Introduction
Colorectal cancer is the third most common human
malignant tumor and is one of the major causes of cancer mortality
in the Western world. Metastatic tumors account for 40 to 50% of
malignancies in newly diagnosed patients (1). The prognosis of metastatic colorectal
cancer (mCRC) remains poor. Among the treatment options for
colorectal liver metastases (CRLM), liver resection is the most
conducive to a cure, with 5-year overall survival rates of 29-48%.
Even for initially unresectable CRLM, effective chemotherapy, along
with targeted therapy, sometimes enables their resection (2,3).
Promising treatments for CRLM include chemotherapy and molecular
agents that target epidermal growth factor receptor (EGFR) and
vascular endothelial growth factor (VEGF). These reports suggest
that the combination of targeted agents and chemotherapy can
increase rates of liver resection and response, thus improving
progression-free and overall survival of patients with CRLM.
However, no studies have compared histopathological type by
treatment with anti-VEGF agents and anti-EGFR agents for wild-type
RAS liver-limited CRLM (4-6).
The present study aimed to compare histopathological
types and heterogeneity of colorectal cancer liver metastasis under
different treatment in patients with liver-limited CRLM treated
with mFOLFOX6 plus anti-VEGF agents vs. mFOLFOX6 plus
anti-EGFR agents
Materials and methods
Patient
This study included 45 patients with CRLM confirmed
to be resectable following neoadjuvant chemotherapy (NACT).
Patients were treated with surgery alone or with surgery following
FOLFOX alone or FOLFOX plus anti-EGFR (cetuximab or panitumumab)
FOLFOX plus anti-VEGF (bevacizumab) as first-line treatment at the
Surgical Oncology Department of Gifu University School of Medicine
(Gifu City/Japan) from January 2006 to August 2015.
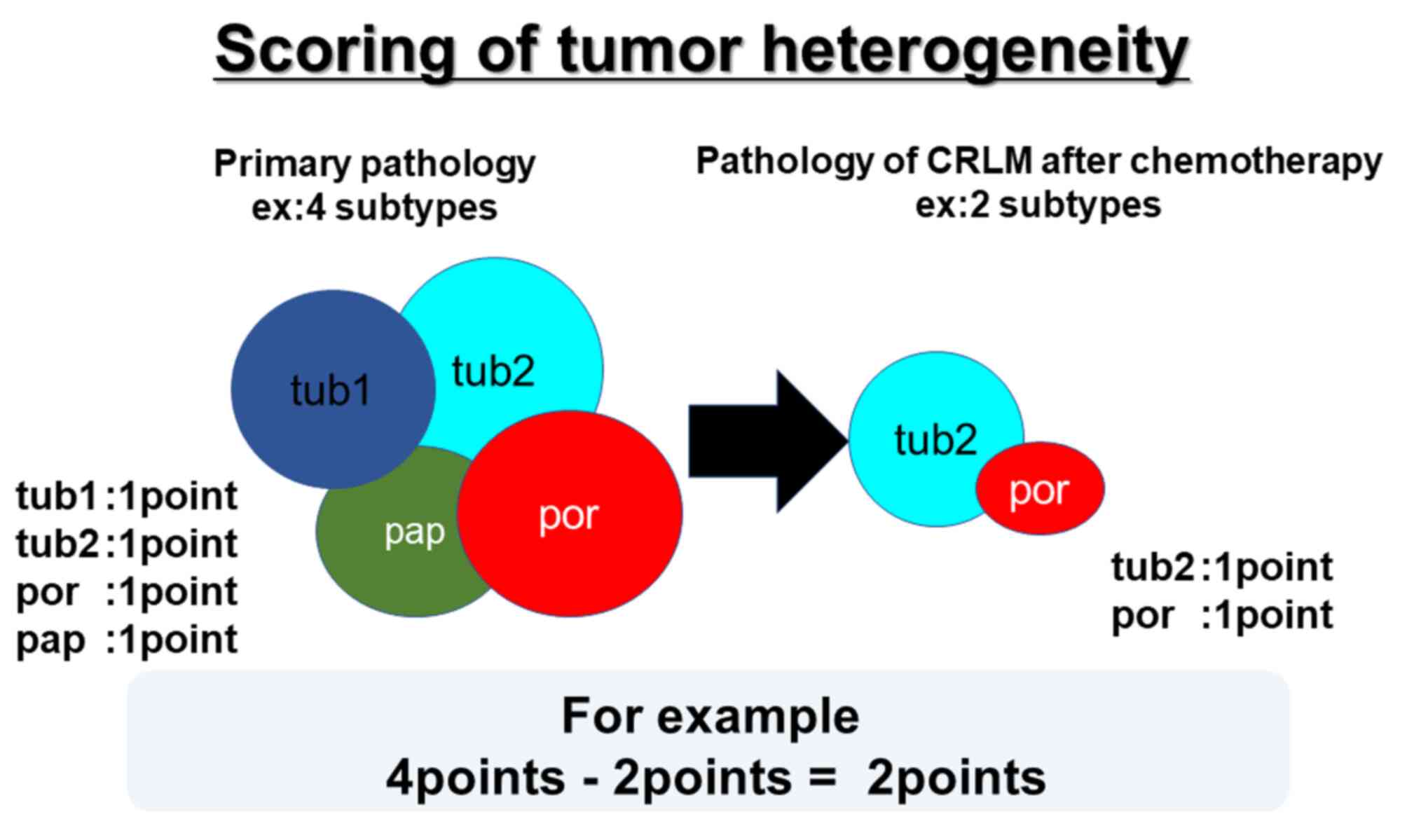
Because an important consequence of intratumoral
heterogeneity is potential differences in histopathology between
primary tumors and their liver metastases, the histopathological
profile of the primary colorectal tumors prior to and after
chemotherapy and that of the CRLMs resected post-chemotherapy were
assessed to investigate the changes between them (Fig. 1A and B).
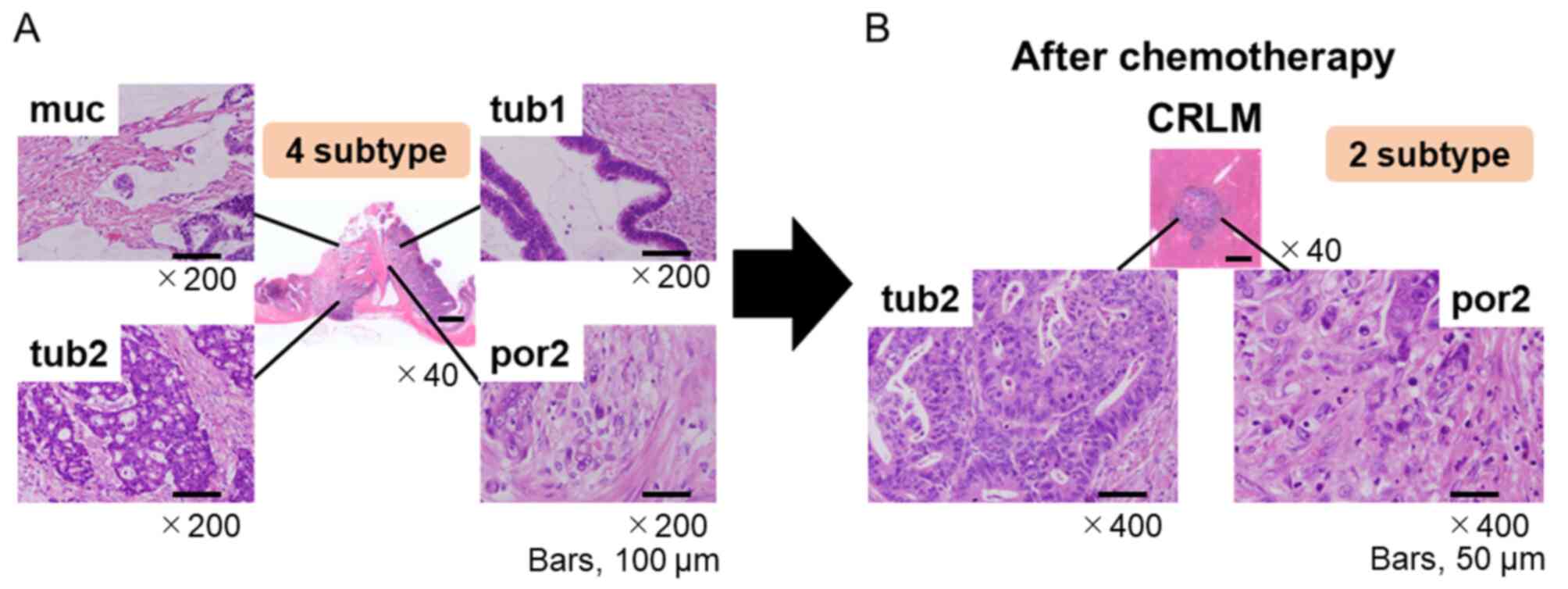 | Figure 1Tumor heterogeneity compared with
primary site and metastatic site. (A) Tumor heterogeneity exhibited
by a primary colorectal tumor [hematoxylin and eosin stain;
magnification, x40 (4 subtype) and x200 (muc, tub1, tub2, por2)].
(B) Tumor heterogeneity of CRLM after chemotherapy. [hematoxylin
and eosin stain; magnification, x40 (CRLM) or x400 (tub2, por2)].
CRLM, colorectal liver metastasis; muc, mucinous adenocarcinoma;
tub, tubular adenocarcinoma; por, poorly differentiated
adenocarcinoma. |
Treatment
In accordance with the Response Evaluation Criteria
in Solid Tumors (RECIST) version 1.1(6), the same methods were used to perform
tumor assessment at baseline and subsequently every 8-12 weeks
using torso contrast-enhanced computed tomography and liver
contrast-enhanced magnetic resonance imaging. The tumor
histopathological response rate was defined as the proportion of
patients with grade Ib or more necrosis in accordance with the
following definition: Grade 0: No necrosis in the tumor; grade 1a:
necrosis in #x003C;33.3% of the tumor; grade 1b: necrosis in
33.3-66.6% of the tumor; grade 2: necrosis in 66.6-#x003C;100% of
the tumor; and grade 3: necrosis in 100% of the tumor. Patients
underwent liver resections if their CRLMs were considered
resectable, based on tumor assessments performed after receiving at
least six cycles of treatment. Liver resection was performed at
least 42 days after the last dose of bevacizumab.
We obtained written informed consent from all
patients enrolled in this study. The study protocol conformed to
the ethical guidelines of the 1975 Declaration of Helsinki. All
study procedures involving humans were conducted in accordance with
the ethical standards required by our institution, and the national
research committee, and were approved by the Institutional Review
Board of the Gifu University Graduate School of Medicine (approval
no. 28-508; March 23, 2017).
Pathological assessment of primary
tumor and CRLMs
Informed consent for histopathological examination
was obtained from all enrolled patients. The postoperative
pathological liver resection specimens were fixed in formalin,
embedded in paraffin, sectioned into 5-mm-thick slices, and stained
with hematoxylin and eosin. In this study, we scored tumor
heterogeneity by each histopathology forms.
The slice revisions of primary tumor and matched
CRLMs were performed by experienced pathologists (Fig. 2).
Statistical analysis
For continuous variables, the data are summarized as
the median with range. For comparisons of variables between groups,
Kruskal-Wallis test and χ2 test were used in independent
cases. Kruskal-Wallis test was used in for continuous variables.
Dunn's post hoc test was performed for comparisons between groups.
Additionally, the χ2 test was used for categorical
variables. A P-value of #x003C;0.05 was considered to indicate
statistical significance. Statistical analysis was performed using
JMP12 software (SAS Institute Inc.).
Results
Patient characteristics
The study comprised 45 patients with mCRC (24 men,
21 women; mean age, 61.9±9.4 years). Locations of primary tumor in
the 45 patients were the right-sided colon in 13 (28.9%) patients
and the left-sided colon in 32 (71.9%) patients. The group treated
with surgery alone included nine patients, whilst those treated
with surgery after FOLFOX alone or plus anti-VEGF or anti-EGFR
included 12 patients each. There were no significant differences in
the 4 categories (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Surgery only
(n=9) | FOLFOX (n=12) | Anti-EGFR + FOLFOX
(n=12) | Anti-VEGF + FOLFOX
(n=12) | P-value |
|---|
| Sex, n | | | | | |
|
Male | 3 | 10 | 5 | 6 | 0.08 |
|
Female | 6 | 2 | 7 | 6 | |
| Age, years | | | | | |
|
Median
(range) | 65.7 (50-81) | 64.8 (49-83) | 59.5 (49-68) | 58.7 (40-68) | 0.45 |
| Location, n | | | | | |
|
Right
side | 2 | 3 | 3 | 5 | 0.78 |
|
Left
side | 7 | 9 | 9 | 7 | |
| RECIST, n | | | | | |
|
SD | - | 3 | 2 | 4 | 0.27 |
|
PR | - | 9 | 10 | 8 | |
|
CR | - | 0 | 0 | 0 | |
| Gradea, n | | | | | |
|
1a/1b | | 3 | 2 | 2 | 0.37 |
|
2 | | 9 | 8 | 8 | |
|
3 | | 0 | 2 | 2 | |
| Histopathology,
n | | | | | |
|
Primary | | | | | |
|
Heterogenous | 6 | 7 | 11 | 12 | 0.04 |
|
Homogenous | 3 | 5 | 1 | 0 | |
|
Liver | | | | | |
|
Heterogenous | 6 | 4 | 6 | 5 | 0.17 |
|
Homogenous | 3 | 8 | 6 | 7 | |
Histopathologic heterogeneity at the
liver metastasis
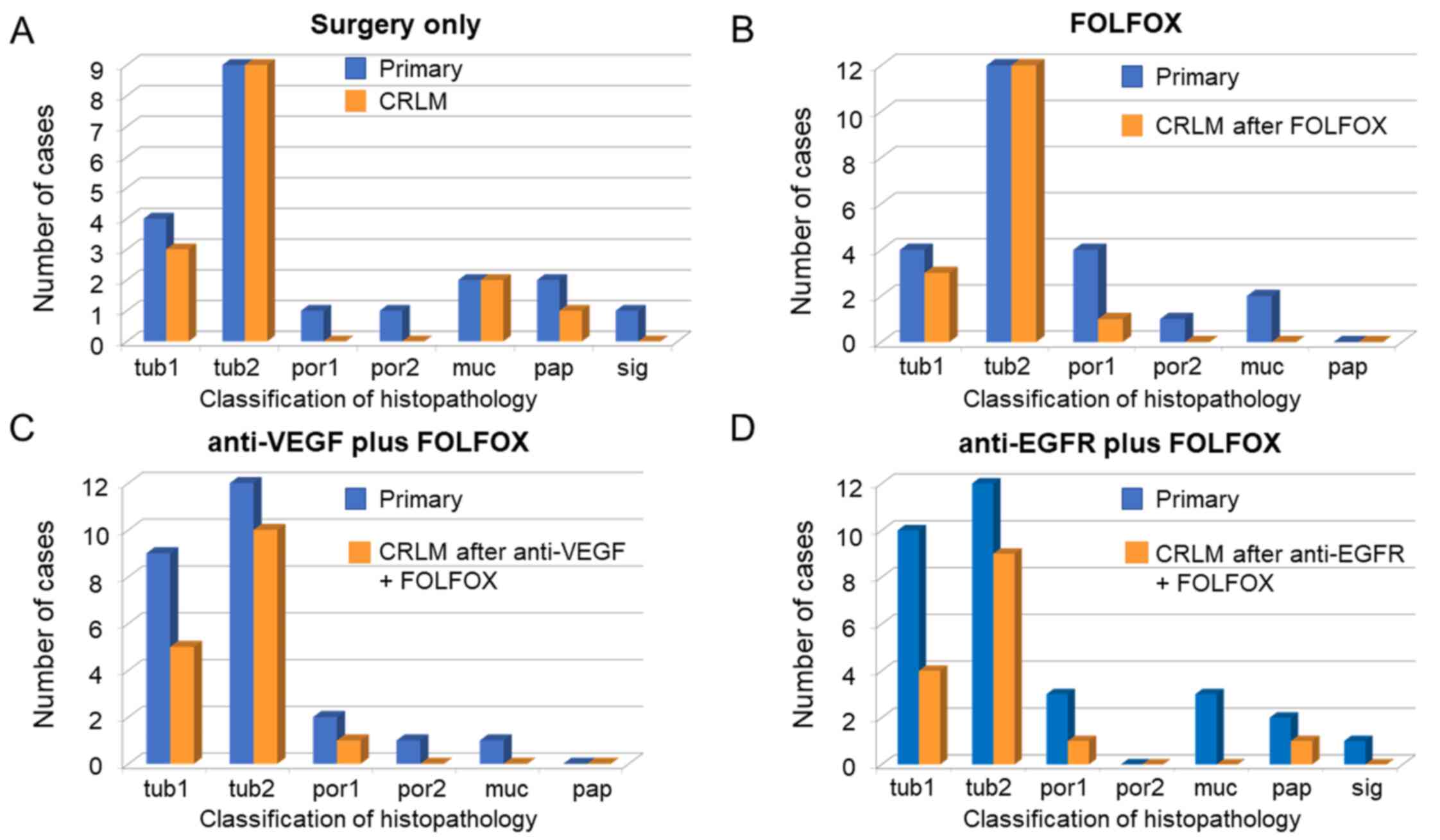
The heterogeneity of the histopathology between the
primary site and the liver metastasis was significantly different
in the group treated with surgery alone compared with the other
groups (P=0.04). However, there were no significant differences
between the three groups or each two groups. In addition, tub2
histopathology of appeared to be a predictive marker between
primary site and posttreatment liver metastasis specimens from
patients with CRLM because the histopathology forms disappeared
after each chemotherapy treatment, except for tub2 (Fig. 3A-D). Though this study didn't
showed data, we assume that Histopathological change greatly
influence long-term survival. We also evaluated patients with CRLM
(31/45) after excluding the patients in whom histopathological
heterogeneity changed to grade 3 in the liver metastasis after
chemotherapy. This study compared the group which underwent
hepatectomy after chemotherapy (n=25) with that which underwent
hepatectomy alone (n=6). In all six of the latter patients,
histopathological heterogeneity at the liver metastasis was
maintained after therapy. However, in 16 out of the 25 patients
(53.3%) who underwent hepatectomy after chemotherapy,
histopathological heterogeneity of liver metastases was lost. We
confirmed the loss of neoplastic cells by chemotherapy and
homogeneity in liver metastases (P=0.04) (Table II).
 | Table IIEvaluation of histopathologic
heterogeneity in patients who received hepatectomy after
chemotherapy versus without chemotherapy. |
Table II
Evaluation of histopathologic
heterogeneity in patients who received hepatectomy after
chemotherapy versus without chemotherapy.
| Treatment | Homogeneous | Heterogeneous | P-value |
|---|
| Hx after Cx, n (%)
(n=25) | 11/25(44) | 14/25(56) | 0.03 |
| Hx alone, n (%)
(n=6) | 0/6 (0) | 6/6(100) | |
Discussion
The present study describes the histopathological
patterns of response of CRLMs to preoperative NACT followed by
liver resection. To our knowledge, this is the first report to
focus on change of histopathological heterogeneity comparing
between primary tumor sites and CRLMs.
Over the past decade, NACT has been widely
recommended for the management of initially resectable CRLMs with
the aim of inducing tumor shrinkage to identify optimal candidates
for subsequent surgical removal. The assessment of tumor regression
has been gradually used to quantify the histopathological response
to NACT and has served as an early parameter predicting prognosis
(7,8).
Discrepancies in the tumor regression patterns in
response to different chemotherapy regimens have been revealed in
previous literature. Rubbia-Brandt et al (9,10)
reported that an oxaliplatin-based regimen improved
histopathological response compared with 5-fluorouracil-, and
irinotecan-based regimens. In terms of monoclonal antibodies, a
bevacizumab-containing regimen provided a better histopathological
response than chemotherapy alone or in combination with cetuximab
(11,12).
Poultsides et al (13) published a large retrospective
analysis of 366 patients (68% treated preoperatively and 32% not)
who underwent CRLM resection. In that study there was no increase
in the degree of necrosis after chemotherapy (13). Nevertheless, it should be noted
that only 69 out of 249 (28%) patients received bevacizumab as part
of the preoperative treatment, and the results in terms of necrosis
for that subgroup were not reported. In other experience, increase
in necrosis seemed to be due to a bevacizumab-related effect.
Additionally, the tumor histopathological response rate was defined
as the proportion of patients with grade ≥Ib (14,15).
Taken together, all of the above-reported
observations of reduced viable cells, fibrosis, and necrosis from a
pathological perspective explained the typical pattern of CLMs
detected using computed tomography scanning in patients receiving
chemotherapy and bevacizumab. Before treatment, the lesions showed
different types of enhancement, a heterogeneous degree of
attenuation, and ill-defined borders that were transformed into
hypo-attenuated and homogeneous metastases with well-defined
borders after treatment (16).
Such histological and morphological characteristics strengthen the
hypothesis that the RECIST criteria are not completely adequate for
evaluating response in patients receiving bevacizumab (17).
In contrast, the anti-EGFR agent cetuximab in
combination with chemotherapy has been reported to increase the
response rate and yield a good curative hepatectomy. In the PEAK
(18), FIRE-3(19) and CALGB/SWOG 80405(20) randomized controlled trials
performed to compare bevacizumab and anti-EGFR therapy for the
progression of recurrent CRC, anti-EGFR was also confirmed to have
a positive effect on survival extension in the presence of
wild-type RAS.
Recently, the multicenter, randomized, phase II ATOM
trial from Japan was designed to evaluate the efficacy and safety of
mFOLFOX6 plus bevacizumab and mFOLFOX6 plus cetuximab in patients
with liver-limited metastasis from wild-type all-RAS CRC. After
study treatment followed by surgical resection of tumors with R0/R1
status, the median progression-free survival of the
bevacizumab-treated arm was 6.5 months [95% confidence interval
(CI)=4.0-13.6 months], whereas that of the cetuximab-treated arm
was 13.8 months (95% CI=8.4 months-not reached; hazard ratio=0.610,
95% CI=0.298-1.245). Of the 57 tumors for which the
histopathological analysis was assessable, the histopathological
response rate (grade 1b/2/3) was 66.6% (20/30) in the
bevacizumab-treated arm and 92.6% (25/27) in the cetuximab-treated
arm (P=0.0229) (16), indicating
that the rate tended to be better in the cetuximab-treated arm
(21).
Falcão et al (22) reported three categories of tumor
growth: i) Replacement growth pattern, in which the tumor permeates
between the liver hepatocytes without disrupting the normal
architecture; ii) desmoplastic growth pattern, in which the tumor
is separated from the liver parenchyma by a band of fibrous tissue
that contains tumor-infiltrating lymphocytes; and iii) pushing
growth pattern, in which the tumor expands and compresses the
surrounding hepatocytes. They reported the pushing growth pattern
to be an independent risk factor for reduced survival (22).
Recently, a tumor-heterogeneity concept that
considers a single tumor to consist of many tumor cell sub-clones
has become an important topic in cancer genomics (23). It is hypothesized to play a
critical role in the progression of many cancer types and is a
major obstacle to precision cancer therapy. During this process,
sub-clones continuously arise via genomic mutation. The presence of
sub-clones has been shown to adversely affect outcome in chronic
lymphocytic leukemia, head and neck cancer, and lung
adenocarcinoma. However, the full complement of factors that lead
to tumor heterogeneity during CRC progression is unknown.
Several promising CRLM treatments have been
reported, including chemotherapy and molecular agents that target
EGFR and VEGF. Anti-EGFR drugs resulted in high response and
resection rates in the CELIM phase II trial and other studies for
initially unresectable CRLM with wild-type KRAS (24,25).
Anti-VEGF regimens, such as mFOLFOX6 or CAPEOX plus bevacizumab,
have also shown high response and resection rates in phase II
studies (19). These reports
suggest that the combination of targeted agents and chemotherapy
can increase the response rate.
A previous study reported a higher pathological
response rate to bevacizumab than for cetuximab. This study
suggested that the loss of intratumoral heterogeneity markedly
affects the response to chemotherapy. But this study suggested that
the heterogeneity of the histopathology between the primary site
and the liver metastasis was significantly different in the group
treated with surgery alone compared with the other groups (P=0.04).
However, there were no significant differences between the three
groups.
In conclusion, the present study highlighted marked
differences between pre and posttreatment specimens from sites in
patients with mCRC. Tub2 of histopathological type appeared to be a
predictive marker in specimens comparing primary site and CRLMs
posttreatment because histopathology types other than tub2
disappeared after chemotherapy treatment. Each NACT agent had an
acceptable safety profile. In the near future, we expect results
from further study to expand the indication for NACT.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NM, HTo and TT conceived the study and its design.
NM, HTa, TT, YI, MFuk, IY, TS, CM, RM, HI, YT, NO, MFut and KY
acquired the data. NM, HTo and SM analyzed and interpreted the data
and drafted the article. NM, HTo, MFut and KY performed critical
revision of the article. HTo, MFut and KY supervised the study. NM
and HTo confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients enrolled in the present study. The study protocol
conformed to the ethical guidelines of the 1975 Declaration of
Helsinki and the guidelines of the regional ethical committees of
Zurich and Basel, Switzerland, and was approved by the
Institutional Review Board of the Gifu University Graduate School
of Medicine (approval no. 28-508; March 23, 2017; Gifu, Japan).
Patient consent for publication
Not applicable.
Competing interests
KY has received honoraria for lectures from Chugai
Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda
Pharmaceutical Co., Ltd., Eli Lilly and Company, Daiichi Sankyo
Co., Ltd., Ono Pharmaceutical Co., Ltd., Merck Serono Co., Ltd.,
Novartis Pharma K.K., and Sanofi K.K.; and research funding from
Ajinomoto Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co.,
Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd.,
Taiho Pharmaceutical Co., Ono Pharmaceutical Co., and Yakult Honsha
Co., Ltd. outside the submitted work. TT has received honoraria for
lectures from Takeda Pharmaceutical Co., Ltd. All remaining authors
declare that they have no competing interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cancer Statistics in Japan-2018.
Foundation for Promotion of Cancer Research. Available from:
https://ganjoho.jp/reg_stat/statistics/brochure/backnumber/2018_jp.html.
|
|
3
|
NCCN GUIDELINES FOR PATIENTS 2018.
Available from: https://www.nccn.org/patients/guidelines/content/PDF/colon-patient.pdf.
|
|
4
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumors:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chua TC, Saxena A, Liauw W, Kokandi A and
Morris DL: Systematic review of randomized and nonrandomized trials
of the clinical response and outcomes of neoadjuvant systemic
chemotherapy for resectable colorectal liver metastases. Ann Surg
Oncol. 17:492–501. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nordlinger B, Van Cutsem E, Gruenberger T,
Glimelius B, Poston G, Rougier P, Sobrero A and Ychou M: European
Colorectal Metastases Treatment Group; Sixth International
Colorectal Liver Metastases Workshop. Combination of surgery and
chemotherapy and the role of targeted agents in the treatment of
patients with colorectal liver metastases: Recommendations from an
expert panel. Ann Oncol. 20:985–992. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rubbia-Brandt L: Hepatic lesions induced
by systemic chemotherapy for digestive cancer. Ann Pathol.
30:421–425. 2010.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
10
|
Rubbia-Brandt L, Giostra E, Brezault C,
Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE,
Soubrane O, et al: Importance of histological tumor response
assessment in predicting the outcome in patients with colorectal
liver metastases treated with neo-adjuvant chemotherapy followed by
liver surgery. Ann Oncol. 18:299–304. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gruenberger B, Tamandl D, Schueller J,
Scheithauer W, Zielinski C, Herbst F and Gruenberger T:
Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy
for patients with potentially curable metastatic colorectal cancer.
J Clin Oncol. 26:1830–1835. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gruenberger B, Schueller J, Heubrandtner
U, Wrba F, Tamandl D, Kaczirek K, Roka R, Freimann-Pircher S and
Gruenberger T: Cetuximab, gemcitabine, and oxaliplatin in patients
with unresectable advanced or metastatic biliary tract cancer: A
phase 2 study. Lancet Oncol. 11:1142–1148. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Poultsides GA, Bao F, Servais EL,
Hernandez-Boussard T, Dematteo RP, Allen PJ, Fong Y, Kemeny NE,
Saltz LB, Klimstra DS, et al: Pathologic response to preoperative
chemotherapy in colorectal liver metastases: Fibrosis, not
necrosis, predicts outcome. Ann Surg Oncol. 19:2797–2804.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Klinger M, Tamandl D, Eipeldauer S, Hacker
S, Herberger B, Kaczirek K, Dorfmeister M, Gruenberger B and
Gruenberger T: Bevacizumab improves pathological response of
colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann
Surg Oncol. 17:2059–2065. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wicherts DA, de Haas RJ, Sebagh M, Saenz
Corrales E, Gorden DL, Levi F, Paule B, Azoulay D, Castaing D and
Adam R: Impact of bevacizumab on functional recovery and histology
of the liver after resection of colorectal metastases. Br J Surg.
98:399–407. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Chun YS, Vauthey JN, Boonsirikamchai P,
Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H,
Charnsangavej C and Loyer EM: Association of computed tomography
morphologic criteria with pathologic response and survival in
patients treated with bevacizumab for colorectal liver metastases.
JAMA. 302:2338–2344. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gruenberger T, Arnold D and Rubbia-Brandt
L: Pathologic response to bevacizumab-containing chemotherapy in
patients with colorectal liver metastases and its correlation with
survival. Surg Oncol. 21:309–315. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schwartzberg LS, Rivera F, Karthaus M,
Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS and Go WY: PEAK: A
randomized, multicenter phase II study of panitumumab plus modified
fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab
plus mFOLFOX6 in patients with previously untreated, unresectable,
wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol.
32:2240–2247. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Venook AP, Niedzwiecki D, Lenz HJ,
Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH,
Atkins JN, et al: Effect of first-line chemotherapy combined with
cetuximab or bevacizumab on overall survival in patients with KRAS
wild-type advanced or metastatic colorectal cancer: A randomized
clinical trial. JAMA. 317:2392–2401. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Oki E, Emi Y, Yamanaka T, Uetake H, Muro
K, Takahashi T, Nagasaka T, Hatano E, Ojima H, Manaka D, et al:
Randomised phase II trial of mFOLFOX6 plus bevacizumab versus
mFOLFOX6 plus cetuximab as first-line treatment for colorectal
liver metastasis (ATOM trial). Br J Cancer. 121:222–229.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Falcão D, Alexandrino H, Caetano Oliveira
R, Martins J, Ferreira L, Martins R, Serôdio M, Martins M, Tralhão
JG, Cipriano MA, et al: Histopathologic patterns as markers of
prognosis in patients undergoing hepatectomy for colorectal cancer
liver metastases-Pushing growth as an independent risk factor for
decreased survival. Eur J Surg Oncol. 44:1212–1219. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li H, Courtois ET, Sengupta D, Tan Y, Chen
KH, Goh JJL, Kong SL, Chua C, Hon LK, Tan WS, et al: Reference
component analysis of single-cell transcriptomes elucidates
cellular heterogeneity in human colorectal tumors. Nat Genet.
49:708–718. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Folprecht G, Gruenberger T, Bechstein WO,
Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher
J, Weitz J, et al: Tumor response and secondary resectability of
colorectal liver metastases following neoadjuvant chemotherapy with
cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol.
11:38–47. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rivera F, Karthaus M, Hecht JR, Sevilla I,
Forget F, Fasola G, Canon JL, Guan X, Demonty G and Schwartzberg
LS: Final analysis of the randomised PEAK trial: Overall survival
and tumor responses during first-line treatment with mFOLFOX6 plus
either panitumumab or bevacizumab in patients with metastatic
colorectal carcinoma. Int J Colorectal Dis. 32:1179–1190.
2017.PubMed/NCBI View Article : Google Scholar
|