Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of non-Hodgkin lymphoma, consisting of ~30-40% of all
non-Hodgkin lymphoma cases (1). A
multicenter cross-sectional study in Indonesia showed that 68.2% of
all patients with non-Hodgkin lymphoma had DLBCL, which more
frequently affected males with a median age of 51 years (2).
Based on the gene-expression profiles, DLBCL is
divided into three subtypes; the B-cell-like germinal center (GCB)
subtype, the activated B-cell-like (ABC) subtype and the third
subtype that does not express genes from either the GCB or ABC
subtypes. The ABC and the third subtypes of DLBCL are collectively
known as the non-germinal center B-cell-like (non-GCB) subtype
(3,4). The germinal-center B-cell-like
subtype is associated with good clinical outcome and expressed
genes characteristic of normal germinal-center B-cells. Meanwhile,
the activated B-cell-like subtype was associated with poorer
clinical outcome and expressed genes characteristic of activated
blood B cells (3).
Tumor cells are known to survive by evading the
immune system of the body. The abilities of cancer cells to escape
the immune system have been extensively studied, including its
immunosuppression capacity (5).
Recently, the PD-1/PD-L1 signaling mechanism has emerged in the
study of tumor cells immunosuppression as enhancing immune
tolerance by inhibition of T cell activation (5).
Programmed cell death-1 (PD-1) or CD279 is a type 1
transmembrane protein of the B7/CD28 family, expressed in several
kinds of immune cells such as peripheral activated B and T cells,
natural killer cells and distinct dendritic cells (DC) (6). PD-1 is also weakly expressed on the
surface of immature T cells and B cells in the thymus and bone
marrow during the developmental process (7). PD-1 has two ligands; a programmed
cell death ligand-1 (PD-L1)/B7H1 and a programmed cell death
ligand-2 (PD-L2)/B7-DC (8). The
CD274 gene encodes PD-L1 and PD-L2 is encoded by PDCD1LG2 gene
(6). The two of them are located
on chromosome 9p.24.1(6). PD-L1 is
expressed on stromal tumor-associated macrophages and lymphocytes.
PD-L2 is primarily expressed in antigen-presenting cells (APCs)
(9).
Cancer cells may express numerous immune inhibitory
signaling proteins that can cause immune cell dysfunction and
apoptosis. PD-L1 is one of the inhibitory molecules which binds to
the programmed cell death-1 (PD-1) molecules expressed on dendritic
cells, T cells, B cells and natural killer T cells to suppress
anti-cancer immunity (10).
The development of an antibody targeting on immune
checkpoint mechanisms such as the PD-1/PD-L1 pathway has led to a
clinically significant antitumor response (11). Previous studies showed that
expression of PD-L1 in DLBCL patients was related to poor prognosis
(12-15).
It was decided to investigate PD-L1 expression in the GCB subtype,
as compared with those in the non-GCB subtype, because numerous
cases of DLBCL are found in Indonesia (2). The hypothesis of the present study
was that non-GCB subtype DLBCL had a higher expression of PD-L1
compared with GCB subtype DLBCL. However, information about PD-L1
expression in DLBCL subtypes in Indonesian patients remains limited
and the role of PD-L1 as targeted therapy in DLBCL has not been
fully elucidated. Therefore, the present study investigated PD-L1
expression in the GCB subtype as compared with those in the non-GCB
subtype of Indonesian DLBCL cases.
Materials and methods
A total of 40 patients samples in the form of
formalin-fixed paraffin-embedded tissues (FFPE) diagnosed as GCB
and non-GCB subtypes of DLBCL in the Department of Anatomical
Pathology, Faculty of Medicine, Universitas Indonesia/Dr. Cipto
Mangunkusumo National Central General Hospital, Jakarta, Indonesia
during the period of 2014 to 2017 were consecutively retrieved from
the archives and reviewed by authors (MH, AH, EH). The tissues were
fixed in 10% neutral buffered formalin for 24 h at room
temperature. Cases without sufficient FFPE materials were excluded
from the study. There were 20 cases of GCB subtype and 20 cases of
non-GCB subtype of DLBCL.
Expressions of PD-L1 in DLBCL FFPE samples were
evaluated according to the standard immunohistochemistry protocols;
chorionic villi (placenta) taken from the archives of the
Department of Anatomical Pathology of Universitas Indonesia/Dr.
Cipto Mangunkusumo National Central General Hospital was used as a
positive control. Unstained sections of 4 µm thickness were cut.
After deparaffinization and rehydration, the slides were blocked
with 3% hydrogen peroxide for 10 min and then washed under running
water for 5 min. Antigen retrieval was conducted with pH 9
Tris-EDTA buffer, in a decloaking chamber, at temperature of 95˚C
for 30 min. After washing in PBS pH 7.4 for 5 min, 10% normal horse
serum (Thermo Fisher Scientific, Inc.) blocking solution was
applied for 30 min at room temperature to block non-specific
protein. Then, each slides was incubated with PD-L1 primary
antibody (Rabbit polyclonal PD-L1 antibody; GeneTex, Inc.; cat. no.
GTX104763) with a dilution of 1:500 for 1 h at room temperature.
After repeated washing, the slides were incubated with a
ready-to-use secondary antibody polymer (Histofine Simple Stain MAX
PO kit; cat. no. 414151F; Nichirei Biosciences Inc.) for 30 min at
room temperature. This secondary antibody conjugated to an amino
acid polymer and multiple enzyme molecules. After repeated washing,
the slides were incubated with diluted diaminobendizine chromogen
buffer substrate for 3 min at room temperature. Counterstaining was
performed with Mayer's hematoxylin for 30 sec at room
temperature.
All slides were evaluated qualitatively in five
representative fields at x400 magnification under a light
microscope (Leica Microsystems GmbH; ≥1,000 tumor cells). Positive
staining of PD-L1 expression was shown by brown color in the tumor
cells membrane (12). ImageJ
software version number 1.51 (National Institutes of Health) was
used for cell counting. Expressions of PD-L1 were evaluated in
tumor cells. The sample was considered positive for PD-L1
expression if the frequency of PD-L1 expressing cells was >30%
(12).
Data obtained were then analyzed by using SPSS
version 20.0 (IBM Corp.). The distribution normality of the data
was determined by using the Shapiro-Wilk test. The statistical
significance value conducted by using a Fisher's exact test and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological characteristics of
DLBCL patients
The present study investigated 40 cases of DLBCL.
The subjects were divided into two groups, consisted of 20 cases of
GCB subtype and 20 cases of non-GCB subtype, which consisted of 21
males and 19 females patients. In the male group, there were 10 GCB
subtype cases and 11 cases of non-GCB subtype. In the female group,
there were nine cases of germinal center B-cell-like (GCB) subtype
and 10 non-GCB subtype cases. The median age of patients with GCB
subtype and non-GCB subtype were 48 and 55 years, respectively. Of
the 20 GCB subtype cases, nine cases were presented in the
extranodal sites and 11 cases in the lymph nodes. Meanwhile, of the
20 non-GCB subtype cases, 10 cases were presented in the extranodal
sites and 10 cases in the lymph nodes (Table I).
 | Table IClinicopathological characteristics of
40 cases with DLBCL investigated. |
Table I
Clinicopathological characteristics of
40 cases with DLBCL investigated.
| Characteristics | GCB subtype | Non-GCB subtype |
|---|
| Total subjects | 20 | 20 |
| Median age
(year-old) | 48 | 55 |
| Sex | | |
|
Male | 10 | 11 |
|
Female | 9 | 10 |
| Sites | | |
|
Nodal | 11 | 10 |
|
Extranodal | 9 | 10 |
Expression of PD-L1 in DLBCL tumor
cells
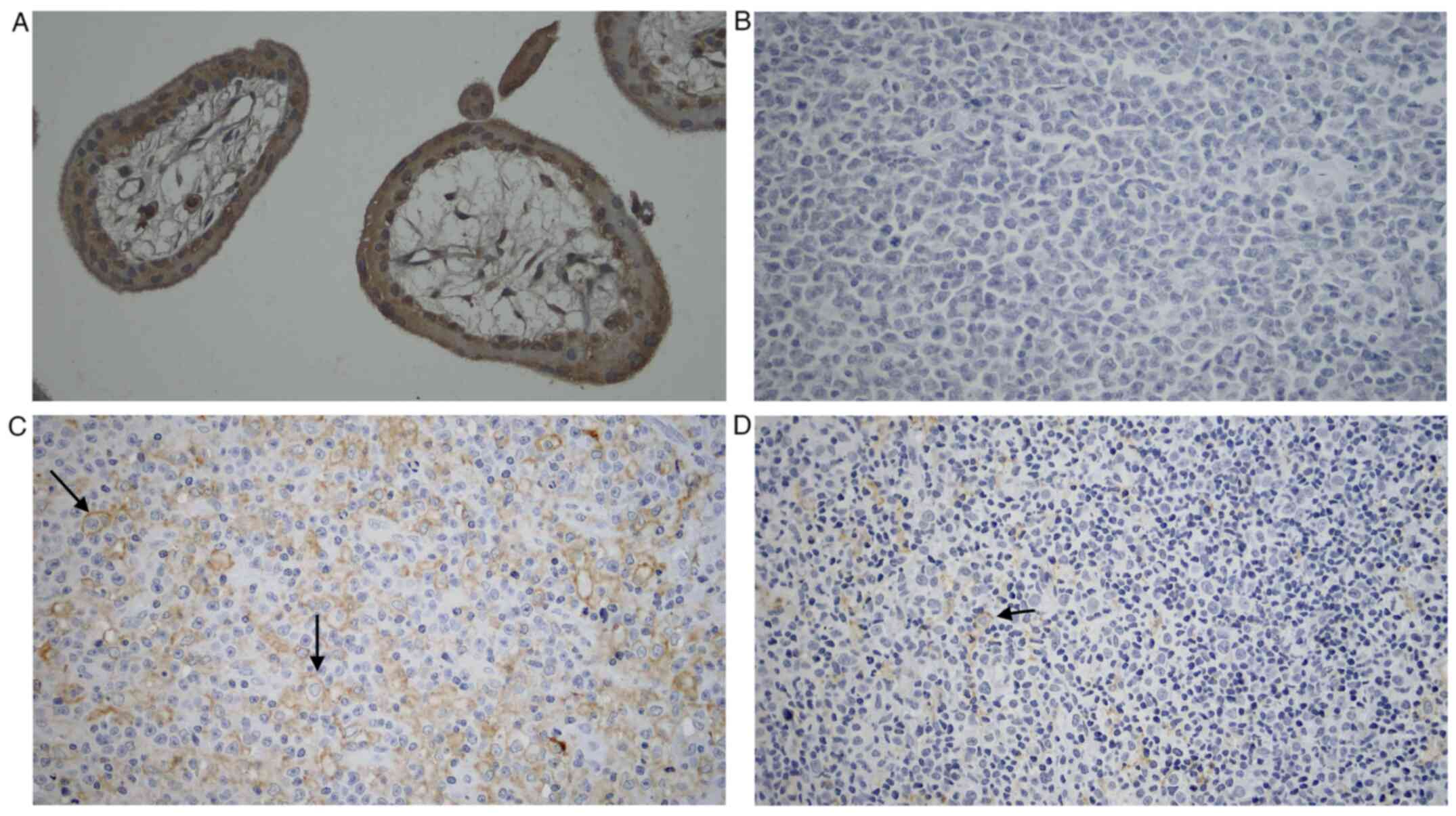
PD-L1 staining was conducted in the GCB subtype and
the non-GCB subtype of DLBCL samples. The result was later compared
with the positive control, which was trophoblastic cells of
chorionic villi (Fig. 1). Marked
expression of PD-L1 was shown on positive control tissue and no
expression in negative control tissue. Meanwhile, there was a more
robust PD-L1 expression in the non-GCB subtype of DLBCL compared
with the GCB subtype.
The distribution of PD-L1 expression in tumor cells
based on the cut-off value of ≥30% positively stained cells was
described in Table II. The
average value for PD-L1 positive expression in tumor cells was
54.725±17.704, while the average for negative PD-L1 expression in
tumor cells was 13.399±9.896. The Fisher's exact test showed
P=0.003; there was a significant difference between the expression
of PD-L1 in the GCB subtype and the non-GCB subtype of nodal and
extranodal DLBCL. Fisher's exact test revealed no significant
difference between PD-L1 expression in GCB and non-GCB of nodal
DLBCL (P=0.361), but revealed significant difference between PD-L1
expression in GCB and non-GCB of extranodal DLBCL (P=0.001).
 | Table IIDistribution of PD-L1 expression in
Diffuse large B-cell Lymphoma subtypes. |
Table II
Distribution of PD-L1 expression in
Diffuse large B-cell Lymphoma subtypes.
| | Expression of PD-L1
in tumor cells |
|---|
| DLBCL Subtypes | Positive | Negative | P-valuea |
|---|
| Nodal and
extranodal | | | |
|
GCB | 3/20 (15%) | 17/20 (85%) | 0.003 |
|
Non-GCB | 13/20 (65%) | 7/20 (35%) | |
| Nodal | | | |
|
GCB | 2/11 (18.2%) | 9/11 (81.8%) | 0.361 |
|
Non-GCB | 4/10 (40%) | 6/10 (60%) | |
| Extranodal | | | |
|
GCB | 1/9 (11.1%) | 8/9 (88.9%) | 0.001 |
|
Non-GCB | 9/10 (90%) | 1/10 (10%) | |
Discussion
In the present study, the incidence of DLBCL in male
patients was not significantly higher compared with in female
patients. It was found that the location of nodal and extranodal
DLBCL in the present study were only slightly different as compared
with other study. Shi et al (16) showed that DLBCL mostly developed in
nodal areas, while only ~37.4% of DLBCL cases were located
extranodal. The present study found that PD-L1 expression in the
DLBCL cases was significantly higher in the non-GCB subtype
compared with GCB subtype. This result was consistent with other
previous Asian population studies, which show a higher expression
of PD-L1 in the non-GCB subtype of DLBCL compared with in GCB
subtypes (12-14,17).
These studies also demonstrated that high PD-L1 expression in DLBCL
is associated with poor clinical outcomes. Higher expression of
PD-L1 in the non-GCB subtype of DLBCL is also associated with poor
clinical outcomes. Kwon et al (14) showed that strong PD-L1 expression
is associated significantly with the presence of B symptoms and
Epstein-Barr virus (EBV) infection. Kiyasu et al (12) note that PD-L1 expression level is
positively correlated with the number of PD-1-positive T cells in
activated B-cell-like (ABC)-subtype DLBCL specimens, but is
negatively correlated with the number of fork-head box P3
(FOXP3)-positive regulatory T cells in GCB-subtype DLBCL specimens.
The poor clinical outcomes of patients with ABC-subtype DLBCL can
be associated with PD-L1 expression in tumor cells. By contrast,
the lack of PD-L1 expression in GCB-subtype DLBCL specimens can be
a possible explanation for the favorable prognosis associated with
this disease subtype (17).
However in a study performed by Kwon et al (14), PD-L1 expression level is not
significantly different between non-GCB subtype of DLBCL and GCB
subtype (P=0.271).
Microenvironment PD-L1 (mPD-L1) can be defined as
non-malignant cells abundantly found in the tumor microenvironment
(12). Some studies have been
conducted to find the association between the PD-1/PD-L1 and its
prognosis in DLBCL (12,17). Patients with a low number of
PD-1+ and PD-L1+ have worse outcomes compared
with PD-L1- or mPD-L1- in DLBCL cases
(12). The finding recommends that
PD-L1 expression in DLBCL might reflect clinical features. The
study also stated that PD-L1+ was significantly
associated with lower overall survival compared with those with
PD-L1-. By contrast, mPD-L1 positivity did not have any
correlation with survival (12).
A previous study performed double staining with
PD-L1/PAX5 as an alternative way to improve the interpretation of
PD-L1 positivity in DLBCL (12).
PAX5 is a broader B-cell marker compared with CD 20. The present
study did not performed PAX5 analysis however, focusing on the
PD-L1 expression. Future study in genetic analysis (chromosome 9
translocation) may consider the involvement of PAX5.
Increased expression of PD-L1 in DLBCL tumor cells
can be considered as an independent prognosis marker. The results
of present study suggested that the PD-L1 expressions in DLBCL may
become a potential immunotherapy marker. DLBCL patients,
particularly those with non-GCB subtype might benefit from
PD-L1/PD-1 axis inhibition. Further investigation by using PD-L1 as
a biomarker of response to such treatment would be recommended.
The present study can be viewed as a preliminary
study to describe PD-L1 expression in DLBCL in Indonesia because it
is taken from the top referral hospital in Indonesia, which
although central, receives lymphoma specimens from remote areas as
well. A future study could be performed using a larger sample sizes
and multicenter study.
A limitation to the present study is that it did not
correlate the immunohistochemical data with the therapeutic outcome
or survival of the patient (due to the position of our
department/laboratory as a central referral center for diagnosis),
while the diagnosed patients were then treated in various hospitals
across the country, some without well-established records of the
outcome. It is hoped that improvements in Indonesian
infrastructures will enable analysis of the treatment outcome as
well.
In conclusion, there was a significant difference in
the expression of PD-L1 protein in the non-GCB subtype of DLBCL as
compared with GCB subtypes of DLBCL. The expression of PD-L1
protein in the non-GCB subtype was higher compared with that in the
GCB subtype.
Acknowledgements
The authors would like to thank Dr Fresia Juwitasari
Wongkar, Department of Anatomical Pathology, Faculty of Medicine,
Universitas Indonesia/Dr. Cipto Mangunkusumo National Central
General Hospital for comments on the manuscript.
Funding
Funding: The authors would like to thank Universitas Indonesia
for funding this research through PUTI Grant with contract number
NKB-1852/UN2.RST/HKP.05.00/2020.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RA designed the study, performed the experiments,
drafted the manuscript, collected various clinical data and
performed the statistical analysis. MFH, AA, ASH and ESRH
contributed to the review of the manuscript, interpreted the data,
assisted with the experiments, revised the manuscript critically
and made substantial contributions regarding manuscript concept and
judgment. RA and ASH confirm the authenticity of all the raw data.
All authors read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Faculty of
Medicine, Universitas Indonesia Research Ethics Committees, with
protocol number 0171/UN2.F1/ETIK/2018.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sethi A, Tandon A, Mishra H and Singh I:
Diffuse large B-cell lymphoma: An immunohistochemical approach to
diagnosis. J Oral Maxillofac Pathol. 23:284–288. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Reksodiputro AH: Multicenter epidemiology
and survival study of B cell non Hodgkin lymphoma patients in
Indonesia. J Blood Disorders Transf. 6(257)2015.
|
|
3
|
Rosenwald A, Wright G, Chan WC, Connors
JM, Campo E, Firsher RI, Gascoyne RD, Muller-Hermenlink K, Smeland
EB, Giltnane JM, et al: The use of molecular profiling to predict
survival after chemotherapy for diffuse large-B-cell lymphoma. N
Engl J Med. 346:1937–1947. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18(10)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nishimura H, Agata Y, Kawasaki A, Sato M,
Imamura S, Minato N, Yagita H, Nakano T and Honjo T:
Developmentally regulated expression of the PD-1 protein on the
surface of double-negative (CD4-CD8-) thymocytes. Int Immunol.
8:773–780. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xia Y, Jeffrey Medeiros L and Young KH:
Signaling pathway and dysregulation of PD1 and its ligands in
lymphoid malignancies. Biochim Biophys Acta. 1865:58–71.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ferrata M, Schad A, Zimmer S, Musholt TJ,
Bahr K, Kuenzel J, Becker S, Springer E, Roth W, Weber MM and
Fottner C: PD-L1 expression and immune cell infiltration in
gastroenteropancreatic (GEP) and Non-GEP neuroendocrine neoplasms
with high proliferative activity. Front Oncol.
9(343)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen J, Jiang CC, Jin L and Zhang XD:
Regulation of PD-L1: A novel role of pro-survival signalling in
cancer. Ann Oncol. 27:409–416. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alsaab HO, Sau S, Alzhrani R, Tatiparti K,
Bhise K, Kashaw SK and Iyer AK: PD-1 and PD-L1 checkpoint signaling
inhibition for Cancer immunotherapy: Mechanism, combinations and
clinical outcome. Front Pharmacol. 8(561)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kiyasu J, Miyoshi H, Hirata A, Arakawa F,
Ichikawa A, Niino D, Sugita Y, Yufu Y, Choi I, Abe Y, et al:
Expression of programmed cell death ligand 1 is associated with
poor overall survival in patients with diffuse large B-cell
lymphoma. Blood. 126:2193–2201. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu LY, Xu XL, Rao HL, Chen J, Lai RC,
Huang HQ, Jiang WQ, Lin TY, Xia ZJ and Cai QQ: Expression and
clinical value of programmed cell death-ligand 1 (PD-L1) in diffuse
large B cell lymphoma: A retrospective study. Chin J Cancer.
36(94)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kwon D, Kim S, Kim PJ, Go H, Nam SJ, Paik
JH, Kim YA, Kim TM, Heo DS, Kim CW and Jeon YK: Clinicopathological
analysis of programmed cell death 1 and programmed cell death
ligand 1 expression in the tumor microenvironments of diffuse large
B cell lymphomas. Histopathology. 68:1079–1089. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xie M, Huang X, Ye X and Qian W:
Prognostic and clinicopathological significance of PD-1/PD-L1
expression in the tumor microenvironment and neoplastic cells for
lymphoma. Int Immunopharmacol. 77(105999)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shi Y, Han Y, Yang J, Liu P, He X, Zhang
C, Zhou S, Zhou L, Qin Y, Song Y, et al: Clinical features and
outcomes of diffuse large B-cell lymphoma based on nodal or
extranodal primary sites of origin: Analysis of 1,085 WHO
classified cases in a single institution in China. Chin J Cancer
Res. 31:152–161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fang X, Xiu B, Yang Z, Qiu W, Zhang L,
Zhang S, Wu Y, Zhu X, Chen X, Xie S, et al: The expression and
clinical relevance of PD-1, PD-L1 and TP63 in patients with diffuse
large B-cell lymphoma. Medicine (Baltimore).
96(e6398)2017.PubMed/NCBI View Article : Google Scholar
|