Introduction
Mixed phenotype acute leukemia (MPAL) is a
heterogeneous and rare subtype of acute leukemia (1-3).
MPAL is characterized by immunophenotypic features of a multi-cell
lineage, and is divided into biphenotypic and biclonal types.
Approximately 25% of MPAL cases have a Philadelphia chromosome (Ph)
(4,5). MPAL commonly diagnosed by the
demonstration of leukemic cells expressing markers of more than one
hematopoietic lineage (3).
Ph-positive MPAL is more common among elderly populations, has
higher white blood cell (WBC) counts at diagnosis, and resulting a
worse prognosis than Ph-negative MPAL (4-7).
Treatment according to the management of Ph-positive acute lymphoid
leukemia (ALL) has usually been provided, but the optimal course of
treatment for Ph-positive MPAL has not yet been established
(8).
Hypocellular acute leukemia is another rare variant
of acute leukemia that occurs more frequently among the elderly and
shows a poor prognosis (9).
Various regimens have been provided for hypocellular acute
leukemia, but consensus remains lacking regarding the optimal
treatment (10). To the best of
our knowledge, no reports have addressed hypocellular MPAL despite
Ph-positive and its treatment.
We report herein the case of an elderly man with
hypocellular Ph-positive biclonal-type MPAL diagnosed by
immunostaining of bone marrow biopsy and genetic analysis not flow
cytometry because of hypocellularity of bone marrow. Dasatinib and
prednisolone were useful for achieving long-term molecular
remission without compromising the quality of life.
Case report
A 77-year-old Japanese man was admitted to our
hospital with malaise and sudden loss of vision in the right eye.
He had been treated for hypertension for the past 25 years, but had
no history of hematological abnormalities. Eastern Cooperative
Oncology Group performance status was 0. Physical examination
revealed conjunctival pallor, grade II/VI ejection systolic murmur
in the left parasternal area in the second to fourth intercostal
space, and petechiae on bilateral upper and lower extremities.
Clinical laboratory data showed: white blood cell count (WBC),
3.4x109/l (neutrophils 71.0%, blasts 5.0%); hemoglobin,
7.6 g/dl; platelet count, 35x109/l; serum ferritin
level, 1,359 ng/ml; serum total protein, 6.1 g/dl; albumin, 3.8
g/dl; aspartate aminotransferase, 34 IU/l; and alanine
aminotransferase, 53 IU/l. However, no other abnormalities were
identified, including lactate dehydrogenase level, renal function,
and markers of disseminated intravascular coagulation. Right optic
neuritis was diagnosed and intravenous methylprednisolone pulse
therapy (1,000 mg/body/day, for 3 days) was immediately started.
After methylprednisolone pulse therapy, oral prednisolone was
continued at 25 mg/day.
He was referred to us for pancytopenia. Bone marrow
aspirate smears showed a very low concentration of cells (nucleated
cell count, 1.0x104/µl), myeloid/erythroid ratio, 0.5;
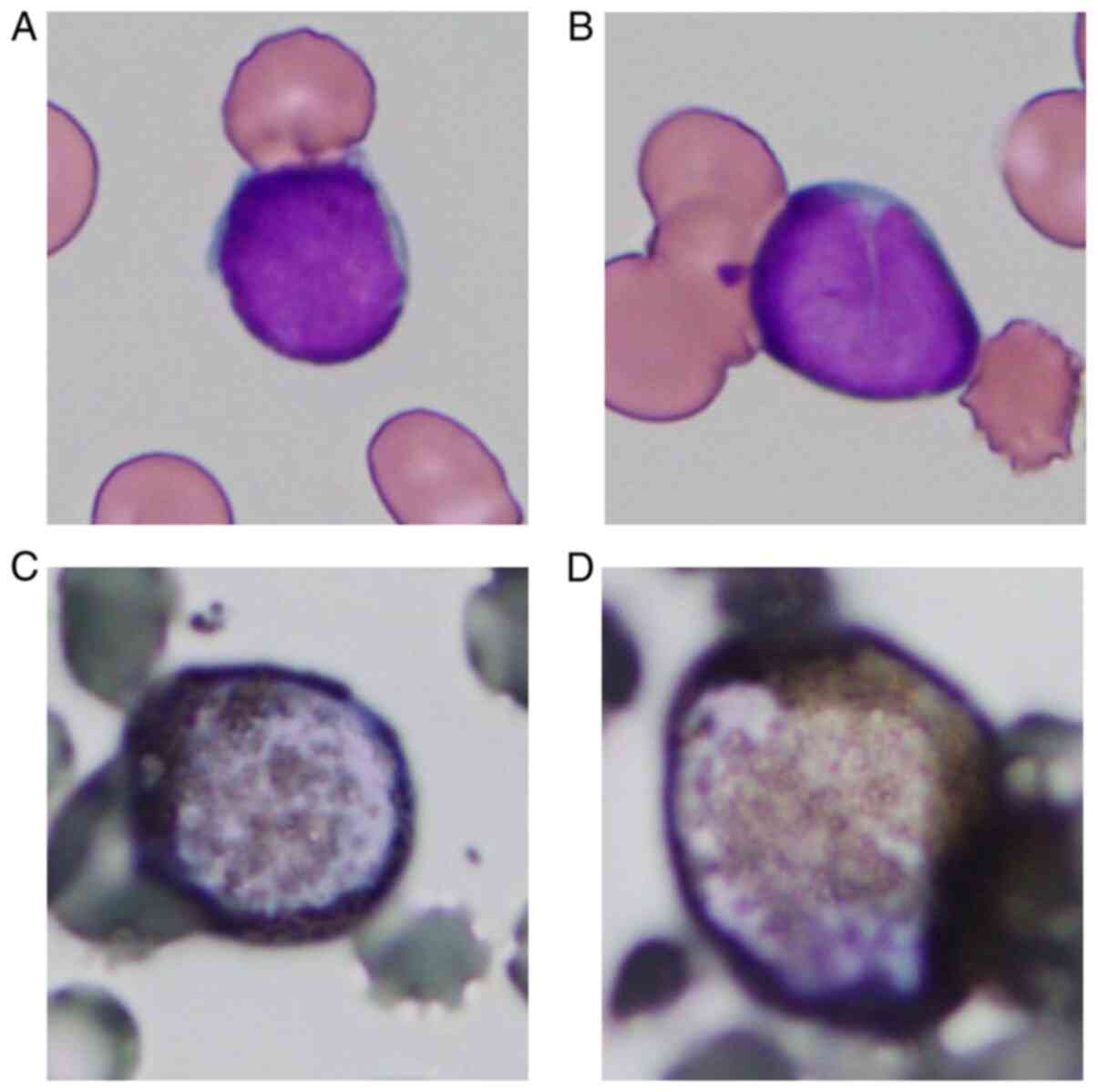
and 28% blasts among non-erythroid cells. Myeloperoxidase staining
of blasts yielded positive results on cytochemistry (Fig. 1). The results of flow cytometry
were unevaluable due to an insufficient number of blood cells
available for measurement. The level of minor BCR-ABL mRNA in bone
marrow was 10,000 copies/µg RNA. G-banding analysis of bone marrow
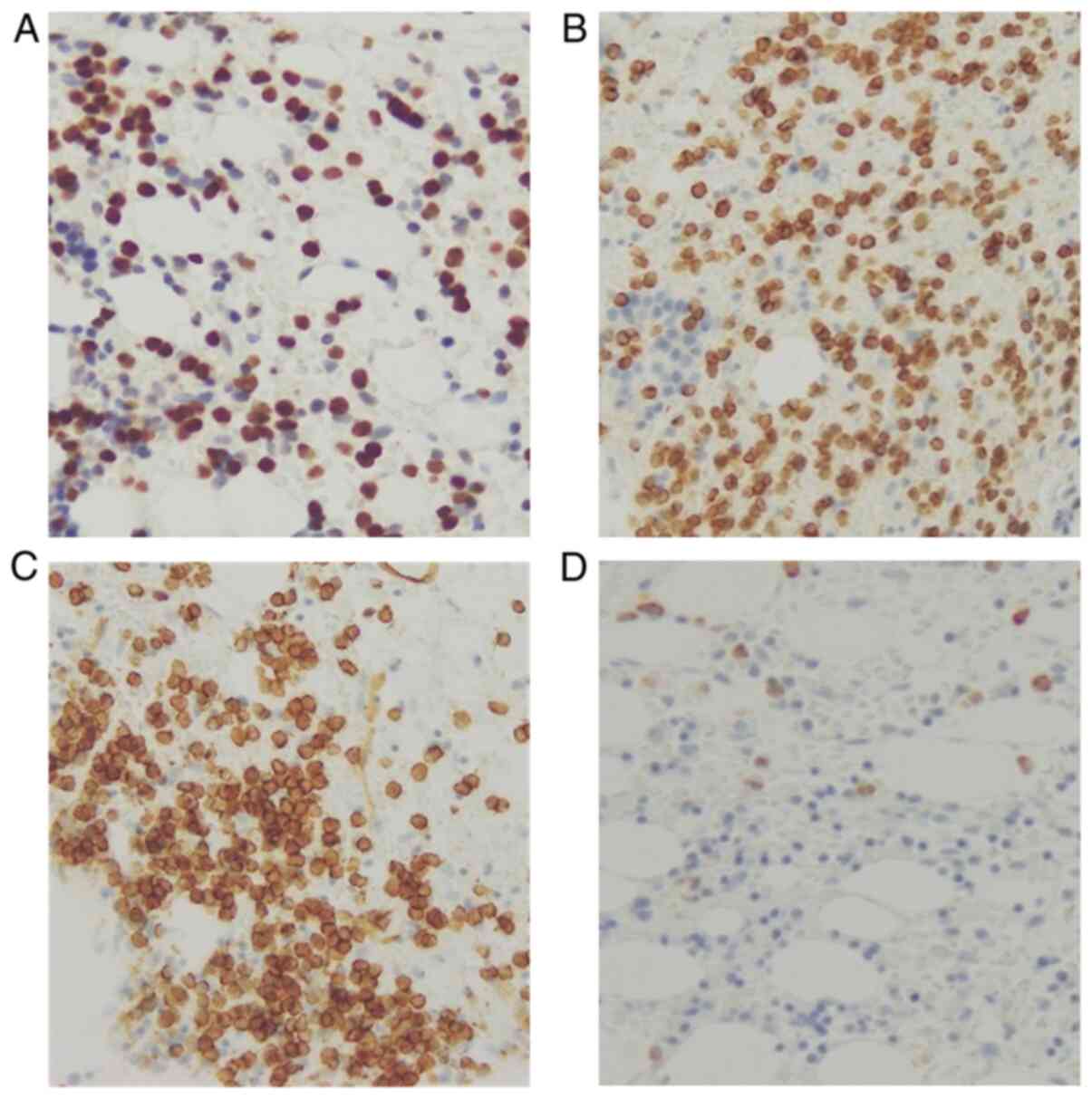
revealed a 46,XY karyotype. Bone marrow biopsy revealed extremely
low cellularity (20%) and proliferation of small round cells with
poor nucleoli that were positive for terminal deoxynucleotidyl
transferase (TdT), CD79a, and CD34, and negative for
myeloperoxidase immunostaining (Fig.
2). The simultaneous presence of two different leukemic clones
was demonstrated, and hypocellular Ph-positive biclonal MPAL was
finally diagnosed.
Before the definitive diagnosis of leukemia, he was
administered a second course of methylprednisolone pulse therapy
for optic neuritis, followed by tapering of oral prednisolone to 10
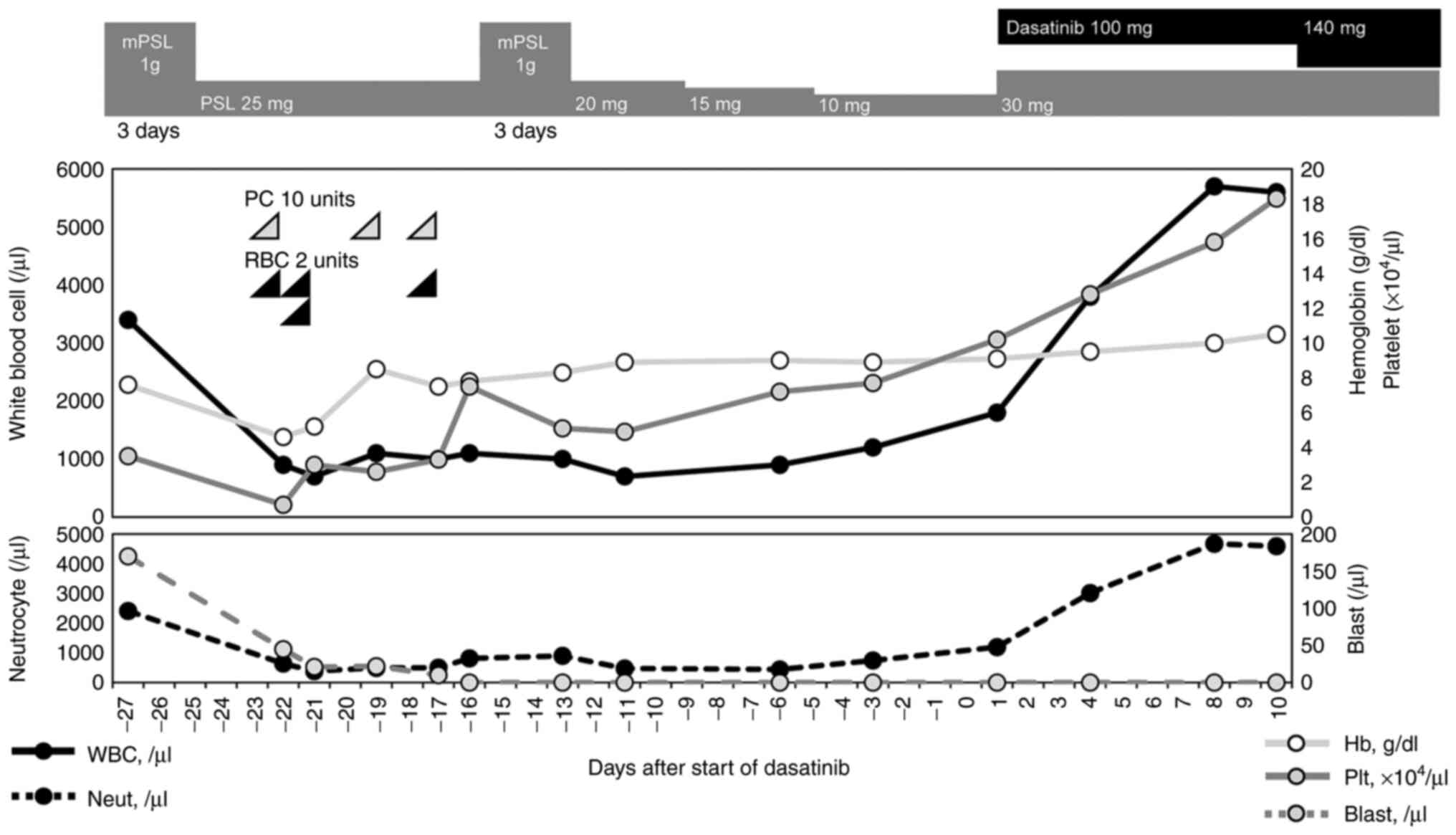
mg/day. Bone marrow examination revealed peripheral blood cells had
already recovered. Twenty-eight days after admission and initial
administration of steroid, dasatinib at 140 mg/day and oral
prednisolone at 30 mg/day were started as induction therapy. After
initiating induction therapy with dasatinib, peripheral blood cell
count immediately recovered significantly and normalized (Fig. 3). The patient was discharged 10
days after starting induction therapy and was continuously treated
with dasatinib and oral prednisolone on an outpatient basis. The
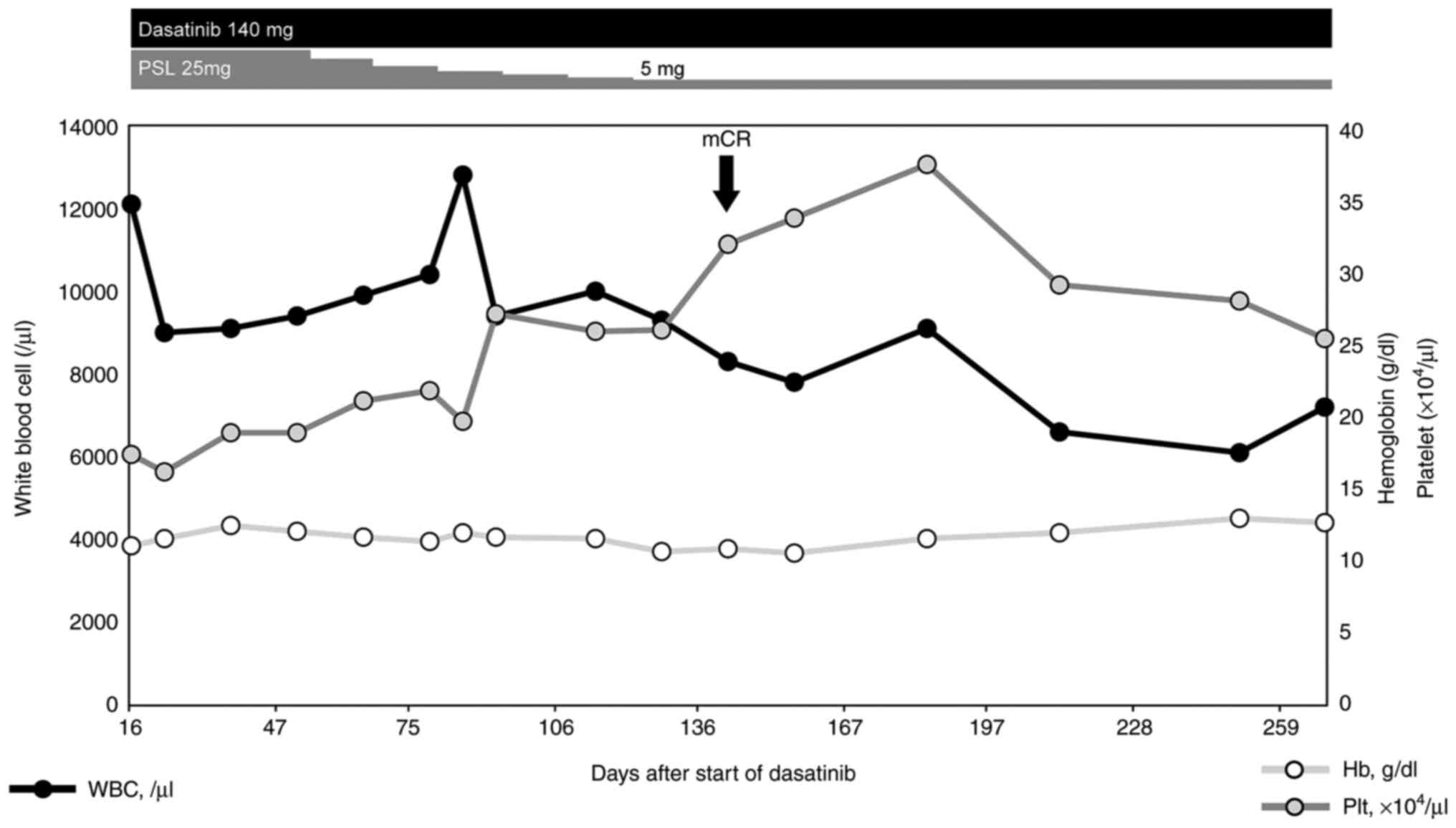
minor BCR-ABL fusion transcript in the bone marrow had disappeared
according to real-time quantitative polymerase chain reaction at
140 days after starting induction therapy. Dasatinib was continued
at 140 mg/day and oral prednisolone was tapered to 5 mg/day
(Fig. 4). After achieving
molecular complete remission (CR), he underwent 4 times of
intrathecal chemotherapy (methotrexate 15 mg, cytarabine 40 mg, and
prednisolone 10 mg) for central nervous system (CNS) prophylaxis.
As of the time of writing, molecular CR has been maintained for 15
months with no serious adverse events and no loss in quality of
life.
Discussion
While hypocellular leukemia has been rarely reported
(9,11), there have been no reports of
hypocellular leukemia with Ph-positive MPAL as in this case. It may
suggest that bone marrow aspiration in a patient of hypocellular
leukemia does not collect a sufficient number of cells, so the cell
origin is not sufficiently evaluated, and even if it is Ph-positive
MPAL, it is not properly diagnosed and underestimated. This case
suggests that hypocellular leukemia found to be
minor-BCR/ABL-positive MPAL may be expected to achieve long-term
survival by combination therapy with TKI and steroids, thus
supporting the usefulness of genetic analysis and immunostaining of
bone marrow biopsy in hypocellular leukemia.
MPAL is a rare leukemic entity that accounts for
1.6-2.8% of adult acute leukemias (1,2), and
is characterized by worse prognosis than single-lineage acute
leukemia. In particular, the presence of the Ph chromosome
adversely influences the prognosis of patients with MPAL (6,12-14).
Although no consensus has been reached regarding treatment
strategies for Ph-positive MPAL, certain cases have been managed
similar to Ph-positive ALL in the recent tyrosine kinase inhibitor
(TKI) era, with dramatically improved outcomes (7,8).
Several reports have described TKI plus prednisolone or
low-intensity chemotherapy as highly effective against elderly
Ph-positive MPAL (15,16). There have been several cases of
optic neuritis as the symptom of CNS involvement by acute leukemia
(17-20).
Steroids have been reported to relieve optic neuritis in ALL
(18). Dasatinib has also been
reported to cross the blood-brain barrier and have an effect on the
optic nerve infiltration of Ph-positive ALL (17,21).
The cause of the optic neuritis of the present case has not been
pathologically proven, but the possibility of leukemic involvement
could not be denied. Dasatinib combined with prednisolone might be
effective, and the leukemic cells in the CSF may have disappeared
at the time of intrathecal chemotherapy after confirmation of
hematological CR. The four times of intrathecal chemotherapy also
seemed to contribute to the prevention of CNS relapse and
maintenance of molecular CR.
Ph-positive leukemias generally present with high
WBC counts because the gene products constantly activate
intracellular signal transduction pathways via markedly increased
tyrosine kinase activity (22).
Despite expression of the Ph chromosome, the patient in our case
presented with pancytopenia and hypocellular bone marrow.
Ph-positive myelodysplastic syndrome has been reported (23,24),
but WBC increased after expression of the Ph chromosome in these
cases (23), and no reports have
described hypocellular bone marrow despite the presence of the Ph
chromosome. The optimal management for hypocellular leukemia has
not yet been established. The case of an elderly woman with
successful control using low-dose granulocyte colony-stimulating
factor and oral prednisolone has been reported (25). In our case, gradual recovery of
hematopoietic function after steroid alone and rapid normalization
of hematopoietic function after addition of dasatinib were
observed. Early administration of steroid might have contributed to
the improved hematopoietic function. In addition, since the Ph
chromosome also inhibits the proliferation of normal blood cells
(22), suppression of Ph-positive
clones by dasatinib may have restored normal hematopoiesis.
We have reported an unusual case of hypocellular
biclonal MPAL despite the Ph expression. Even in cases of
hypocellular leukemia, physicians should keep in mind of the
Ph-chromosome expression. Genetic analysis and immunostaining of
bone marrow biopsy can aid the correct diagnosis of the origin of
leukemia blasts when it is unanalyzable by flow cytometry due to
the hypocellular bone marrow. The clinical course of our patient
demonstrated the efficacy and tolerability of dasatinib combined
with steroid therapy for elderly patients with hypocellular
Ph-positive MPAL.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SL contributed to the conception and the design of
the study, the literature review and manuscript writing, data
interpretation and manuscript revision. KF and YK contributed to
data interpretation, manuscript discussion, and figure creation.
HW, TH and HT helped with the design of the study, data
interpretation, manuscript discussion and manuscript revision, and
approved of the final manuscript version to be published. SL and KF
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wolach O and Stone RM: Optimal therapeutic
strategies for mixed phenotype acute leukemia. Curr Opin Hematol.
27:95–102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Weinberg OK and Arber DA: Mixed-phenotype
acute leukemia: Historical overview and a new definition. Leukemia.
24:1844–1851. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J (eds): WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. Vol 2. Revised 4th
edition. IARC Publications, Lyon, 2017.
|
|
4
|
Matutes E, Pickl WF, Van't Veer M, Morilla
R, Swansbury J, Strobl H, Attarbaschi A, Hopfinger G, Ashley S,
Bene MC, et al: Mixed-phenotype acute leukemia: Clinical and
laboratory features and outcome in 100 patients defined according
to the WHO 2008 classification. Blood. 117:3163–3171.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Weinberg OK, Seetharam M, Ren L, Alizadeh
A and Arber DA: Mixed phenotype acute leukemia: A study of 61 cases
using World health organization and European Group for the
immunological classification of leukaemias criteria. Am J Clin
Pathol. 142:803–808. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Weir EG, Ali Ansari-Lari M, Batista DA,
Griffin CA, Fuller S, Smith BD and Borowitz MJ: Acute bilineal
leukemia: A rare disease with poor outcome. Leukemia. 21:2264–2270.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Y, Gu M, Mi Y, Qiu L, Bian S and Wang
J: Clinical characteristics and outcomes of mixed phenotype acute
leukemia with Philadelphia chromosome positive and/or bcr-abl
positive in adult. Int J Hematol. 94:552–555. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shimizu H, Yokohama A, Hatsumi N, Takada
S, Handa H, Sakura T and Nojima Y: Philadelphia chromosome-positive
mixed phenotype acute leukemia in the imatinib era. Eur J Haematol.
93:297–301. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park HW, Lee JH, Choi SJ, Lee JH, Seol M,
Lee YS, Ryu SG, Kim KH, Jang H, Seo EJ, et al: Hypoplastic acute
myeloid leukemia. Blood. 108(4493)2006.
|
|
10
|
Needleman SW, Burns CP, Dick FR and
Armitage JO: Hypoplastic acute leukemia. Cancer. 48:1410–1414.
1981.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Berdeaux DH, Glasser L, Serokmann R, Moon
T and Durie BG: Hypoplastic acute leukemia: Review of 70 cases with
multivariate regression analysis. Hematol Oncol. 4:291–305.
1986.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Killick S, Matutes E, Powles RL, Hamblin
M, Swansbury J, Treleaven JG, Zomas A, Atra A and Catovsky D:
Outcome of biphenotypic acute leukemia. Haematologica. 84:699–706.
1999.PubMed/NCBI
|
|
13
|
Legrand O, Perrot JY, Simonin G, Baudard
M, Cadiou M, Blanc C, Ramond S, Viguié F, Marie JP and Zittoun R:
Adult biphenotypic acute leukaemia: An entity with poor prognosis
which is related to unfavourable cytogenetics and P-glycoprotein
over-expression. Br J Haematol. 100:147–155. 1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Heesch S, Neumann M, Schwartz S, Bartram
I, Schlee C, Burmeister T, Hänel M, Ganser A, Heuser M, Wendtner
CM, et al: Acute leukemias of ambiguous lineage in adults:
Molecular and clinical characterization. Ann Hematol. 92:747–758.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kawajiri C, Tanaka H, Hashimoto S, Takeda
Y, Sakai S, Takagi T, Takeuchi M, Ohwada C, Sakaida E, Shimizu N
and Nakaseko C: Successful treatment of Philadelphia
chromosome-positive mixed phenotype acute leukemia by appropriate
alternation of second-generation tyrosine kinase inhibitors
according to BCR-ABL1 mutation status. Int J Hematol. 99:513–518.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Takata H, Ikebe T, Sasaki H, Miyazaki Y,
Ohtsuka E, Saburi Y, Ogata M and Shirao K: Two elderly patients
with philadelphia chromosome positive mixed phenotype acute
leukemia who were successfully treated with dasatinib and
prednisolone. Intern Med. 55:1177–1181. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Satake A, Okada M, Asada T, Fujita K,
Ikegame K, Tamaki H, Fujimori Y and Ogawa H: Dasatinib is effective
against optic nerve infiltration of Philadelphia
chromosome-positive acute lymphoblastic leukemia. Leuk Lymphoma.
51:1920–1922. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Townsend JH, Dubovy SR, Pasol J and Lam
BL: Transient optic perineuritis as the initial presentation of
central nervous system involvement by pre-B cell lymphocytic
leukemia. J Neuroophthalmol. 33:162–164. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Quann KA and Redner RL: The eyes have it:
CNS leukemia presenting as optic neuritis. Int J Hematol.
113:311–312. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Myers KA, Nikolic A, Romanchuk K, Weis E,
Brundler MA, Lafay-Cousin L and Costello F: Optic neuropathy in the
context of leukemia or lymphoma: Diagnostic approach to a
neuro-oncologic emergency. Neurooncol Pract. 4:60–66.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Porkka K, Koskenvesa P, Lundán T,
Rimpiläinen J, Mustjoki S, Smykla R, Wild R, Luo R, Arnan M,
Brethon B, et al: Dasatinib crosses the blood-brain barrier and is
an efficient therapy for central nervous system Philadelphia
chromosome-positive leukemia. Blood. 112:1005–1012. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gotoh A and Broxmeyer HE: The function of
BCR/ABL and related proto-oncogenes. Curr Opin Hematol. 4:3–11.
1997.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fukunaga A, Sakoda H, Iwamoto Y, Inano S,
Sueki Y, Yanagida S and Arima N: Abrupt evolution of Philadelphia
chromosome-positive acute myeloid leukemia in myelodysplastic
syndrome. Eur J Haematol. 90:245–249. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Keung YK, Beaty M, Powell BL, Molnar I,
Buss D and Pettenati M: Philadelphia chromosome positive
myelodysplastic syndrome and acute myeloid leukemia-retrospective
study and review of literature. Leuk Res. 28:579–586.
2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Islam A: Hypoplastic acute myeloid
leukemia in an elderly patient. A long-term partial remission with
low-dose prednisone and G-CSF. Clin Case Rep. 7:1285–1290.
2019.PubMed/NCBI View Article : Google Scholar
|