease progression, an
NGS-based liquid biopsy was used to examine the possible mechanism
underlying resistance development. Despite the disappearance of the
EGFR exon 21 L858R mutation, a new EGFR-KDD mutation and CDK4 gene
amplification were identified.
Previous studies have reported evidence that
afatinib treatment achieved promising effects in patients with
NSCLC who harbored uncommon EGFR mutations, such as those with
EGFR-KDD (11,13,14).
In addition, the results from our previous clinical practice
indicated that several patients with NSCLC who had the EGFR G719X
mutation and acquired resistance to gefitinib, nonetheless
responded well to afatinib. Therefore, the patient in the present
case was treated with afatinib. After 1 month, the patient reported
no chest tightness, and the pleural effusion had disappeared. This
was accompanied by prolonged stable disease for 10 months.
To ascertain whether the EGFR-KDD mutation
identified was a primary mutation, NGS on surgically resected
tissue was performed. There was no evidence of other mutations,
except those in EGFR exon 21 (L858R) and TP53.
SMARCA4 alterations are the most common recurrent
genomic alterations in NSCLC, and they have been found to be
associated with poor patient outcome (15). However, the present case exhibited
a decreased abundance of the SMARCA4 mutation following EGFR-TKI
treatment. This result suggested that a SMARCA4 mutation did not
play a central role in the progression of NSCLC in our patient.
When the patient's condition worsened again, a
decreased abundance of the EGFR-KDD mutation and an increase in
CDK4 amplification were identified. Consequently, it was
hypothesized that the EGFR-KDD mutation may have conferred
resistance to gefitinib, subsequently becoming the new driver
mutation, instead of the initial EGFR L858R mutation.
Various mechanisms of acquired resistance to
first-generation EGFR-TKIs in patients with NSCLC who had EGFR
mutations have been previously described, and the T790M mutation is
the most common known mechanism underlying resistance (16). Chemotherapy remains the standard
therapy for patients with T790M-negative NSCLC who have acquired
resistance to EGFR-TKIs. However, the patient described herein had
an acquired EGFR-KDD mutation, and yet responded well to afatinib
following failure of gefitinib. This rare mutation was detected by
liquid biopsy using an NGS assay, which is a non-invasive
technology that can provide dynamic monitoring of gene mutations
for targeted therapy of patients with NSCLC (17). NGS is not widely available in China
due to the high cost. However, the PIONEER study showed that over
half of Asian patients with NSCLC harbor EGFR mutations (18). Therefore, more widespread use of
NGS should enable the detection of more rare acquired mutations,
thereby identifying new targets for the development of novel
therapies.
In conclusion, the EGFR-KDD mutation in NSCLC may be
a secondary EGFR mutation that confers resistance to treatment with
first-generation EGFR-TKIs, such as gefitinib, but appears to be
sensitive to the second-generation EGFR-TKI, afatanib.
Identification of this mutation in additional patients with NSCLC
will confirm its role in acquired resistance.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and analyzed during the
current study are not publicly available, as it is not allowed to
share the sequencing data of Chinese patient without the permission
according to the Criminal Law of The People's Republic of China,
but are available from the corresponding author on reasonable
request.
Authors' contributions
CH: Writing the original draft of the manuscript;
YW: Conceptualization, supervision, writing, review and editing of
the manuscript. Both authors have seen and can confirm the
authenticity of the raw data. Both authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written consent for the
publication of the case details and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lim ZF and Ma PC: Emerging insights of
tumor heterogeneity and drug resistance mechanisms in lung cancer
targeted therapy. J Hematol Oncol. 12(134)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee SM, Khan I, Upadhyay S, Lewanski C,
Falk S, Skailes G, Marshall E, Woll PJ, Hatton M, Lal R, et al:
First-line erlotinib in patients with advanced non-small-cell lung
cancer unsuitable for chemotherapy (TOPICAL): A double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 13:1161–1170.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ko B, Paucar D and Halmos B: EGFR T790M:
Revealing the secrets of a gatekeeper. Lung Cancer (Auckl).
8:147–159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Normanno N, Maiello MR, Chicchinelli N,
Iannaccone A, Esposito C, De Cecio R, D'alessio A and De Luca A:
Targeting the EGFR T790M mutation in non-small-cell lung cancer.
Expert Opin Ther Targets. 21:159–165. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gallant JN, Sheehan JH, Shaver TM, Bailey
M, Lipson D, Chandramohan R, Red Brewer M, York SJ, Kris MG,
Pietenpol JA, et al: EGFR kinase domain duplication (EGFR-KDD) is a
novel oncogenic driver in lung cancer that is clinically responsive
to afatinib. Cancer Discov. 5:1155–1163. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lababede O and Meziane MA: The eighth
edition of TNM staging of lung cancer: Reference chart and
diagrams. Oncologist. 23:844–848. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nie H, Zhou X, Shuzhang D, Nie C, Zhang X
and Huang J: Palbociclib overcomes afatinib resistance in non-small
cell lung cancer. Biomed Pharmacother. 109:1750–1757.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Du Z, Gallant JN, Sheehan J, Meiler J and
Lovly CM: Intramolecular dimerization of EGFR kinase domain
duplication as a novel activation mechanism. J Thoracic Oncol. 12
(Suppl)(S1536)2017.
|
|
11
|
Baik CS, Wu D, Smith C, Martins RG and
Pritchard CC: Durable response to tyrosine kinase inhibitor therapy
in a lung cancer patient harboring epidermal growth factor receptor
tandem kinase domain duplication. J Thoracic Oncol. 10:e97–e99.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu YC, Wang WX, Xu CW, Tan QH, Li JY,
Zhuang W, Song ZB, Du KQ, Chen G, Lv TF and Song Y: Lung
adenocarcinoma patient with an EGFR kinase domain duplication (KDD)
and the response to icotinib. J Thorac Dis. 10:E359–E363.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang J, Li X, Xue X, Ou Q, Wu X, Liang Y,
Wang X, You M, Shao YW, Zhang Z and Zhang S: Clinical outcomes of
EGFR kinase domain duplication to targeted therapies in NSCLC. Int
J Cancer. 144:2677–2682. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Russo A, Franchina T, Ricciardi G,
Battaglia A, Picciotto M and Adamo V: Heterogeneous responses to
epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors
(TKIs) in patients with uncommon EGFR mutations: New insights and
future perspectives in this complex clinical scenario. Int J Mol
Sci. 20(1431)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schoenfeld AJ, Bandlamudi C, Lavery JA,
Montecalvo J, Namakydoust A, Rizvi H, Egger J, Concepcion CP, Paul
S, Arcila ME, et al: The genomic landscape of SMARCA4 alterations
and associations with outcomes in patients with lung cancer. Clin
Cancer Res. 26:5701–5708. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Westover D, Zugazagoitia J, Cho BC, Lovly
CM and Paz-Ares L: Mechanisms of acquired resistance to first- and
second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 29
(Suppl 1):i10–i19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rolfo C, Mack PC, Scagliotti GV, Baas P,
Barlesi F, Bivona TG, Herbst RS, Mok TS, Peled N, Pirker R, et al:
Liquid biopsy for advanced non-small cell lung cancer (NSCLC): A
statement paper from the IASLC. J Thorac Oncol. 13:1248–1268.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014.PubMed/NCBI View Article : Google Scholar
|