1. Introduction
Osteosarcoma (OS), a malignant tumor type that
originates in mesenchymal tissue, is most common in children and
adolescents. OS occurs in the metaphysis of long bones with a rich
blood supply. It is highly malignant and characterized by early
metastasis, rapid disease progression, a high mortality rate and
frequent clinical treatment failure (1,2). At
present, OS is primarily treated by a combination of neoadjuvant
chemotherapy, surgery and postoperative chemotherapy. Surgical
treatment alone is ineffective and associated with frequent
recurrence and lung metastasis; the 5-year survival rate of
patients is only 15-20% (3). The
combination of chemotherapy and surgery is an important treatment
strategy for OS; however, multidrug resistance frequently leads to
failure of chemotherapy for OS. The causes of drug resistance of OS
cells are diverse and include low drug absorption, evasion of
apoptosis, abnormal function of microRNAs (miRNAs), autophagy,
altered membrane permeability and the DNA damage response. Changes
in autophagy are thought to cause drug resistance in OS and the
proliferation of malignant cells may result in treatment
failure.
In autophagy, cells create autophagolysosomes from
lysosomes to degrade damaged organelles, such as mitochondria and
macromolecules. Autophagy has an important regulatory role in cell
growth, development, differentiation and death (4-7).
According to certain researchers, autophagic death may be an
important way to eliminate tumor cells resistant to apoptosis due
to gene mutations (8). The primary
mechanism of action of chemotherapy drugs is the induction of tumor
cell apoptosis; however, chemotherapeutics may also induce
autophagy of tumor cells. Apoptosis and autophagy are related but
independent processes (9).
Regulating the autophagy of OS cells to reduce their resistance to
chemotherapeutic drugs is an important consideration in the
development of novel treatment strategies for OS.
2. Autophagy and chemotherapy
resistance
Basic concepts
Autophagy is an evolutionarily conserved process
that involves lysosomal enzymatic degradation of damaged organelles
and proteins to maintain cellular homeostasis. Under stress
conditions and during cell death, high levels of autophagy are
induced; thus, it is thought that autophagy may initiate cell
death, although this has been controversial. Autophagy is complex,
involving various signaling pathways that promote cell death
(10). Cells may undergo autophagy
due to a lack of nutrients, blood oxygen and growth factors, and
due to cellular toxicity caused by proteins or organelles, and
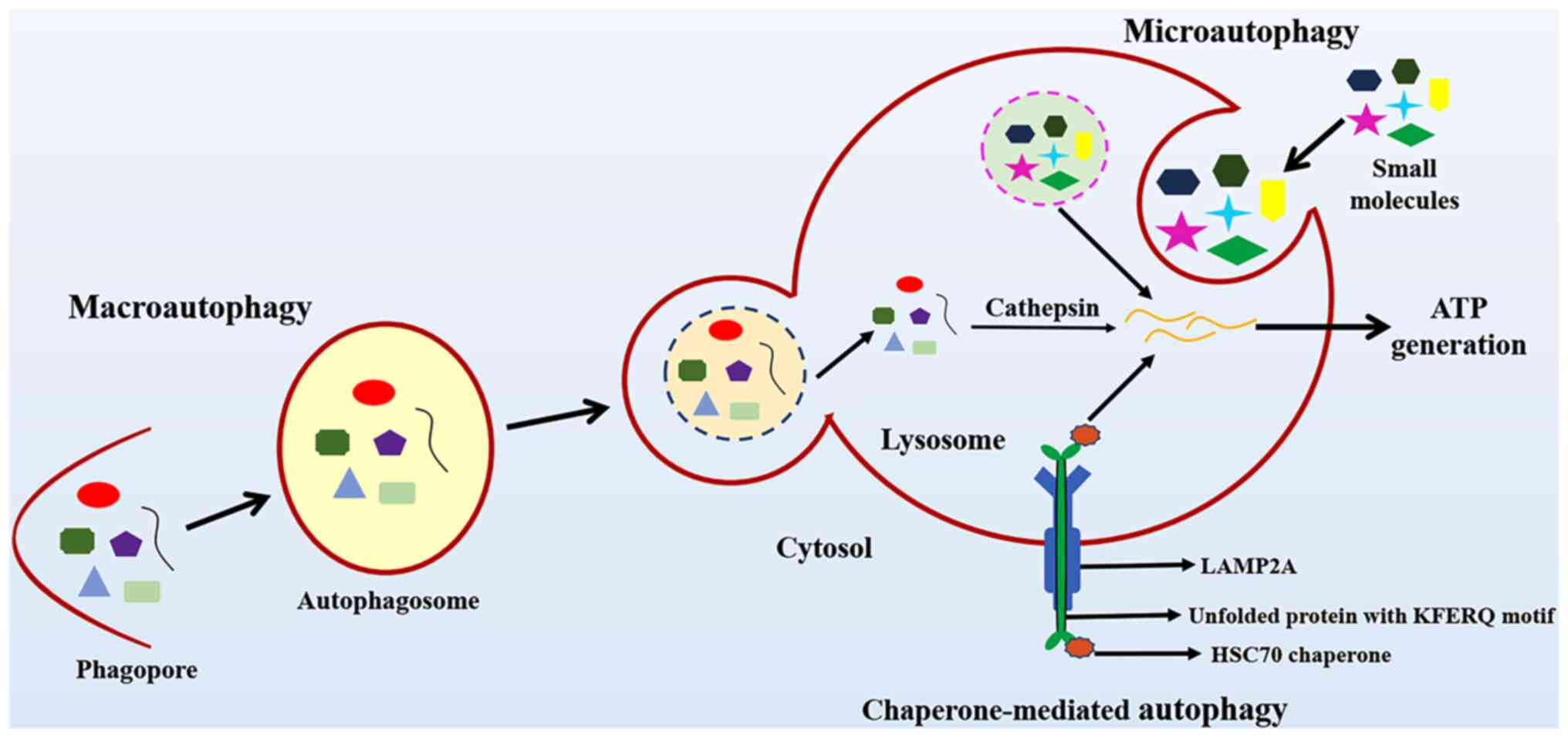
disturbances in their internal environment (10). Autophagy is classified into
macroautophagy, microautophagy and chaperone-mediated autophagy.
The different types of autophagy are presented in Fig. 1.
Chemotherapy, surgery and radiotherapy are the three
major treatments for cancer. Chemotherapy involves the use of drugs
to kill cancer cells and is currently one of the most effective
treatments. Chemotherapeutics, as systemic treatments, diffuse
throughout the body via the blood circulation. As such, for tumors
that have a tendency to spread throughout the body, as well as for
advanced tumors that have undergone metastasis, chemotherapy is the
primary treatment (11).
Role of autophagy in inhibiting tumor
progression
DNA and cell organelles may be damaged by physical,
chemical and biological carcinogens, such as radiation, aflatoxin
and viruses, respectively, which disrupt cellular metabolism. The
accumulation of metabolic waste and a lack of cellular energy
activates autophagy-related genes (ATGs), which initiate autophagy.
Autophagy may eliminate damaged organelles, degrade self-folded
proteins and maintain genomic stability, thereby inhibiting tumor
development. It has been indicated that, in early tumor cells,
autophagy is either present at low levels or absent. Loss of
autophagy may cause accumulation of mutations, leading to tumor
formation and metastasis. Therefore, promoting autophagy may
inhibit tumor formation and development (12). Mele et al (13) determined that curcumin may increase
Beclin-1 and microtubule-associated protein 1 light chain 3β (LC3B)
protein levels and inhibit AKT-mammalian target of rapamycin (mTOR)
and other pathways, thereby inducing autophagy.
During tumor development, tumor cells proliferate
rapidly; this process requires high levels of nutrients. However,
rapid growth is not possible when there are insufficient blood
vessels in the tumor tissue. When the energy demands of solid tumor
cells are not met due to a lack of oxygen and nutrients, Liver
kinase B1 is activated to phosphorylate adenosine
monophosphate-activated kinase (AMPK). Activated AMPK
phosphorylates Ser863, while TSC2 is able to activate Ras homolog,
mTORC1 binding to inhibit mTORC1 and induce autophagy (14). When in a hypoxic state, tumor cells
obtain energy from anaerobic glycolysis, which generates high
levels of reactive oxygen species that further promote autophagy
(15). During tumor development,
cells are able to resist various environmental stressors by
inducing autophagy. Autophagy may degrade damaged organelles and
misfolded proteins, provide energy for tumor cells and promote the
spread of tumor cells. Furthermore, tumor cell death induced by
autophagy may lead to moderate inflammation; this promotes new
blood vessels that may dilate into the tumor tissue to provide
nutrition supporting tumor growth. When environmental stress is
severe, tumor cells may enter into a state of reversible dormancy
due to autophagy and exist in the host for a long period of time
(16). Therefore, moderate levels
of autophagy are conducive to the survival and development of tumor
cells in vivo.
Autophagy and chemotherapy
tolerance
It is thought that autophagy has an important role
in the occurrence, development and treatment of tumors. Although
chemotherapy has a significant beneficial effect in numerous
patients, acquired drug resistance has become a major reason for
treatment failure. Numerous studies have indicated that a variety
of chemotherapeutic drugs may induce autophagy (10,17).
Furthermore, there is a correlation between autophagy and tumor
chemoresistance.
Chemotherapy induces apoptosis of cancer cells;
however, cancer cells frequently protect themselves by inducing
autophagy, thereby avoiding apoptosis, which markedly reduces the
efficacy of chemotherapy. Liu et al (18) used MTT and Hoechst 33342 staining,
as well as flow cytometry, to detect apoptosis of A549 lung cancer
cells after chemotherapy; they also used the autophagy inhibitor
3-methyladenine (3-MA) to study the relationship between autophagy
and apoptosis in cancer cells. Their experiments indicated that
cisplatin (DDP) and paclitaxel may induce autophagy and apoptosis
of A549 lung cancer cells. Studies have also indicated that
salivary gland adenoid cystic cancer cells caused by autophagy are
resistant to DDP, which frequently leads to chemotherapy failure
(19). Transmission electron
microscopy is able to detect the expression of the autophagy marker
LC3 and trace amounts of p62 also indicate autophagy induced by
DDP. Furthermore, downregulation of Beclin-1 via 3-MA or RNA
interference may enhance DDP-induced apoptosis. Therefore, the
induction of protective autophagy by chemotherapy enhances the
chemotherapeutic resistance of tumor cells.
3. Regulation of autophagy in OS
mTOR
mTOR has important roles in cell growth and
metabolism, as well as in the regulation of autophagy. mTOR is an
atypical serine/threonine protein kinase that is able to control
intracellular mRNA translation and protein synthesis. Changes in
mTOR signaling are common in numerous tumor types, including OS.
Kim et al (20) indicated
that when the body is in a normal nutritional state, the
PI3K/AKT/mTOR pathway is activated to inhibit autophagy and cells
proliferate normally. However, when cells are underfed or stressed,
mTOR is inhibited and autophagy thus activated, causing
uncontrolled cell growth and proliferation, as well as inhibition
of apoptosis, eventually leading to tumor progression and
metastasis (21).
High mobility group protein 1
(HMGB1)
HMGB1 is associated with damage to chromatin and is
also involved in the construction and stabilization of nucleosomes
and DNA damage repair. Furthermore, HMGB1 is an important regulator
of autophagy in chemoresistant OS (22,23).
Zhang et al (24) indicated
that HMGB1 competitively binds Beclin-1 during autophagy and
regulates autophagy by controlling the dissociation of the
Beclin1/Bcl-2 complex. HMGB1 also binds cell surface receptors,
activating downstream signaling pathways to stimulate cell
proliferation and migration, as well as autophagy. In short, the
high expression of HMGB1 observed in OS tissue is related to the
occurrence and development of tumors. Downregulation of HMGB1 may
hinder tumor cell metastasis (23); thus, HMGB1 has an important
regulatory role in tumor progression.
miRNAs
miRNAs are highly conserved non-coding RNAs (~22
oligonucleotides) that repress gene expression by binding a target
mRNA at the 3'-untranslated region, thus inhibiting translation or
inducing degradation. miRNAs regulate cell differentiation and
development, the nervous system, immunity, viral infection, DNA
repair, cell junctions, cell-to-cell communication, cellular
reprogramming and metabolism (25,26).
Numerous studies have demonstrated the important role of miRNAs in
the occurrence, regulation and progression of human OS. Certain
miRNAs act as tumor suppressors, while others act as oncogenes
(27). In recent years, the
correlation between miRNAs and autophagy has attracted much
attention. While most miRNAs are downregulated in OS cell lines,
Mutlu et al (28) reported
that, following suppression of autophagy-associated miRNAs by
adriamycin and rapamycin, the expression of certain miRNAs,
including miR-3141, miR-4296, miR-133b and miR-720, was markedly
increased. Chen et al (29)
and Niu et al (23)
indicated that miRNAs have an important regulatory role in
autophagy. Numerous miRNAs have also been reported to suppress the
development of resistance and sensitivity to drugs by controlling
and blocking autophagy. According to various studies (23,29,30),
miR-101 not only has a significant inhibitory effect on OS cell
proliferation, but may also promote apoptosis, while reducing the
expression of the autophagy-related proteins Beclin1 and LC3B;
these results suggest that miR-101 affects the proliferation and
apoptosis of OS cells by regulating the expression of autophagy
genes. miR-22, which has an important role in the regulation of
autophagy, functions as both a tumor suppressor gene and
proto-oncogene. It has a key role in cell growth, proliferation,
migration, invasion and aging (31). Wang et al (32) and Li et al (33) indicated that miR-22 regulates
HMGB1-induced autophagy and has an important role in the
proliferation and migration of OS. Overexpression of miR-22
inhibits cell proliferation and the formation of OS cell colonies
in patients treated with anti-tumor drugs, suggesting that miR-22
has potential for reducing the development of drug resistance
during OS chemotherapy.
p53
p53 regulates the cell cycle and apoptosis and
affects the efficacy of chemotherapeutic drugs (34). As an important tumor suppressor
gene, p53 is involved in the regulation of autophagy. Cytoplasmic
p53 inhibits autophagy. Pitolli et al (35) determined that nutritional
deficiencies, changes in the cellular environment and DNA damage
activate p53 and AMPK pathways in the nucleus, resulting in the
phosphorylation of tuberous sclerosis complex, inhibition of mTOR
activity and the induction of autophagy. p53 is also able to
activate pro-apoptotic proteins to dissociate the Beclin1-Bcl-2
complex, thereby promoting autophagy.
Beclin-1
The most common mechanism of autophagy induction in
patients with OS is the activation of Beclin-1 via upstream
mediators (30). Beclin-1 was the
first mammalian-related autophagy regulatory gene to be identified.
Beclin-1 dysfunction may lead to immune dysfunction and
tumorigenesis. Zhang et al (36) reported that chemotherapeutic drugs,
such as DDP, doxorubicin and methotrexate, induce upregulation of
Beclin-1 expression in OS cells, while knocking down the Beclin-1
gene inhibited OS cell proliferation, metastasis and invasion. OS
cells are more sensitive to chemotherapy when the Beclin-1 gene is
knocked down or an autophagy inhibitor is used (37). Beclin-1 has an important role in OS
cell proliferation and tumor progression, and inhibition of
autophagy may improve the efficacy of chemotherapy.
Atg-4B
There are two major autophagy pathways in OS: The
mTOR and class III phosphatidylinositol kinase (Ptdins3K) pathways
(38). mTOR stimulates Ptdins3k
activity and inhibits the formation of the mammalian orthologs of
yeast Atg1 (ULK1/2) complex. The mTOR inhibitor rapamycin induces
autophagy-mediated cell death in gliomas. Ptdins3K synthesizes
PI3K, which provides a binding site for ATGs during the formation
of autophagosomes. These two pathways regulate the formation of
LC3B liposomes by regulating the activity of Atg4 and Atg7. Atg4B,
which activates LC3B, catalyzes the cleavage of the carboxyl end of
LC3B (39).
Shi et al (40) indicated that the anti-tumor effect
of the drug NSC185058 is related to Atg4B function and inhibition
of autophagy. A high concentration of NSC185058 reduces the
viability of Saos-2 cells. Knocking down Atg4B leads to autophagic
defects in Saos-2 OS cells. In addition, the use of Atg4B protein
antagonists to reduce Saos-2 OS cell viability was linked to
inhibition of autophagy; the antagonists had no effect on
Atg4B-deficient OS cell lines. Thus, inhibition of autophagy is
considered the primary mechanism underlying the anti-tumor activity
of the drug NSC185058(41).
4. Drugs affecting autophagy and
chemotherapy efficacy for OS
Certain drugs promote autophagy, while others
inhibit it. Autophagy-inhibiting drugs include chloroquine and
3-MA. Autophagy inducers increase autophagy and killing of OS
cells, i.e., they both promote protective autophagy and cause
autophagic death. Autophagy inhibitors also increase tumor cell
death, indicating that the regulation of autophagy may increase
sensitivity to chemotherapeutic drugs, particularly in the presence
of particular gene mutations and in tumor cells resistant to
apoptosis. Most of the available drugs enhance sensitivity to
chemotherapeutics by inhibiting protective autophagy.
Autophagy inducers Tripterygium
wilfordii
Tripterygium wilfordii is a traditional
Chinese medicine that exerts pharmacological effects, including
immunosuppression. Recently, Tripterygium wilfordii has been
reported to also exert anti-tumor effects; it kills leukemia,
multiple myeloma, liver cancer, melanoma and breast cancer cells
(42-45).
Hou et al (46) indicated
that Tripterygium wilfordii inhibits the proliferation of OS
cells but is not toxic to normal cells. Tripterygium
wilfordii has been demonstrated to induce apoptotic and
autophagic death of OS cells, thus significantly inhibiting OS cell
proliferation; these effects were partially ameliorated by
autophagy inhibitors.
Arsenic trioxide
Arsenic trioxide (As2O3) was
first used in the treatment of acute promyelocytic leukemia. It
exhibits short-term efficacy in the treatment of stage III OS
(47). Hashmi and Nishihori
(48) reported that
AS2O3 promoted autophagic cell death in human
OS (HOS), but as the level of autophagy increases, so too does the
level of HOS apoptosis. AS2O3 may also
increase the level of autophagy in multidrug-resistant cells, such
as MG63 cells. However, as the As2O3
concentration increases, the level of cell autophagy decreases
following an initial increase, eventually reaching the basal level.
A marker protein of early apoptosis was identified at this stage.
The effects of As2O3 vary among OS cells with
different characteristics. In multidrug-resistant cells, such as
MG63 cells, As2O3 induces protective
autophagy, which partially alleviates cell death, while in
chemotherapy-sensitive cells, such as HOS cells,
As2O3 induces autophagic death. Zhang et
al (49) indicated that
As2O3 induced protective autophagy in gastric
cancer cells and the addition of autophagy inhibitors markedly
increased apoptosis.
Rapamycin
The immunosuppressive agent rapamycin is primarily
used to prevent immune rejection after organ transplantation.
Recently, rapamycin was reported to exert significant anti-tumor
effects (50,51). mTOR, an upstream regulator of
autophagy, phosphorylates ULK1 and ULK2, thereby inhibiting
autophagy (52). As an mTOR
inhibitor, rapamycin inhibits the transition of the cell cycle from
G0/G1 to S, as well as protein transcription and translation,
thereby inhibiting tumor cell proliferation (53). Rapamycin is also an effective
autophagy inducer. Protective autophagy inhibits the death of
certain tumor cells (54). Saraf
et al (55) indicated that
the autophagy inhibitor chloroquine, used in combination with
rapamycin, inhibited protective autophagy in OS and increased
rapamycin-mediated inhibition of tumor cell proliferation,
ultimately increasing the sensitivity of chemotherapeutic
drugs.
Autophagy inhibitors
Autophagy inhibitors have not yet been used on their
own in the clinical setting due to a lack of specificity, i.e., due
to their toxicity to normal cells. However, the efficacy of
chemotherapeutic drugs is commonly enhanced by adding autophagy
inhibitors to the regimen. Kocaturk et al (56) indicated that the level of autophagy
in MG63 cells was significantly reduced by the autophagy inhibitor
3-MA; its use in combination with DDP significantly increased MG63
cell death. After U2OS cells had been pretreated with the autophagy
inhibitor chloroquine, treatment with the Akt kinase inhibitor
MK-2206 further inhibited their activity (57). Saraf et al (55) reported that treatment of OS cells
with chloroquine and rapamycin inhibited protective autophagy,
thereby inhibiting tumor cell proliferation and enhancing
sensitivity to chemotherapeutic drugs. Further research on
autophagy inhibitors is important as a means of increasing the
sensitivity to, and thus the efficacy of, chemotherapeutic
drugs.
Survivin inhibitors
Survivin, an inhibitor of apoptosis protein,
regulates mitosis and apoptosis. Survivin has been detected in most
types of tumor tissues and may increase tumor cell apoptosis and
chemotherapy sensitivity (58);
thus, survivin has been considered a target in OS treatment. YM155,
a specific inhibitor of survivin, inhibits the proliferation of
various tumor cell types and is considered safe and effective
(59). Khan et al (60) indicated that YM155 inhibits
proliferation, induces autophagy and apoptosis, and reduces the
expression of survivin mRNA in F5M2 cells. Waligórska-Stachura
et al (59) determined that
survivin is highly expressed in OS cells and is related to the
degree of malignancy. YM155 inhibited Saos-2 and MG63 cell
proliferation and invasion and promoted apoptosis. It also
increased sensitivity to the chemotherapeutic doxorubicin. Coumar
et al (61) demonstrated
that YM155 inhibited the proliferation of liver cancer cells and is
involved in the induction of autophagic death of stem cancer cells.
Church and Talbot (62) indicated
that YM155 induced autophagy in the breast cancer cell line
MDA-MB-231; this induction of autophagy also promoted apoptosis.
The mechanism underlying the effect of YM155 on malignant tumors
has emerged as an important research target. The relationship
between YM155 and autophagy in OS remains to be further elucidated.
In particular, it remains to be determined whether autophagy
inducers are able to increase the efficacy of YM155.
Photodynamic therapy (PDT)
PDT involves intravenous or local injection of
photosensitizers into the body. Selective aggregation of tumor
cells occurs when using a particular wavelength of laser
irradiation; this causes tumor cells to produce large quantities of
cytotoxic singlet oxygen and oxygen free radicals, which may
inhibit tumor cell proliferation and spread (63,64).
PDT damages organelles, which in turn induces apoptosis and
autophagy. A certain level of autophagy may improve cell viability
under stress. Furthermore, when apoptosis genes are mutated or
suppressed, autophagic death becomes the primary mechanism of cell
death. Aloe-emodin, an anti-tumor drug and photosensitizer, induces
autophagy in MG63 cells, which leads to an early anti-apoptotic
effect (65). The effects of the
autophagy induced by PDT vary by cell type and dose; increasing the
efficacy of PDT is an important goal of future research.
5. Summary
Autophagy has an important role in the onset,
progression and treatment of OS. The relationship between autophagy
and tumor behavior is complex; autophagy exerts different
regulatory effects according to the stage of the tumor. At present,
clinical treatments of OS are not ideal. High-dose adjuvant
chemotherapy may induce protective autophagy and lead to drug
resistance, and is accompanied by serious side effects.
Chemotherapy resistance may markedly affect treatment outcomes.
Autophagy-related factors such as mTOR, Beclin-1, miRNA, HMG family
proteins and ATGs are involved in chemoresistance in OS. Modulating
the autophagy pathway to reduce chemoresistance and increase tumor
sensitivity to therapeutic drugs should improve the outcomes of OS.
Although there are no clinical trials on osteosarcoma and
autophagy, the combined application of autophagy inhibitors and
chemotherapeutic drugs is receiving increased attention in the
field of cancer treatment. Autophagy may reverse multidrug
resistance, thereby increasing the sensitivity of tumor cells to
drugs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
Conceptualization and methodology: SW;
investigation: YP and JW; writing-original draft: YP;
writing-review and editing: YP, JW and SW; visualization: SW;
project administration: SW. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu KH, Lu EW, Lin CW, Yang JS and Yang SF:
New insights into molecular and cellular mechanisms of zoledronate
in human osteosarcoma. Pharmacol Ther. 214(107611)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Otoukesh B, Abbasi M, Gorgani HO, Farahini
H, Moghtadaei M, Boddouhi B, Kaghazian P, Hosseinzadeh S and Alaee
A: MicroRNAs signatures, bioinformatics analysis of miRNAs, miRNA
mimics and antagonists, and miRNA therapeutics in osteosarcoma.
Cancer Cell Int. 20(254)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Z, Xu D, Chen X, Li S, Chan MTV and Wu
WKK: LINC01133: An emerging tumor-associated long non-coding RNA in
tumor and osteosarcoma. Environ Sci Pollut Res Int. 27:32467–32473.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mendes AC, Ciccone M, Gazolla B and Bahia
D: Epithelial haven and autophagy breakout in gonococci infection.
Front Cell Dev Biol. 8(439)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ma W, Wei S, Zhang B and Li W: Molecular
mechanisms of cardiomyocyte death in drug-induced cardiotoxicity.
Front Cell Dev Biol. 8(434)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lin Y, Zhao WR, Shi WT, Zhang J, Zhang KY,
Ding Q, Chen XL, Tang JY and Zhou ZY: Pharmacological activity,
pharmacokinetics, and toxicity of timosaponin AIII, a natural
product isolated from anemarrhena asphodeloides bunge: A review.
Front Pharmacol. 11(764)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu W, Meng Y, Zong C, Zhang S and Wei L:
Autophagy and tumorigenesis. Adv Exp Med Biol. 1207:275–299.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Blondy S, David V, Verdier M, Mathonnet M,
Perraud A and Christou N: 5-Fluorouracil resistance mechanisms in
colorectal cancer: From classical pathways to promising processes.
Cancer Sci. 111:3142–3154. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Condello M, Mancini G and Meschini S: The
exploitation of liposomes in the inhibition of autophagy to defeat
drug resistance. Front Pharmacol. 11(787)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Whelan JS and Davis LE: Osteosarcoma,
chondrosarcoma, and chordoma. J Clin Oncol. 36:188–193.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lim J and Murthy A: Targeting autophagy to
treat cancer: Challenges and opportunities. Front Pharmacol.
11(590344)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mele L, Del Vecchio V, Liccardo D, Prisco
C, Schwerdtfeger M, Robinson N, Desiderio V, Tirino V, Papaccio G
and La Noce M: The role of autophagy in resistance to targeted
therapies. Cancer Treat Rev. 88(102043)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang J, Ueharu H and Mishina Y: Energy
metabolism: A newly emerging target of BMP signaling in bone
homeostasis. Bone. 138(115467)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tian Y, Song W, Xu D, Chen X, Li X and
Zhao Y: Autophagy induced by ROS aggravates testis oxidative damage
in diabetes via breaking the feedforward loop linking p62 and Nrf2.
Oxid Med Cell Longev. 2020(7156579)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kulka LAM, Fangmann PV, Panfilova D and
Olzscha H: Impact of HDAC inhibitors on protein quality control
systems: Consequences for precision medicine in malignant disease.
Front Cell Dev Biol. 8(425)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ashrafizadeh M, Tavakol S, Ahmadi Z,
Roomiani S, Mohammadinejad R and Samarghandian S: Therapeutic
effects of kaempferol affecting autophagy and endoplasmic reticulum
stress. Phytother Res. 34:911–923. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Liu F, Liu D, Yang Y and Zhao S: Effect of
autophagy inhibition on chemotherapy-induced apoptosis in A549 lung
cancer cells. Oncol Lett. 5:1261–1265. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tan Q, Liu Y, Deng X, Chen J, Tsai PJ,
Chen PH, Ye M, Guo J and Su Z: Autophagy: A promising process for
the treatment of acetaminophen-induced liver injury. Arch Toxicol.
94:2925–2938. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim WK, Pyee Y, Chung HJ, Park HJ, Hong
JY, Son KH and Lee SK: Antitumor activity of spicatoside A by
modulation of autophagy and apoptosis in human colorectal cancer
cells. J Nat Prod. 79:1097–1104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yecies JL and Manning BD: mTOR links
oncogenic signaling to tumor cell metabolism. J Mol Med (Berl).
89:221–228. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tang D, Loze MT, Zeh HJ and Kang R: The
redox protein HMGB1 regulates cell death and survival in cancer
treatment. Autophagy. 6:1181–1183. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Niu J, Yan T, Guo W, Wang W and Zhao Z:
Insight into the role of autophagy in osteosarcoma and its
therapeutic implication. Front Oncol. 9(1232)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang J, Kou YB, Zhu JS, Chen WX and Li S:
Knockdown of HMGB1 inhibits growth and invasion of gastric cancer
cells through the NF-κB pathway in vitro and in vivo. Int J Oncol.
44:1268–1276. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Berindan-Neagoe I, Monroig Pdel C,
Pasculli B and Calin GA: MicroRNAome genome: A treasure for cancer
diagnosis and therapy. CA Cancer J Clin. 64:311–336.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gulino R, Forte S, Parenti R, Memeo L and
Gulisano M: MicroRNA and pediatric tumors: Future perspectives.
Acta Histochem. 117:339–354. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Llobat L and Gourbault O: Role of
MicroRNAs in human osteosarcoma: Future perspectives. Biomedicines.
9(463)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mutlu H, Mutlu S and Bostancıklıoğlu M:
Profiling of autophagy-associated microRNAs in the osteosarcoma
cell line of U2OS. Anticancer Agents Med Chem. 21:1732–1737.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen R, Wang G, Zheng Y, Hua Y and Cai Z:
Drug resistance-related microRNAs in osteosarcoma: Translating
basic evidence into therapeutic strategies. J Cell Mol Med.
23:2280–2292. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jamali Z, Taheri-Anganeh M, Shabaninejad
Z, Keshavarzi A, Taghizadeh H, Razavi ZS, Mottaghi R, Abolhassan M,
Movahedpour A and Mirzaei H: Autophagy regulation by microRNAs:
Novel insights into osteosarcoma therapy. IUBMB Life. 72:1306–1321.
2020.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Xia H and Hui KM: Mechanism of cancer drug
resistance and the involvement of noncoding RNAs. Curr Med Chem.
21:3029–3041. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang G, Shen N, Cheng L, Lin J and Li K:
Downregulation of miR-22 acts as an unfavorable prognostic
biomarker in osteosarcoma. Tumour Biol. 36:7891–7895.
2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li X, Wang S, Chen Y, Liu G and Yang X:
miR-22 targets the 3' UTR of HMGB1 and inhibits the
HMGB1-associated autophagy in osteosarcoma cells during
chemotherapy. Tumour Biol. 35:6021–6028. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu S, Gong Y, Yin Y, Xing H and Zhang N:
The multiple function of long noncoding RNAs in osteosarcoma
progression, drug resistance and prognosis. Biomed Pharmacother.
127(110141)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pitolli C, Wang Y, Candi E, Shi Y, Melino
G and Amelio I: p53-Mediated tumor suppression: DNA-damage response
and alternative mechanisms. Cancers (Basel).
11(1983)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang W, Li Q, Song C and Lao L: Knockdown
of autophagy-related protein 6, Beclin-1, decreases cell growth,
invasion, and metastasis and has a positive effect on
chemotherapy-induced cytotoxicity in osteosarcoma cells. Tumour
Biol. 36:2531–2539. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xu R, Liu S, Chen H and Lao L:
MicroRNA-30a downregulation contributes to chemoresistance of
osteosarcoma cells through activating Beclin-1-mediated autophagy.
Oncol Rep. 35:1757–1763. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li M, Hou Y, Wang J, Chen X, Shao ZM and
Yin XM: Kinetics comparisons of mammalian Atg4 homologues indicate
selective preferences toward diverse Atg8 substrates. J Biol Chem.
286:7327–7338. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shi M, Zhang T, Sun L, Luo Y, Liu DH, Xie
ST, Song XY, Wang GF, Chen XL, Zhou BC and Zhang YZ: Calpain, Atg5
and Bak play important roles in the crosstalk between apoptosis and
autophagy induced by influx of extracellular calcium. Apoptosis.
18:435–451. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Guo Y, Huang C, Li G, Chen T, Li J and
Huang Z: Paxilitaxel induces apoptosis accompanied by protective
autophagy in osteosarcoma cells through hypoxia-inducible factor-1α
pathway. Mol Med Rep. 12:3681–3687. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Peng B, Xu L, Cao F, Wei T, Yang C, Uzan G
and Zhang D: HSP90 inhibitor, celastrol, arrests human monocytic
leukemia cell U937 at G0/G1 in thiol-containing agents reversible
way. Mol Cancer. 9(79)2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kannaiyan R, Manu KA, Chen L, Li F,
Rajendran P, Subramaniam A, Lam P, Kumar AP and Sethi G: Celastrol
inhibits tumor cell proliferation and promotes apoptosis through
the activation of c-Jun N-terminal kinase and suppression of PI3
K/Akt signaling pathways. Apoptosis. 16:1028–1041. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sethi G, Ahn KS, Pandey MK and Aggarwal
BB: Celastrol, a novel triterpene, potentiates TNF-induced
apoptosis and suppresses invasion of tumor cells by inhibiting
NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB
activation. Blood. 109:2727–2735. 2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang H, Chen D, Cui QC, Yuan X and Dou QP:
Celastrol, a triterpene extracted from the Chinese ‘Thunder of God
Vine,’ is a potent proteasome inhibitor and suppresses human
prostate cancer growth in nude mice. Cancer Res. 66:4758–4765.
2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hou W, Liu B and Xu H: Celastrol:
Progresses in structure-modifications, structure-activity
relationships, pharmacology and toxicology. Eur J Med Chem.
189(112081)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Beauchamp EM and Uren A: A new era for an
ancient drug: Arsenic trioxide and Hedgehog signaling. Vitam Horm.
88:333–354. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hashmi H and Nishihori T: Role of
hematopoietic cell transplantation in relapsed acute promyelocytic
leukemia. Clin Transplant. 34(e14009)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang G, Liu J, Zhang Y, Qu J, Xu L, Zheng
H, Liu Y and Qu X: Cbl-b-dependent degradation of FLIP(L) is
involved in ATO-induced autophagy in leukemic K562 and gastric
cancer cells. FEBS Lett. 586:3104–3110. 2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jóźwiak S, Sadowski K, Kotulska K and
Schwartz RA: Topical use of mammalian target of rapamycin (mTOR)
inhibitors in tuberous sclerosis complex-A comprehensive review of
the literature. Pediatr Neurol. 61:21–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Garza-Lombó C and Gonsebatt ME: Mammalian
target of rapamycin: Its role in early neural development and in
adult and aged brain function. Front Cell Neurosci.
10(157)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Pulakat L and Chen HH: Pro-senescence and
anti-senescence mechanisms of cardiovascular aging: Cardiac
MicroRNA regulation of longevity drug-induced autophagy. Front
Pharmacol. 11(774)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang J, Li X, Zhong M, Wang Y, Zou L, Wang
M, Gong X, Wang X, Zhou C, Ma X and Liu M: miR-301a suppression
within fibroblasts limits the progression of fibrosis through the
TSC1/mTOR pathway. Mol Ther Nucleic Acids. 21:217–228.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cao L and Niu Y: Triple negative breast
cancer: special histological types and emerging therapeutic
methods. Cancer Biol Med. 17:293–306. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Saraf AJ, Fenger JM and Roberts RD:
Osteosarcoma: Accelerating progress makes for a hopeful future.
Front Oncol. 8(4)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kocaturk NM, Akkoc Y, Kig C, Bayraktar O,
Gozuacik D and Kutlu O: Autophagy as a molecular target for cancer
treatment. Eur J Pharm Sci. 134:116–137. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ebrahimi S, Hosseini M, Shahidsales S,
Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM and Avan A:
Targeting the Akt/PI3K signaling pathway as a potential therapeutic
strategy for the treatment of pancreatic cancer. Curr Med Chem.
24:1321–1331. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bernardo PS, Lemos LGT, de Moraes GN and
Maia RC: Unraveling survivin expression in chronic myeloid
leukemia: Molecular interactions and clinical implications. Blood
Rev. 43(100671)2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Waligórska-Stachura J, Jankowska A, Waśko
R, Liebert W, Biczysko M, Czarnywojtek A, Baszko-Błaszyk D, Shimek
V and Ruchała M: Survivin-prognostic tumor biomarker in human
neoplasms-review. Ginekol Pol. 83:537–540. 2012.PubMed/NCBI
|
|
60
|
Khan Z, Khan AA, Yadav H, Prasad GBKS and
Bisen PS: Survivin, a molecular target for therapeutic
interventions in squamous cell carcinoma. Cell Mol Biol Lett.
22(8)2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Coumar MS, Tsai FY, Kanwar JR, Sarvagalla
S and Cheung CH: Treat cancers by targeting survivin: Just a dream
or future reality? Cancer Treat Rev. 39:802–811. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Church DN and Talbot DC: Survivin in solid
tumors: Rationale for development of inhibitors. Curr Oncol Rep.
14:120–128. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Calabrò G, Patalano A, Lo Conte V and
Chianese C: Photodynamic chemotherapy in the treatment of
superficial mycoses: An evidence-based evaluation. G Ital Dermatol
Venereol. 148:639–648. 2013.PubMed/NCBI
|
|
65
|
Carina V, Costa V, Sartori M, Bellavia D,
De Luca A, Raimondi L, Fini M and Giavaresi G: Adjuvant biophysical
therapies in osteosarcoma. Cancers (Basel). 11(348)2019.PubMed/NCBI View Article : Google Scholar
|