Introduction
Pleuroparenchymal fibroelastosis (PPFE) is a rare
form of interstitial lung disease (ILD), characterized by upper
zone pleural fibrosis with subpleural intra-alveolar fibrosis and
alveolar septal elastosis. It was first described in 2004 by
Frankel et al and is considered to be an independent entity
among ILDs in the 2013 Classification of Idiopathic Interstitial
Pneumonias by the American Thoracic Society/European Respiratory
Society (1,2).
PPFE affects both sexes and may be diagnosed in
young patients. The clinical course varies as it can progress
rapidly in certain cases or have an indolent course (3).
Although its physiopathology remains unclear,
certain factors have been associated with PPFE, including
chemotherapy. Several cases previously described the association
between PPFE and drugs used to treat malignancy, especially
alkylating drugs, such as cyclophosphamide and carmustine (3-4). Given
the predicted increasing prevalence of patients treated with
chemotherapeutic agents, PPFE may become more common in this
population (5). However, there is
no effective pharmacological treatment and lung transplant must be
considered in certain cases. Hence, it is important to raise
awareness of this disease among clinicians.
Case report
A female patient (age, 34 years) was diagnosed with
nodular sclerosing Hodgkin lymphoma in 2016 (stage II-A). The
patient was treated in Hospital Center of São João (Porto,
Portugal) with first line chemotherapy (doxorubicin, bleomycin,
vinblastine and dacarbazine) and mediastinal and right axillary
radiotherapy. The patient had no other relevant comorbidities (such
as auto-immune disease), no history of smoking, no known relevant
occupational or environmental exposure and was not prescribed
regular medication. In 2018, the patient presented with insidious
onset exertional dyspnea and reported a respiratory tract infection
that was treated with antibiotics (amoxicillin and clavulanic
acid). Physical examination of the patient did not result in any
relevant findings. Laboratory blood tests and blood gas analysis
were normal. The patient's lung volumes in lung functional tests
were within normal range, but the diffusing capacity for carbon
monoxide (53%) and adjusted alveolar volume (54%) were decreased
(6). High-resolution computed
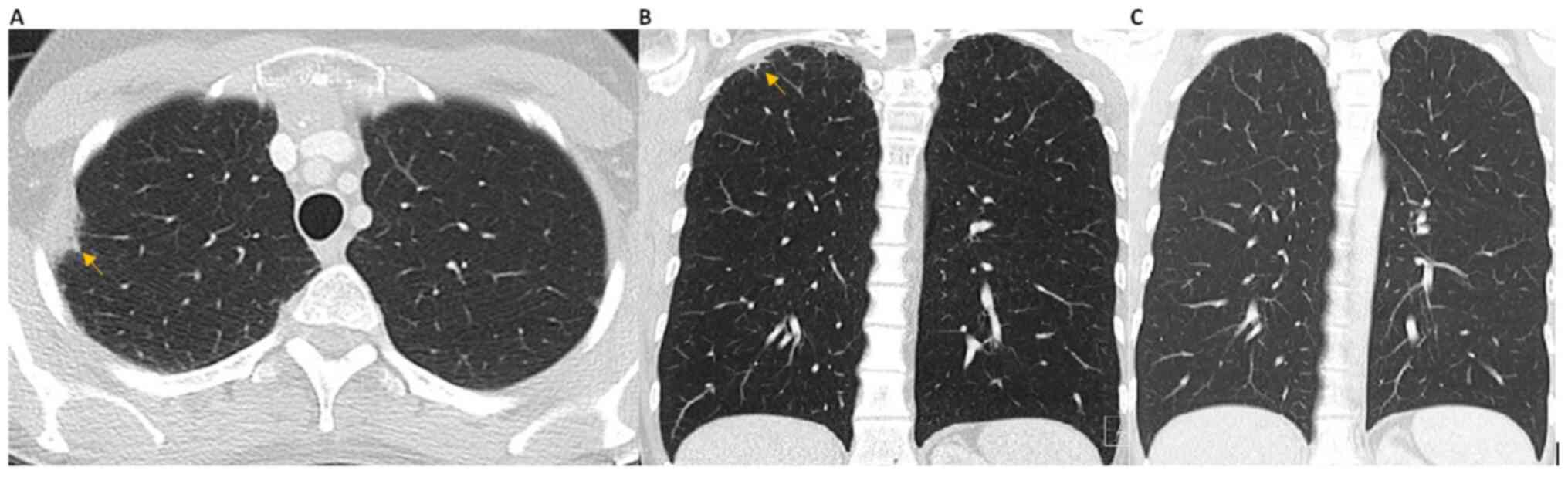
tomography (HRCT) of the chest revealed biapical subpleural opacity
with interstitial thickening and slight upper lobe volume loss
(Fig. 1). These features were not
present in previous scans. These changes were subtle, which is also
the reason why lung volumes in the respiratory function test were
normal. It was hypothesized that the diffusing capacity for carbon
monoxide was depressed due to sequelae from previous radiotherapy
that resulted in changes in lung function that may not be seen in
CT scan. Given her past history of malignancy, a percutaneous
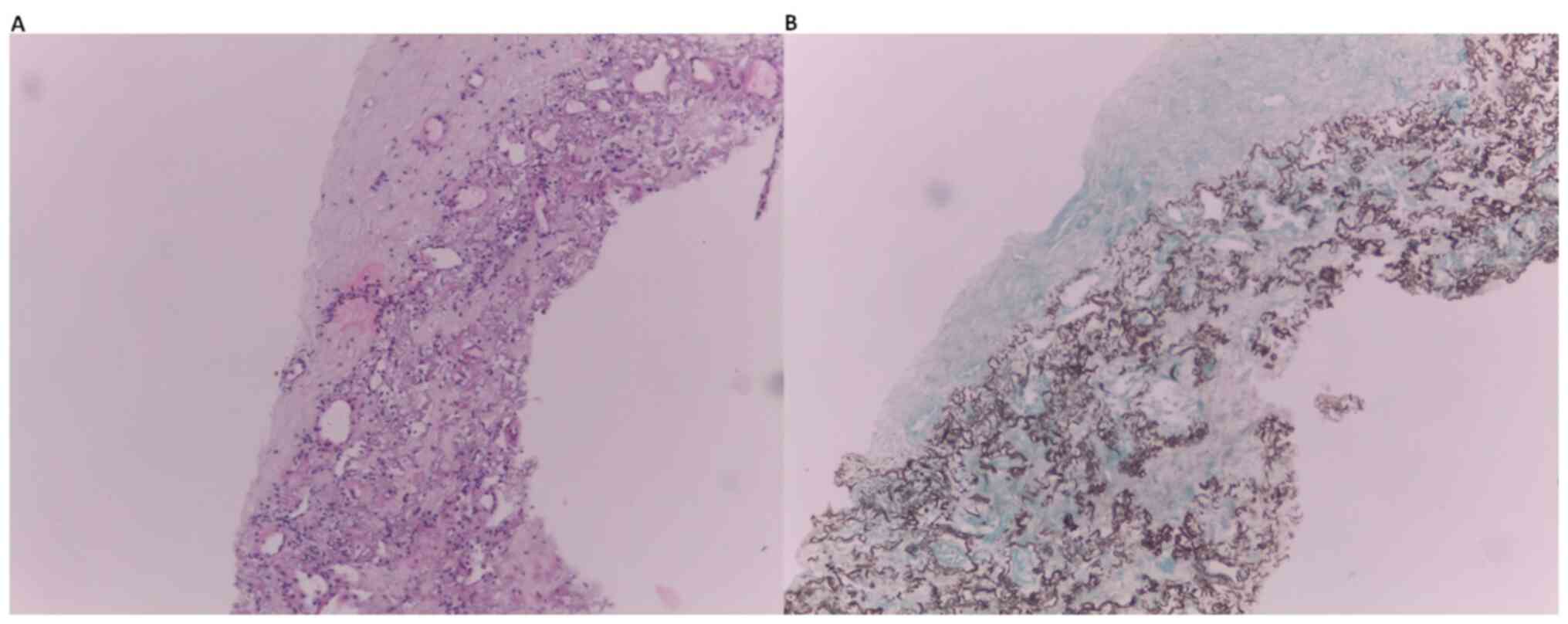
transthoracic lung biopsy was performed, which revealed pleural
fibrosis and subpleural and parenchymal fibroelastosis (Fig. 2). Following evaluation and
discussion among the ILD multidisciplinary team, the patient was
diagnosed with PPFE. To date, the patient remains clinically and
functionally stable in follow-up appointments. Hence, the patient
has not yet been treated with immunosuppressive therapy.
Discussion
PPFE is a rare and recently described clinical
entity. However, the number of diagnosed cases has increased as
clinicians become more aware of this disease (7). Although PPFE physiopathology remains
unclear, its distinct pattern of fibrosis with definable and
reproducible clinical, radiological and histopathological criteria
allowed its classification as an independent entity in the 2013
American Thoracic Society and European Respiratory Society
classification of Idiopathic Interstitial Pneumonias, specifically
as a rare interstitial pneumonia (8,9).
Several factors have been associated with PPFE,
including chemotherapy, lung and bone marrow transplant, connective
tissue disease and recurrent pulmonary infections (9). It was hypothesized that PPFE may
represent a common final pattern of response of the lung to
multiple types of injury, such as recurrent infection, allergen
exposure and chemotherapy (9). An
idiopathic form of PPFE has also been described, as well as
familial cases.
Clinically, PPFE equally affects both sexes, with a
proportion of patients being relatively young. PPFE develops and
progresses rapidly in some cases, whereas other patients present
with radiological changes years before symptoms have occurred
(9) The most common features of
PPFE are dyspnea on exertion, insidious onset dry cough and weight
loss (3). Pneumothorax is common
due to pleural alterations and can be a complication that is
difficult to manage following lung biopsy. If radiological features
are typical of PPFE and no other diagnosis is suspected, one should
consider the risks and benefits of performing a lung biopsy for
each individual patient since histology is not mandatory for PPFE
diagnosis (10).
Imaging and histological criteria for the diagnosis
of PPFE were described in 2012 (10,11).
HRCT imaging criteria of PPFE can be defined as either ‘definite’
or ‘consistent with’. ‘Definite’ criteria consist of pleural
thickening and subpleural fibrosis concentrated in the upper lobes,
whereby lower lobe involvement is less marked or absent.
‘Consistent with’ criteria include upper lobe thickening and
subpleural fibrosis; however, the distribution of changes are not
concentrated in the upper lobes, or features of coexisting disease
are present elsewhere (10).
The histological criteria of PPFE are also defined
as either ‘definite’ and ‘consistent with’. ‘Definite’ criteria
include upper zone pleural fibrosis with subjacent intra-alveolar
fibrosis, accompanied by alveolar septal elastosis. ‘Consistent
with’ criteria include intra-alveolar fibrosis, but not those
associated with significant pleural fibrosis, not predominantly
present beneath the pleura or not present in an upper lobe biopsy
(10).
Drugs associated with lung injury and pleural
effusion, pleuritis, pleural thickening or fibrosis have been
reported in this context (7). PPFE
has been identified as a complication of chemotherapy with
alkylating agents such as cyclophosphamide or carmustine. The
disease can develop months to years after treatment and in some
cases, it may be difficult to determine a causal connection. Data
that support this association are: Pre-therapy normal chest
imaging, the existence of reports from different countries over
multiple years that describe the same disease associated with the
same drugs, different alkylating agents resulting in the same
disease and the development of PPFE in young individuals, as
fibrotic disease is more common in the elderly.
Bleomycin is used in models of pleural fibrosis and
is instilled in the pleural space (7). To the best of our knowledge, bleomycin
and the other chemotherapy drugs used in the present case report
has not previously been associated with PPFE (7). However, to date, no effective
treatment has been able to modify the natural course of PPFE.
Corticosteroids and immunosuppressive agents have been used, but
with limited efficacy. Lung transplants must be considered in
selected patients with advanced disease (8).
Although bleomycin and the other chemotherapy agents
used to treat the present patient have not been implied in the
etiology of PPFE thus far, this association seems plausible since
it was observed in a young patient with no known contact with other
possible causative agents and normal chest imaging prior to
chemotherapy.
PPFE was diagnosed 2 years after treatment cessation
in the present case report. The disease may develop several years
after chemotherapy treatment. A study has shown that the interval
between the time of drug exposure to onset of PPFE symptoms or
radiological features ranges from 6 months to 16 years (7). The present patient also underwent
radiotherapy; this treatment can induce pneumonitis/fibrose or
organizing pneumonia. However, since PPFE lesions were observed
outside of the radiation plan (which excludes the possibility of
radiation pneumonitis) and PPFE has never been reported in this
scenario, this does not indicate any relevant role of radiation in
this particular clinical case.
PPFE has been increasingly diagnosed over recent
years due to growing awareness of this disease among clinicians.
This will facilitate greater understanding of the etiology of this
disease and the development of novel treatment approaches to
improve patient quality of life and survival rate in the
future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ACRV, JRMF and JSVDC contributed to the planning,
conduct, reporting, conception, design and acquisition of data and
drafting the manuscript. AM, NM, PCM, HNEB, JMP and CS contributed
to planning, conduct, reporting, conception, design and acquisition
of data and revision of the article. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The authors certify that this study involving a
human subject was performed in accordance with the Declaration of
Helsinki 1975, as revised in 2000, and was approved by the Ethical
Committee of Hospital Center of São João and Faculty of Medicine,
University of Oporto (approval no. CE-OP-70-2020). Additionally,
informed written consent was obtained from the patient.
Patient consent for publication
Informed written consent was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frankel SK, Cool CD, Lynch DA and Brown
KK: Idiopathic pleuroparenchymal fibroelastosis. Description of a
novel clinicopathologic entity. Chest. 126:2007–2013.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Travis W, Costabel U, Hansell DM, King TE
Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU,
et al: An Official American Thoracic Society/European Respiratory
Society Statement: Update of the International Multidisciplinary
Classification of the Idiopathic Interstitial Pneumonias. Am J
Respir Crit Care Med. 88:733–748. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Portillo K, Guasch Arriaga I and
Ruiz-Manzano J: Pleuroparenchymal Fibroelastosis: Is it Also an
Idiopathic Entity? Arch Bronconeumol. 51:509–514. 2015.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
4
|
Beynat-Mouterde C, Beltramo G, Lezmi G,
Pernet D, Camus C, Fanton A, Foucher P, Cottin V and Bonniaud P:
Pleuroparenchymal fibroelastosis as a late complication of
chemotherapy agents. Eur Respir J. 44:523–527. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wilson BE, Jacob S, Yap ML, Ferlay J, Bray
F and Barton MB: Estimates of global chemotherapy demands and
corresponding physician workforce requirements for 2018 and 2040: A
population-based study. Lancet Oncol. 20:769–780. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pellegrino R, Viegi G, Brusasco V, Crapo
RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson
P, Hankinson J, et al: Interpretative strategies for lung function
tests. Eur Respir J. 26:948–968. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Camus P, Thusen J, Hansell D and Colby T:
Pleuroparenchymal fibroelastosis: One more walk on the wild side of
drugs? Eur Respir J. 44:289–296. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bonifazi M, Montero MA and Renzoni EA:
Idiopathic Pleuroparenchymal Fibroelastosis. Curr Pulmonol Rep.
6:9–15. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Thüsen JH: Pleuroparenchymal
Fibroelastosis: Its pathological characteristics. Curr Respir Med
Rev. 9:238–247. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Reddy TL, Tominaga M, Hansell DM, von der
Thusen J, Rassl D, Parfrey H, Guy S, Twentyman O, Rice A, Maher TM,
et al: Pleuroparenchymal Fibroelastosis: A spectrum of
histopathological and imaging phenotypes. Eur Respir J. 40:377–385.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Redondo MT, Melo N, Mota PC, Jesus JM,
Moura CS, Guimarães S and Morais A: Idiopathic pleuroparenchymal
fibroelastosis: A rare but increasingly recognized entity. Rev Port
Pneumol (2006). 21:41–44. 2015.PubMed/NCBI View Article : Google Scholar
|