Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common malignant tumor type worldwide and the most
common malignancy originating in the mucosal epithelium of the oral
cavity, pharynx, larynx and hypopharynx (1). Smoking and excessive alcohol
consumption have been associated with the tumorigenesis of HNSCC
(2). Human papillomavirus has been
associated with tumors arising in the oropharynx (3). Various cases are usually diagnosed at
a later stage, which is associated with high mortality and
morbidity rates (2). Although
multimodality approaches, such as EGFR monoclonal antibody in
combination with chemotherapy, are used for more advanced-stage
disease, 30-40% of patients develop distant metastases within 5
years (3); therefore,
understanding the molecular characteristics and immunological
profile of HNSCC may help overcome certain obstacles associated
with targeted therapies and prove beneficial for the patients.
The tumor microenvironment significantly affects
tumor aggressiveness and response to treatment (4). Tumor-infiltrating lymphocytes (TILs)
are an important histopathological characteristic of HNSCC
(5). Tumor infiltration by T
lymphocytes is a highly informative prognostic factor for
predicting the clinical outcome of the disease. In several studies,
TILs of different types and locations in primary tumors are of
prognostic value for overall survival (OS) and disease-free
survival (DFS) (6,7). However, different subsets of
lymphocytes have different functions. CD4+ T-helper
cells stimulate cytotoxic CD8+ T cells to enhance the
antitumor immune response (8),
while CD4+ regulatory T cells (Tregs) are considered to
serve as suppressors of antitumor immune response (9). CD3+ T cells have been
considered as an important T-cell marker for the classification of
malignant lymphomas and leukemias (10). Although TILs have been extensively
investigated, the prognostic significance of specific TIL
subgroups, such as CD3+ or CD4+, is different
due to the complexity of the composition and function of TILs, and
has been inconsistent across different studies. The role of TILs in
immune surveillance in HNSCC remains to be clarified and previous
studies have reported conflicting results.
The immune checkpoint molecules programmed death 1
(PD-1) and programmed death 1 ligand 1 (PD-L1) have been evaluated
in a number of cancer types (11,12).
PD-1 is mainly expressed on the surface of T and B cells during
activation. PD-1 signaling is mediated through binding to its two
ligands, PD-L1 and PD-L2, which are mainly expressed by cancer
cells (13). As an inhibitory
immune checkpoint molecule, PD-1 mediates the immune escape of
tumor cells (14). PD-L1 is also
referred to as cluster of differentiation 274, which is a protein
encoded by the CD274 gene (15).
There is increasing evidence that tumor cells express various
levels of PD-L1 in numerous types of cancer, including HNSCC.
However, the currently available results remain controversial. The
clinical prognostic significance of PD-L1 expression in HNSCC
tissues varies across different studies, which may be due to
different sample sources and intratumor heterogeneity, different
antibodies and staining protocols, and inconsistent cut-off values,
among others (16). In three
recent randomized phase III trials, the PD-1 targeting antibodies
nivolumab and pembrolizumab, which have been used to treat patients
with advanced HNSCC, exhibited superior efficacy and lower toxicity
compared with traditional chemotherapy and radiotherapy alone
(17). Pembrolizumab combined with
chemotherapy was determined to be superior to cisplatin and
5-fluorouracil (18). Furthermore,
pembrolizumab monotherapy was more effective as a first-line
treatment in patients with HNSCC whose tumors express PD-L1
compared with those with non-PD-L1-expressing tumors (17). However, a large proportion of
patients do not respond to treatment, while certain initial
responders eventually develop resistance; thus, further research is
required.
The aim of the present study was to investigate the
frequency of TILs expressing CD3 and CD4 and the expression of
PD-L1 in tumor cells using formalin-fixed paraffin-embedded (FFPE)
samples from patients with HNSCC. Furthermore, the association of
TILs and PD-L1 with clinical characteristics and prognosis was
assessed in order to evaluate the predictive value of TILs and
PD-L1 expression.
Materials and methods
Patient cohort
A total of 63 FFPE tissue specimens were collected
from patients with pathologically confirmed HNSCC at the Second
Hospital of Jilin University (Changchun, China) between May 2008
and July 2019. Patients who had previously received conventional
radiotherapy and/or chemotherapy prior to surgery were excluded.
All tumor specimens were obtained from primary resections. The
study protocol was approved by the Medical Ethics Committee of
Jilin University (Changchun, China; no. 2021-157). Approximately
half of the patients provided written informed consent for the
study. For the other half of the patients, a telephone interview
was conducted and verbal consent was obtained.
The clinical characteristics of the patients are
summarized in Table I. The
majority of the patients were male [45 (71.4%)] and the median age
was 64.6 years (range, 40-76.8 years). Approximately half of the
patients had advanced disease with T stage 3/4 (52.4%), N stage 2/3
(54%) and clinical stage III/IV (55.6%). All studied tumour
specimens were assessed on HE-stained slides using standard
diagnostic criteria.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Value |
|---|
| Age, years [median
(range)] | 64.6 (40-76.8) |
| Sex | |
|
Male | 45 (71.4) |
|
Female | 18 (28.6) |
| T
classification | |
|
T1 | 11 (17.5) |
|
T2 | 19 (30.1) |
|
T3/4 | 33 (52.4) |
| N
classification | |
|
N0 | 13 (20.6) |
|
N1 | 16 (25.4) |
|
N2/3 | 34(54) |
| Clinical stage | |
|
I | 3 (0.05) |
|
II | 25 (39.7) |
|
III/IV | 35 (55.6) |
| PD-L1 expression
score | |
|
High,
>5 | 28 (45.2) |
|
Low, ≤5 | 34 (56.8) |
| CD3 expression
score | |
|
High,
>26.8 | 30(50) |
|
Low,
≤26.8 | 30(50) |
| CD4 expression
score | |
|
High,
>17.3 | 29 (48.3) |
|
Low,
≤17.3 | 31 (51.7) |
Immunohistochemistry (IHC)
CD3+ and CD4+ TILs were
determined by IHC. Archival FFPE HNSCC tumor blocks were cut into
4-µm sections, deparaffinized with xylene and rehydrated through a
decreasing ethanol gradient. Boiling in 10 mM citrate buffer, pH
6.0 (Novocastra; Leica Microsystems, Ltd.) in a microwave was used
for epitope retrieval. After endogenous peroxidase blocking (0.3%
H2O2; Thermo Fischer Scientific, Inc.) at
room temperature for 30 min, the sections were incubated with
primary antibodies against CD3 (1:200 dilution; clone A0452; cat.
no. A045229-2; Dako; Agilent Technologies, Inc.) and CD4 (1:500
dilution; clone 4B12; cat. no. NCL-L-CD4-368; Novocastra; Leica
Microsystems, Ltd.) at 4˚C overnight. The CD4 antibody was used to
stain all CD4+-expressing cells, including Tregs.
Secondary detection was performed using the Level-2 Ultra
Streptavidin (horseradish peroxidase) system (Dako; Agilent
Technologies, Inc.) at room temperature, which included anti-rabbit
for CD3 or anti-mouse for CD4 for 30 min. Streptavidin was applied
to all sections for 30 min at room temperature. Color detection was
performed by using 3,3'-diaminobenzidine for 5 min at room
temperature. The slides were counterstained with hematoxylin and
mounted with coverslips.
PD-L1 was detected by using the mouse monoclonal
anti-human PD-L1 antibody (1:100 dilution; CD274, clone UMAB229;
cat. no. UM800121; OriGene Technologies, Inc.) at 4˚C overnight.
Accel Retrieval Solution (GBI Labs) was used for antigen retrieval.
Human tonsillar samples known to be positive for CD3, CD4 and PD-L1
expression served as positive controls. Sections without primary
antibodies were used as negative controls.
Evaluation of TILs and PD-L1
expression
A semi-quantitative method was utilized to assess
the CD3 and CD4 expression of TILs inside the infiltrated tumor
tissues. As described previously (6,19),
three of the most densely stained fields were randomly selected,
with necrotic areas excluded. The proportion of cells with positive
staining (range, 0-100%) and staining intensity (0, negative; 1,
weak; 2, moderate; and 3, strong) were assessed and recorded. The
final score was calculated by multiplying the percentage of
positive cells with the average staining intensity.
The abundance and location of PD-L1 expression
within the tumor were also determined semi-quantitatively. PD-L1
expression was membranous, with variable cytoplasmic staining. A
four-level score was used to quantify the proportion of total cells
stained and the staining intensity on the membrane: 0 (0-<5%
positive cells), 1 (5-10% positive cells), 2 (10-20% positive
cells), 3 (20-50% positive cells) and 4 (>50% positive cells).
The staining intensity was determined as 0, 1, 2 or 3(20). The final score was calculated by
multiplying the proportion of stained cells (scored as 0, 1-4) by
the staining intensity (scored as 0, 1-3) (21). A Leica light microscope linked with
a camera was used for capturing and analysing images
(magnification, x200). All evaluations were performed in a blinded
manner regarding the clinical outcome of the patients.
RNA extraction and quantification of
PD-L1 expression by reverse transcription-quantitative
(RT-q)PCR
A total of 60 available FFPE surgical samples from
the same cohort of patients with HNSCC and 10 adjacent normal
tissue samples were collected. Based on HE-stained tissue sections,
only samples containing >60% tumor cells were considered. The
expression of PD-L1 at the mRNA level was measured by using
RT-qPCR. A total of 5-10 8-µm sections were obtained from each
sample and total RNA was extracted from the samples using the
Recover All Total Nucleic Acid Isolation kit for FFPE (Thermo
Fisher Scientific, Inc.). In brief, the sections were
deparaffinized with xylene and rehydrated through a decreasing
ethanol gradient. After washing with 100% ethanol, the air-dried
pellets were incubated with 200 µl digestion buffer and 4 µl of
protease K (included in the kit) in heat blocks for 15 min at 50˚C
and then 15 min at 80˚C. After adding 100% ethanol, the mixture was
loaded onto a filter cartridge, centrifuged, the flow-through was
discarded and the filter cartridge was washed twice. Subsequently,
60 µl DNase was added, followed by incubation at room temperature
for 30 min. After two additional washes, the RNA was eluted with
nuclease-free water. According to the manufacturer's protocol, RT
of RNA was performed with Super Script III reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.). Primer 3 (v.0.4.0;
https://bioinfo.ut.ee/primer3-0.4.0/)
was used to design the primers. The primers for PD-L1 were as
follows: Forward, 5'-GTGGCATCCAAGATACAAACTCAA-3' and reverse,
5'-TCCTTCCTCTTGTCACGCTCA-3'. The primers for GAPDH were as follows:
Forward, 5'-GTCTCCTCTGACTTCAACAGCG-3' and reverse,
5'-ACCACCCTGTTGCTGTAGCCAA-3'. Amplification was performed using
SYBR Green Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and the ABI PRISM 7900 Sequence Detection System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The 10-µl
reaction volume contained 50 ng cDNA (either from FFPE tumor
samples or normal adjacent tissue samples), 200 nmol/l of each
primer and 5 µl of SYBR Green Master Mix (Thermo Fisher Scientific,
Inc.). The thermocycling conditions included initial denaturation
at 94˚C for 2 min; 40 cycles of denaturation at 94˚C for 15 sec;
and annealing and extension at 60˚C for 1 min. Relative
quantification of PD-L1 mRNA levels was performed by using the
2-∆∆Cq method (22). As
calculated from the duplicate reactions for each tested sample, the
mean Cq value was used. The relative fold change of mRNA
expression, in which the mean of the ∆Cq values of the target
amplicon was normalized to the endogenous gene GAPDH, compared with
normal tissue specimens. Each experiment was performed three
times.
Statistical analysis
Fisher's exact test was used to assess differences
between categorical variables. The associations between the
expression of each detected biomarker and various
clinicopathological parameters were assessed. The Kaplan-Meier
method was used to calculate OS and DFS and the log-rank test was
used for comparisons. The Cox proportional hazard model was used to
perform univariate and multivariate analyses. Factors with
prognostic significance in the univariate model were further
analyzed in a multivariate Cox proportional hazards regression
model. P<0.05 was considered to indicate statistically
significant differences. Pearson's correlation coefficient analysis
was used to determine the correlation between PD-L1 expression and
CD3+ or CD4+ TILs. Analyses were performed
using the R 4.1.2 package (R Foundation for Statistical
Computing).
Results
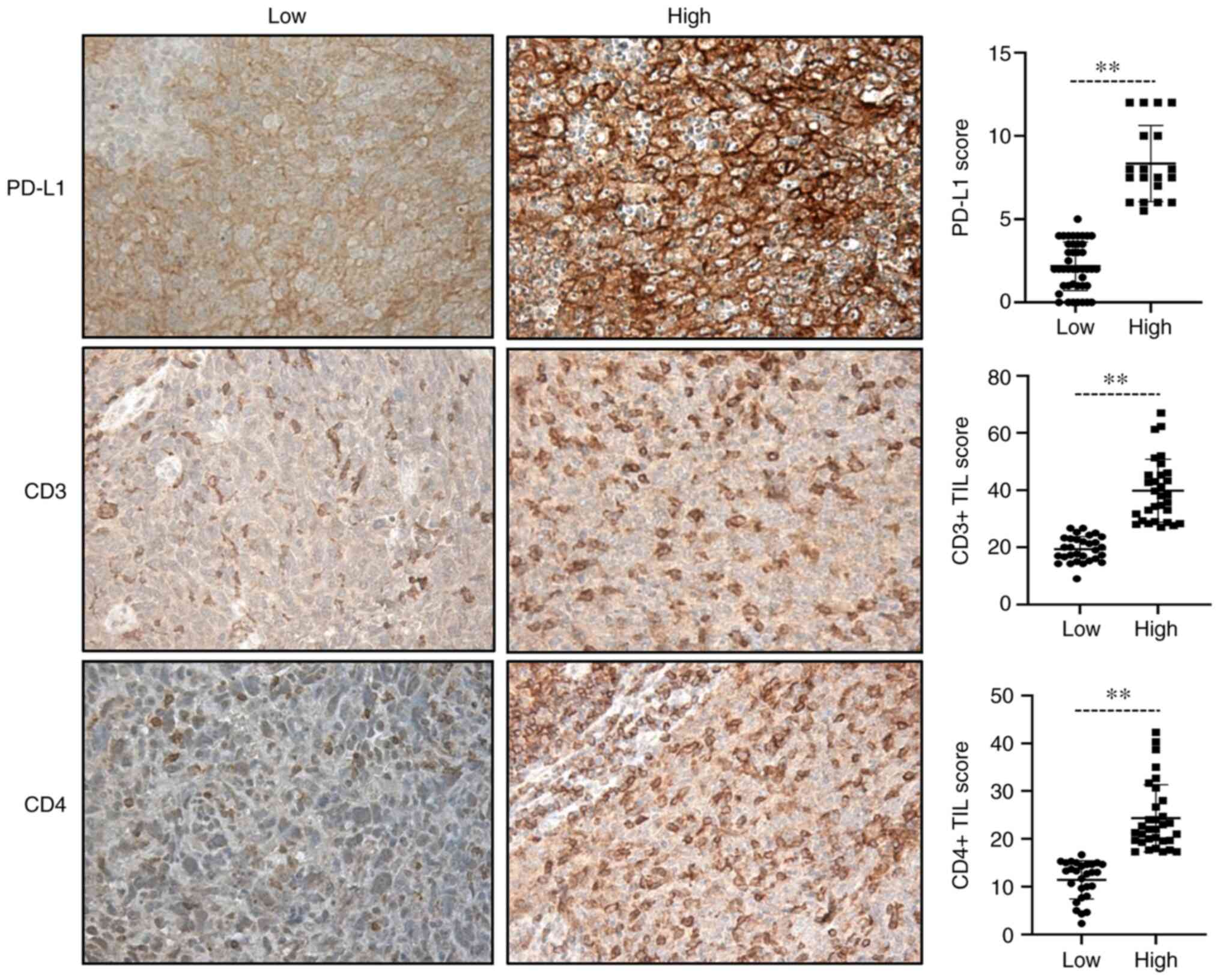
TILs in HNSCC
The expression of CD3 and CD4 on TILs was evaluated
in the tumor parenchyma of patients with HNSCC. Of the 63 tumor
samples, 61 were available for CD3 and CD4 assessment. A total of
two samples were excluded due to the small sample size or poor
tissue morphology after staining. CD3 and CD4 were expressed in
100% of the samples. With a range of 9.0-61.3 for CD3 and 2.3-42.3
for CD4, the median score of CD3 and CD4 expression was 26.8 and
17.3, respectively. As a dichotomous variable, patients were
divided into CD3 expression high and low groups. According to the
median, high was defined as a score >26.8 and low was defined as
a score ≤26.8. Using a similar method, patients were assigned to
CD4 high (>17.3) and low (≤17.3) groups (Table I). IHC analysis revealed that the
expression of CD3 was abundant in tumors, while the expression of
CD4 was relatively low.
Evaluation of PD-L1 expression by
IHC
Of the 61 available tumors, PD-L1 was expressed in
53 (86.0%, score ≥1). According to the median score, PD-L1
expression was divided into low and high groups, with 28 samples
(45.2%) exhibiting high PD-L1 expression (score >5) and 34
samples exhibiting low expression (score ≤5; Table I). In addition, PD-L1 expression
was observed in 53 samples in which TILs were present.
Representative results on CD3, CD4 and PD-L1 immunostaining and
quantification in tumor cells are provided in Fig. 1.
Evaluation of PD-L1 expression by
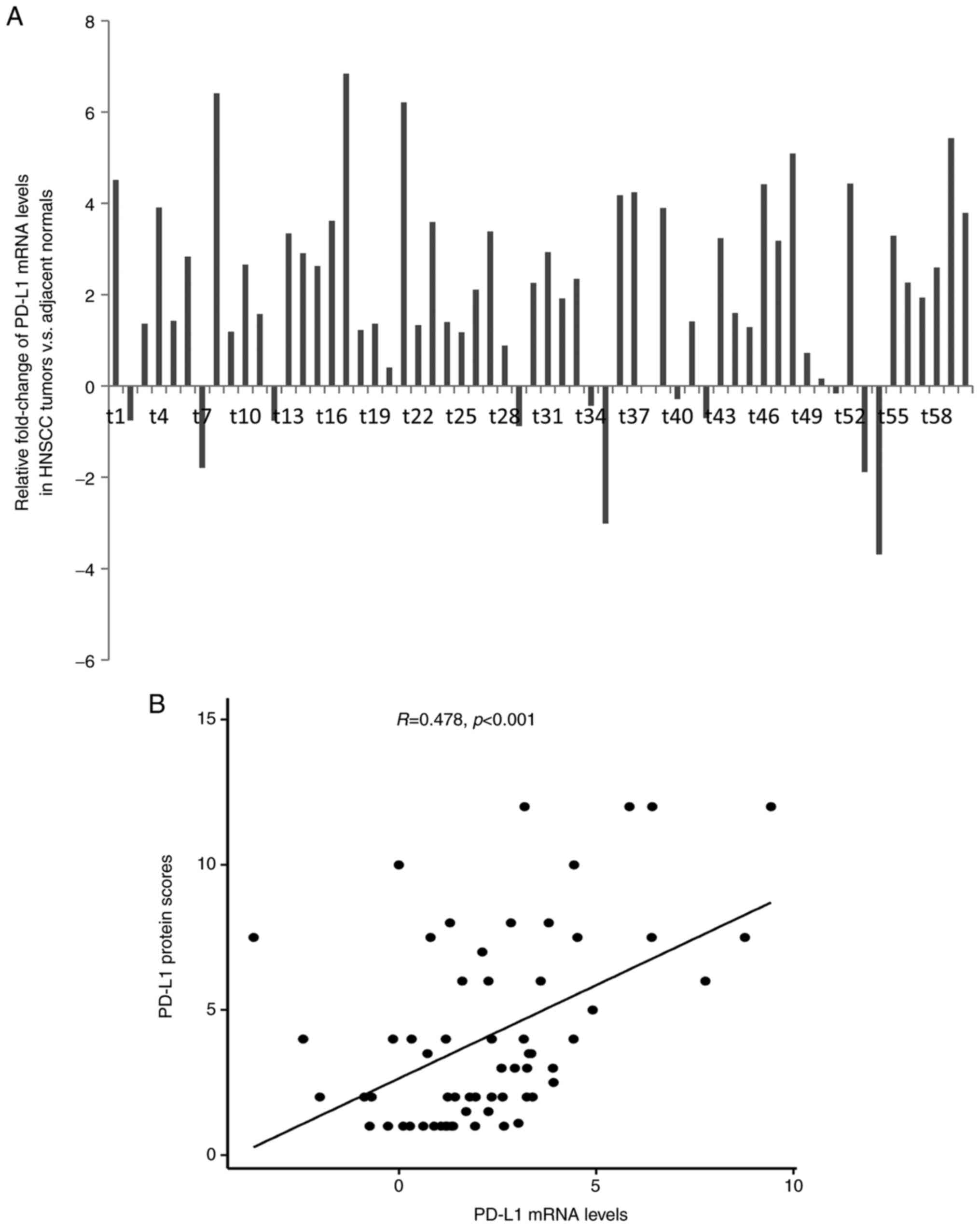
RT-qPCR
The mRNA expression of PD-L1 was evaluated by
RT-qPCR in 60 available patient samples. By comparing with 10
normal tissue samples, the mean expression level of PD-L1 in HNSCC
was 2.07-fold higher compared with that in normal tissues (range,
-3.69- to 6.84-fold). PD-L1 mRNA expression was detected in 49 of
60 patients (81.7%; Fig. 2A),
highlighting the overexpression of PD-L1 gene in tumor cells.
Furthermore, PD-L1 mRNA levels were indicated to be significantly
correlated with PD-L1 protein levels (R=0.461; P<0.001; Fig. 2B). However, there was no
significant association between PD-L1 mRNA levels and patient
clinicopathological characteristics.
Association of TILs and PD-L1
expression with clinicopathological characteristics
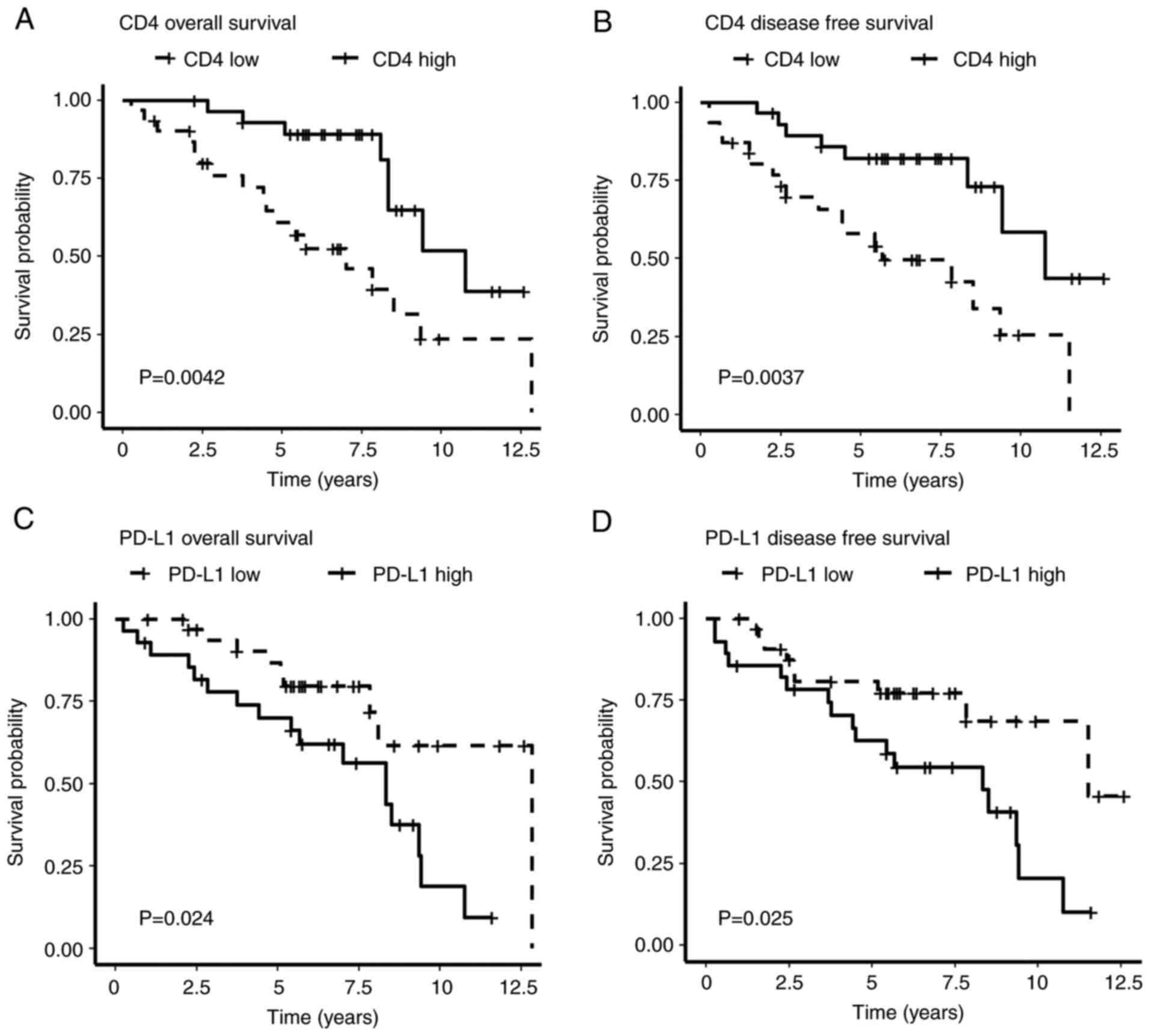
The clinical value of TILs and PD-L1 protein
expression was further evaluated. With a mean follow-up of 72
months (range, 3-151 months), constructed Kaplan-Meier curves
revealed that patients with high CD4 expression were associated
with improved OS (P=0.004) and DFS (P=0.004), which was confirmed
by univariate Cox regression analysis [HR=0.31, 95% confidence
interval (CI): 1.13-0.72, P=0.004 for OS; and HR=0.30, 95% CI:
0.13-0.71, P=0.006 for DFS; Fig.
3A and B; Table II].
 | Table IIUnivariate and multivariate analyses
of factors associated with survival in patients with head and neck
squamous cell carcinoma. |
Table II
Univariate and multivariate analyses
of factors associated with survival in patients with head and neck
squamous cell carcinoma.
| | Overall
survival | Disease-free
survival |
|---|
| | Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (> vs. ≤64.6
years) | 2.62
(1.09-6.27) | 0.03 | 1.45
(0.56-3.71) | 0.003 | 2.29
(0.98-5.27) | 0.05 | 1.32
(0.53-3.27) | 0.004 |
| Sex (female vs.
male) | 0.67
(0.28-1.56) | 0.357 | | | 0.83
(0.36-1.91) | 0.667 | | |
| T classification
(T1/2 vs. T3/4) | 0.76
(0.20-2.93) | 0.698 | | | 0.66
(0.17-2.53) | 0.548 | | |
| N classification
(N0/1 vs. N2/3) | 1.94
(0.86-4.37) | 0.11 | | | 1.71
(0.76-3.87) | 0.193 | | |
| Clinical stage
(I/II vs. III/IV) | 1.65
(0.73-3.71) | 0.229 | | | 1.65
(0.74-3.69) | 0.223 | | |
| CD3 expression
(score > vs. ≤26.8) | 0.59
(0.27-1.29) | 0.183 | | | 0.56
(0.27-1.26) | 0.175 | | |
| CD4 expression
(score > vs. ≤17.3) | 0.31
(1.13-0.72) | 0.004 | 0.35
(0.15-0.86) | 0.006 | 0.30
(0.13-0.71) | 0.006 | 0.33
(0.14-0.80) | 0.009 |
| PD-L1 expression
(score > vs. ≤5) | 2.47
(1.12-5.46) | 0.024 | 2.29
(0.89-5.86) | 0.025 | 2.57
(1.17-5.66) | 0.015 | 2.24
(0.90-5.57) | 0.051 |
As a categorical variable, PD-L1 was also used to
divide the patients into low and high expression groups.
Kaplan-Meier survival analysis demonstrated that high PD-L1
expression was significantly associated with unfavorable OS and DFS
(P=0.024 and P=0.025, respectively; Fig. 3C and D), compared with low PD-L1 expression.
Univariate Cox regression analysis revealed significant differences
between the high and low expression groups, [HR=2.47, 95%
confidence interval (CI): 1.12-5.46, P=0.024 for OS; and HR=2.57,
95% CI: 1.17-5.66, P=0.015 for DFS (Table II).
Furthermore, multivariate analysis using the Cox
proportional hazards model indicated that CD4 and PD-L1 were
significantly associated with OS and DFS after adjusting for age
(for OS, CD4: HR=0.35, 95% CI: 0.15-0.86 and P=0.006; and PD-L1:
HR=2.29, 95% CI: 0.89-5.86, P=0.025; for DFS, CD4: HR=0.33, 95% CI:
0.14-0.80 and P=0.009; and PD-L1: HR=2.24, 95% CI: 0.90-5.57 and
P=0.051; Table II). However, CD3
expression was not indicated to be significantly associated with
disease outcome. Furthermore, no significant associations of
patient sex and tumor stage with CD4 or PD-L1 expression were
observed.
To elucidate the correlation between PD-L1
expression and CD3+ or CD4+ TILs, a Pearson's
correlation coefficient analysis was performed. The scatter plot
demonstrated that the expression of CD4+ TILs had a
negative, if insignificant, correlation with the PD-L1 score
(R=-0.22, P=0.08). No significant correlation between
CD3+ TILs and PD-L1 expression was obtained (Fig. S1). Overall, these results
indicated that CD4+ TILs and PD-L1 have a role as
independent prognostic factors for patients with HNSCC.
Discussion
The infiltration of the tumor parenchyma by a large
number of TILs has been associated with clinical outcomes in
various types of cancer. Tumor immune checkpoint-targeted therapies
have achieved responses in multiple cancer types (23), including HNSCC (16). However, the clinical response rates
vary widely and the reasons for this have yet to be fully
elucidated. In the present study, the distribution of TILs and the
expression of PD-L1 were quantitatively analyzed in a population of
patients with HNSCC. Both PD-L1 protein and mRNA levels were
analyzed. The clinical significance of these immune parameters and
the clinical characteristics of the patients were assessed. It was
demonstrated that CD3+ and CD4+ TILs were
present in 100% of the samples. Patients whose tumors were
infiltrated by high numbers of CD4+ T cells exhibited
significantly improved OS and DFS compared with patients exhibiting
poor tumor infiltration. In addition, when a score of >1 of
tumor cells was considered positive, PD-L1 expression was detected
in 86% of the samples and when a score of >5 of cells was
considered positive, PD-L1 expression was observed in 45% of the
samples. Of note, PD-L1 mRNA levels were indicated to be
significantly associated with PD-L1 protein levels (R=0.461;
P<0.001). It is worth noting that high PD-L1 expression was
significantly associated with unfavorable OS and DFS. These results
were independent of clinicopathological characteristics and were of
predictive value for disease outcome.
There is growing interest in evaluating TILs in
solid tumors. It has been demonstrated that the immune attack or
immune escape of tumor cells induces the immune response in the
tumor microenvironment and serves an important role in the response
to cancer therapy (1). However, as
different subsets of lymphocytes have different functions in the
tumor microenvironment, there is currently no standardized method
for evaluating TILs. Previous studies analyzed the peritumoral
stroma and tumor core area separately, and evaluated the numbers
and percentage of TILs in solid tumors with different results. In
many cancers, CD3+ or CD8+ TILs is positively
correlated with favorable clinical outcomes (6,24,25),
whereas others suggest that CD4+ or CD8+ TILs
is associated with better prognosis (26) or has no directly correlation with
patient survival, independent of HPV status (27). Therefore, the focus of the present
study was to assess the numbers of CD3+ and
CD4+ TILs in the tumor parenchyma and determine their
association with the clinical variables of the patients.
It has been indicated that CD3+ T cells
are the major subtype of TILs in gastric cancer (28). In laryngeal squamous cell
carcinoma, a high density of CD3+ cells in the tumor
core area was associated with a lower risk of metastasis (29). CD3+ and CD8+
T cells were observed to be associated with improved disease
outcome in patients with head and neck cancer treated with
chemoradiotherapy (30). The
results of the present study consistently demonstrated that
CD3+ cells were the predominant TIL subtype in the
present HNSCC cohort, although the results did not exhibit any
clinical significance, possibly due to the small sample size.
The complexity of the CD4+ T cell
response has led to ambiguous results. CD4+ T cells are
able to promote antitumor immunity through Th1 and Th2 cells
(31). Other subtypes, such as
Th17 cells, are mainly characterized by the production of high
cytokine IL-17A levels, which has been linked to antitumor immunity
in mouse models (32). However,
CD4+ forkhead box (Fox)p3+ Tregs contribute
to the inhibition of autoimmunity and prevent excessive immune
response to pathogens (33).
CD4+ TIL infiltration has been evaluated in several
studies on HNSCCs. RT-qPCR analysis indicated that CD4 mRNA
expression levels were significantly correlated with the
CD4+ cell infiltration score in cancer epithelium and
cancer stroma (8). Higher
CD4+ TIL numbers in patients with HNSCC are associated
with improved OS and relapse-free survival (26,34).
This phenomenon was also observed in the present study. The present
results indicated that patients whose tumors were infiltrated by
high numbers of CD4+ T cells had superior OS and DFS
compared with patients exhibiting lower tumor infiltration. By
contrast, another study demonstrated that neither CD4 nor Foxp3
expression intratumoral or in the stroma area showed significance
for the clinical outcome (6).
Immunotherapy by using PD-1 and PD-L1 immune
checkpoint blockade has been used in different cancer clinical
trials with certain success. With regard to HNSCC, Schneider et
al (35) reported that PD-L1
expression was present in 36% of primary carcinomas and the
presence of lymph node metastasis further indicated that PD-L1
expression may be associated with reduced OS and DFS. However, in a
meta-analysis of 3,105 patients from 23 studies, no significant
difference between patients with PD-L1-positive and -negative HNSCC
was obtained in terms of OS and DFS (36), while a recently reported randomized
phase III clinical study (KEYNOTE-048) (18) demonstrated that pembrolizumab
(anti-PD-L1) plus platinum treatment is suitable for PD-L1-positive
recurrent or metastatic HNSCC. The results of the present study
supported this evidence, as it was demonstrated that >80% of
tumors have a PD-L1 score ≥1 and indicated that increased PD-L1
expression may be associated with unfavorable OS and DFS in
HNSCC.
PD-1/PD-L1 immune checkpoint therapy has
revolutionized the traditional cytotoxic anticancer treatments in
recent years and has achieved a long-lasting therapeutic response
in various types of cancer (37).
However, these therapies still pose significant clinical challenges
for patients with HNSCC. In order to further understand the
mechanism of the PD-1/PD-L1 pathway, the associations between
PD-L1, CD3 and CD4 were investigated, but there were limitations in
detecting PD-1 expression due to the availability of samples;
hence, a new cohort of patients may be required for further
study.
In conclusion, the data of the present study
demonstrated that the numbers of CD4+ TILs as detected
by IHC were associated with the clinical outcome of patients with
HNSCC. PD-L1 is commonly expressed in HNSCC at the protein and mRNA
levels. In particular, high expression of PD-L1 on IHC examination
may reflect the deterioration of OS and DFS and indicate the
opportunity for immune checkpoint blocking therapy in patients with
HNSCC.
Supplementary Material
Correlation between PD-L1 expression
and CD3+ or CD4+ tumor-infiltrating
lymphocytes. (A) CD3+ vs. PD-L1; (B) CD4+ vs.
PD-L1. PD-L1, programmed death ligand 1.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Health Special
Project of Jilin Province (grant no. 2020SCZT002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GFG designed the study. ZMF and GFG are responsible
for confirming the integrity and authenticity of the data and the
accuracy of the data analysis. ZMF, DJZ, YYG, KWS and NY collected
and analyzed the patient data. SH, FG and YNW evaluated and
interpreted the clinicopathological data. DJZ, YYG and JB were
responsible for immunohistochemically staining and evaluated the
results. FG and YNW performed the statistical analysis and
interpreted the results. ZMF and DJZ wrote the manuscript. ZMF and
GFG revised the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The authors are accountable for all aspects of the
work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved. The study protocol was approved by the Medical Ethics
Committee of Jilin University (no. 2021-157) and all patients
consented to participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6(92)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stein AP, Saha S, Kraninger JL, Swick AD,
Yu M, Lambert PF and Kimple RJ: Prevalence of human papillomavirus
in oropharyngeal cancer: A systematic review. Cancer J. 21:138–146.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu
Y, Gong Z, Zhang S, Zhou J, Cao K, et al: Role of tumor
microenvironment in tumorigenesis. J Cancer. 8:761–773.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
de Ruiter EJ, Ooft ML, Devriese LA and
Willems SM: The prognostic role of tumor infiltrating T-lymphocytes
in squamous cell carcinoma of the head and neck: A systematic
review and meta-analysis. Oncoimmunology.
6(e1356148)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Balermpas P, Michel Y, Wagenblast J, Seitz
O, Weiss C, Rödel F, Rödel C and Fokas E: Tumour-infiltrating
lymphocytes predict response to definitive chemoradiotherapy in
head and neck cancer. Br J Cancer. 110:501–509. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hendry S, Salgado R, Gevaert T, Russell
PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV,
Gonzalez-Ericsson PI, et al: Assessing tumor-infiltrating
lymphocytes in solid tumors: A practical review for pathologists
and proposal for a standardized method from the International
Immunooncology Biomarkers Working Group: Part 1: Assessing the host
immune response, TILs in invasive breast carcinoma and ductal
carcinoma in situ, metastatic tumor deposits and areas for further
research. Adv Anat Pathol. 24:235–251. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nishimura T, Iwakabe K, Sekimoto M, Ohmi
Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M and Ohta A:
Distinct role of antigen-specific T helper type 1 (Th1) and Th2
cells in tumor eradication in vivo. J Exp Med. 190:617–627.
1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Neisig A, Vangsted A, Zeuthen J and
Geisler C: Assembly of the T-cell antigen receptor. Participation
of the CD3 omega chain. J Immunol. 151:870–879. 1993.PubMed/NCBI
|
|
11
|
Zhang JY, Yan YY, Li JJ, Adhikari R and Fu
LW: PD-1/PD-L1 based combinational cancer therapy: Icing on the
cake. Front Pharmacol. 11(722)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rahimi Kalateh Shah Mohammad G,
Ghahremanloo A, Soltani A, Fathi E and Hashemy SI: Cytokines as
potential combination agents with PD-1/PD-L1 blockade for cancer
treatment. J Cell Physiol. 235:5449–5460. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang X, Yang X, Zhang C, Wang Y, Cheng T,
Duan L, Tong Z, Tan S, Zhang H, Saw PE, et al: Tumor cell-intrinsic
PD-1 receptor is a tumor suppressor and mediates resistance to PD-1
blockade therapy. Proc Natl Acad Sci USA. 117:6640–6650.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sharpe AH and Pauken KE: The diverse
functions of the PD1 inhibitory pathway. Nat Rev Immunol.
18:153–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Qiao XW, Jiang J, Pang X, Huang MC, Tang
YJ, Liang XH and Tang YL: The evolving landscape of PD-1/PD-L1
pathway in head and neck cancer. Front Immunol.
11(1721)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cramer JD, Burtness B and Ferris RL:
Immunotherapy for head and neck cancer: Recent advances and future
directions. Oral Oncol. 99(104460)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Burtness B, Zhang Y, Harrington KJ and
Rischin D: Further clinical interpretation and implications of
KEYNOTE-048 findings-Authors' reply. Lancet. 396:379–380.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Larbcharoensub N, Mahaprom K, Jiarpinitnun
C, Trachu N, Tubthong N, Pattaranutaporn P, Sirachainan E and
Ngamphaiboon N: Characterization of PD-L1 and PD-1 expression and
CD8+ tumor-infiltrating lymphocyte in epstein-barr
virus-associated nasopharyngeal carcinoma. Am J Clin Oncol.
41:1204–1210. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Badoual C, Hans S, Merillon N, Van Ryswick
C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A,
Besnier N, et al: PD-1-expressing tumor-infiltrating T cells are a
favorable prognostic biomarker in HPV-associated head and neck
cancer. Cancer Res. 73:128–138. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cho YA, Yoon HJ, Lee JI, Hong SP and Hong
SD: Relationship between the expressions of PD-L1 and
tumor-infiltrating lymphocytes in oral squamous cell carcinoma.
Oral Oncol. 47:1148–1153. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Upadhaya S, Neftelino ST, Hodge JP, Oliva
C, Campbell JR and Yu JX: Combinations take centre stage in
PD1/PDL1 inhibitor clinical trials. Nat Rev Drug Discov.
20:168–169. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Maibach F, Sadozai H, Seyed Jafari SM,
Hunger RE and Schenk M: Tumor-infiltrating lymphocytes and their
prognostic value in cutaneous melanoma. Front Immunol.
11(2105)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Canning M, Guo G, Yu M, Myint C, Groves
MW, Byrd JK and Cui Y: Heterogeneity of the head and neck squamous
cell carcinoma immune landscape and its impact on immunotherapy.
Front Cell Dev Biol. 7(52)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Spector ME, Bellile E, Amlani L, Zarins K,
Smith J, Brenner JC, Rozek L, Nguyen A, Thomas D, McHugh JB, et al:
Prognostic value of tumor-infiltrating lymphocytes in head and neck
squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg.
145:1012–1019. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
van Kempen PM, Noorlag R, Swartz JE,
Bovenschen N, Braunius WW, Vermeulen JF, Van Cann EM, Grolman W and
Willems SM: Oropharyngeal squamous cell carcinomas differentially
express granzyme inhibitors. Cancer Immunol Immunother. 65:575–585.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang D, He W, Wu C, Tan Y, He Y, Xu B,
Chen L, Li Q and Jiang J: Scoring system for tumor-infiltrating
lymphocytes and its prognostic value for gastric cancer. Front
Immunol. 10(71)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Höing B, Kanaan O, Altenhoff P, Petri R,
Thangavelu K, Schlüter A, Lang S, Bankfalvi A and Brandau S:
Stromal versus tumoral inflammation differentially contribute to
metastasis and poor survival in laryngeal squamous cell carcinoma.
Oncotarget. 9:8415–8426. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Barnes TA and Amir E: HYPE or HOPE: The
prognostic value of infiltrating immune cells in cancer. Br J
Cancer. 117:451–460. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schüler T, Qin Z, Ibe S, Noben-Trauth N
and Blankenstein T: T helper cell type 1-associated and cytotoxic T
lymphocyte-mediated tumor immunity is impaired in interleukin
4-deficient mice. J Exp Med. 189:803–810. 1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Martin-Orozco N, Muranski P, Chung Y, Yang
XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW and Dong C: T
helper 17 cells promote cytotoxic T cell activation in tumor
immunity. Immunity. 31:787–798. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Quezada SA, Peggs KS, Curran MA and
Allison JP: CTLA4 blockade and GM-CSF combination immunotherapy
alters the intratumor balance of effector and regulatory T cells. J
Clin Invest. 116:1935–1945. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nguyen N, Bellile E, Thomas D, McHugh J,
Rozek L, Virani S, Peterson L, Carey TE, Walline H, Moyer J, et al:
Tumor infiltrating lymphocytes and survival in patients with head
and neck squamous cell carcinoma. Head Neck. 38:1074–1084.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schneider S, Kadletz L, Wiebringhaus R,
Kenner L, Selzer E, Füreder T, Rajky O, Berghoff AS, Preusser M and
Heiduschka G: PD-1 and PD-L1 expression in HNSCC primary cancer and
related lymph node metastasis-impact on clinical outcome.
Histopathology. 73:573–584. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang WF, Wong MCM, Thomson PJ, Li KY and
Su YX: The prognostic role of PD-L1 expression for survival in head
and neck squamous cell carcinoma: A systematic review and
meta-analysis. Oral Oncol. 86:81–90. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Marin-Acevedo JA, Kimbrough EO and Lou Y:
Next generation of immune checkpoint inhibitors and beyond. J
Hematol Oncol. 14(45)2021.PubMed/NCBI View Article : Google Scholar
|