Introduction
Immune checkpoint inhibitors represent novel,
promising and effective therapeutic approaches for many cancers.
However, such therapies are frequently followed by immune-related
adverse events that may affect variable systems and organs
including colitis, dermatitis, hepatitis, pneumonitis and
thyroiditis. Most of them occur within the first few months of
immunotherapy. Oncologists may often need collaboration with
physicians from other specialties, like gastroenterologists,
dermatologists, pulmonologists, endocrinologists, in cases of high
grade or persistent toxicity.
Oral cavity may also be affected by drug related
toxicity (1,2). Clinician often describe oral
toxicities with general terminologies like mucositis or stomatitis
(2). Stomatitis has been recorded
as a potential side effect in phase 3 trials with checkpoint
inhibitors like nivolumab (1,3).
However, in these great randomized trials there was no search for
the exact type of stomatitis, perhaps due to the fact that there
were no serious adverse events regarding oral cavity (4). A few cases of oral lichenoid
reactions have been described in the literature as potential
‘stomatitis’ lesions following anti-PD-1 therapy (5-9).
These anti-PD1-related toxicities were histologically consistent
with oral lichenoid reactions, however tended to display a greater
histiocytic infiltrate compared to those described in
nondrug-induced lichenoid responses (8).
Moreover, specific etiologic factors like foreign
materials, infective organisms, and immunologic agents are
responsible for the development of chronic granulomatous
inflammation in oral cavity (10).
Interestingly, anti-PD1 treatment has also been associated with
development of certain granulomatous disease including sarcoid-like
granulomatous reaction, granuloma annulare and granulomatous
inflammation of the pleura (11-13).
Furthermore, lichenoid granulomatous reactions
(LGR), observed in skin and oral mucosa, and reported as lichenoid
granulomatous dermatitis (LGD) and lichenoid granulomatous
stomatitis (LGS), respectively, represent mixed entities that
display a combination of granulomatous inflammation with a
histologic type consistent with lichenoid lesions (14). LGR may evolve as a result of
certain medications or other etiologic factors like common
pathogens or foreign materials (15-18).
This study presents for the first time a case of LGR
in the oral mucosa, as a side-effect of immune checkpoint inhibitor
treatment and it refers to a patient who received nivolumab.
Nivolumab is a known human IgG4 monoclonal antibody that blocks
PD-1 and releases T-cell activated immunity against cancer
cells.
Case report
A 67-year-old female patient was diagnosed 2 years
ago with cutaneous melanoma of inner right thigh, Clark III,
Breslow 3.5 mm, mitotic rate 15/mm2, with ulceration,
pT3bN0M0, stage IIB. Her medical history was free, with absence of
allergies and autoimmune disorders. Following initial diagnosis,
the patient underwent wide excision and sentinel inguinal lymph
node biopsy and was set under regular observation. After one and a
half year, she presented with a suspicious nodule of right groin,
at the area of the sentinel lymph node biopsy scar. There was a
surgical excision and the histological examination revealed
subcutaneous melanoma metastasis. The patient started adjuvant
immunotherapy with nivolumab every two weeks. One week after the
first cycle, she complained of intense mouth pain. Nevertheless,
the patient received the second cycle of nivolumab and was further
evaluated by the oncology team. Stomatitis grade 2 was initially
diagnosed, showing no involvement of face or body skin area. Local
antifungal treatment was prescribed, but stomatitis persisted and
got worse. The patient had severe pain and eating discomfort.
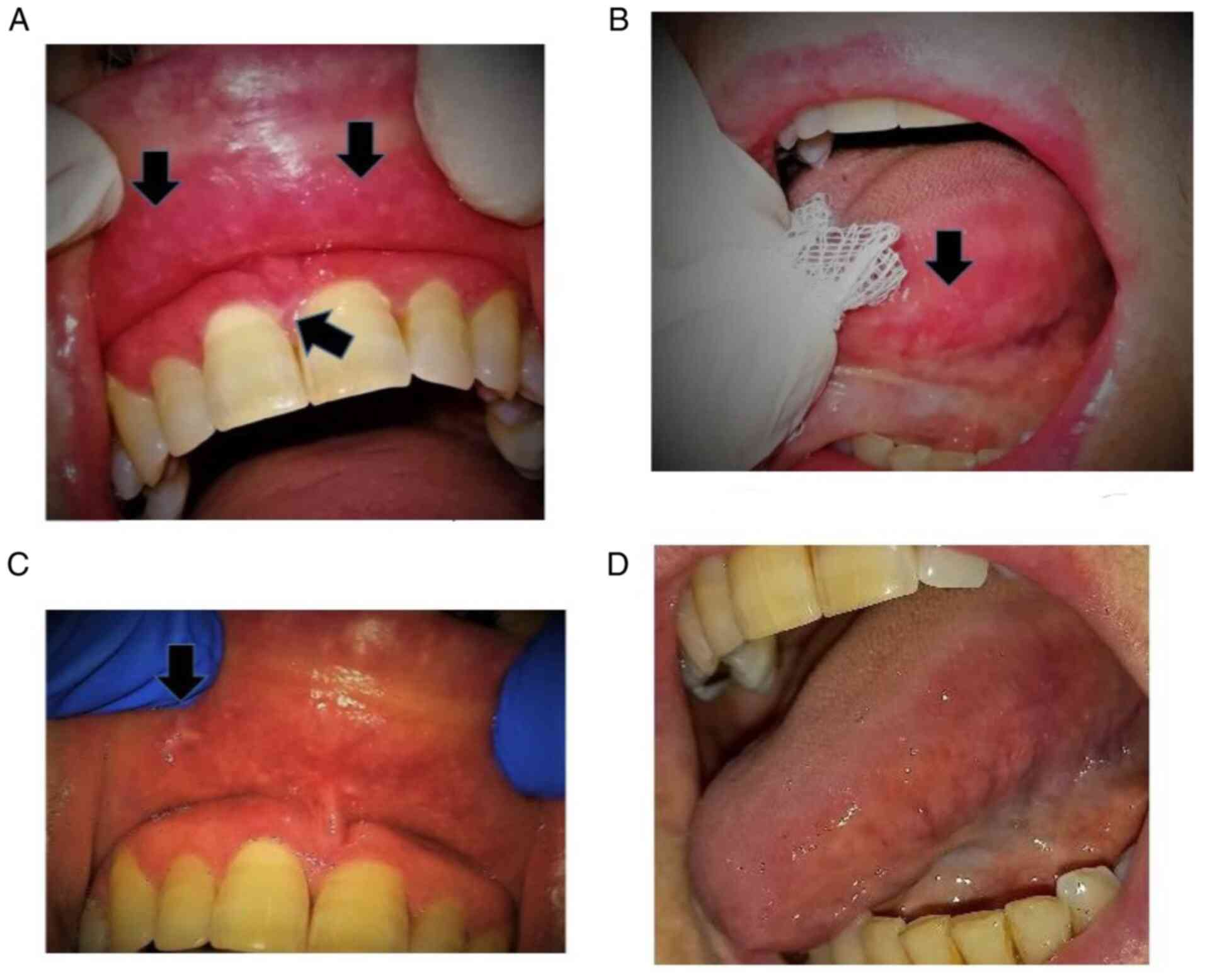
Consequently, she was referred to an Oral Medicine specialist who
observed several atrophic, erythematous areas with central
ulcerations of varying degrees mostly located bilaterally at the
sides of the tongue and at the mucosa of both upper and lower lip.
Moreover, at the periphery of the atrophic regions, a mild white
radiating striae was hardly noticed (Fig. 1).
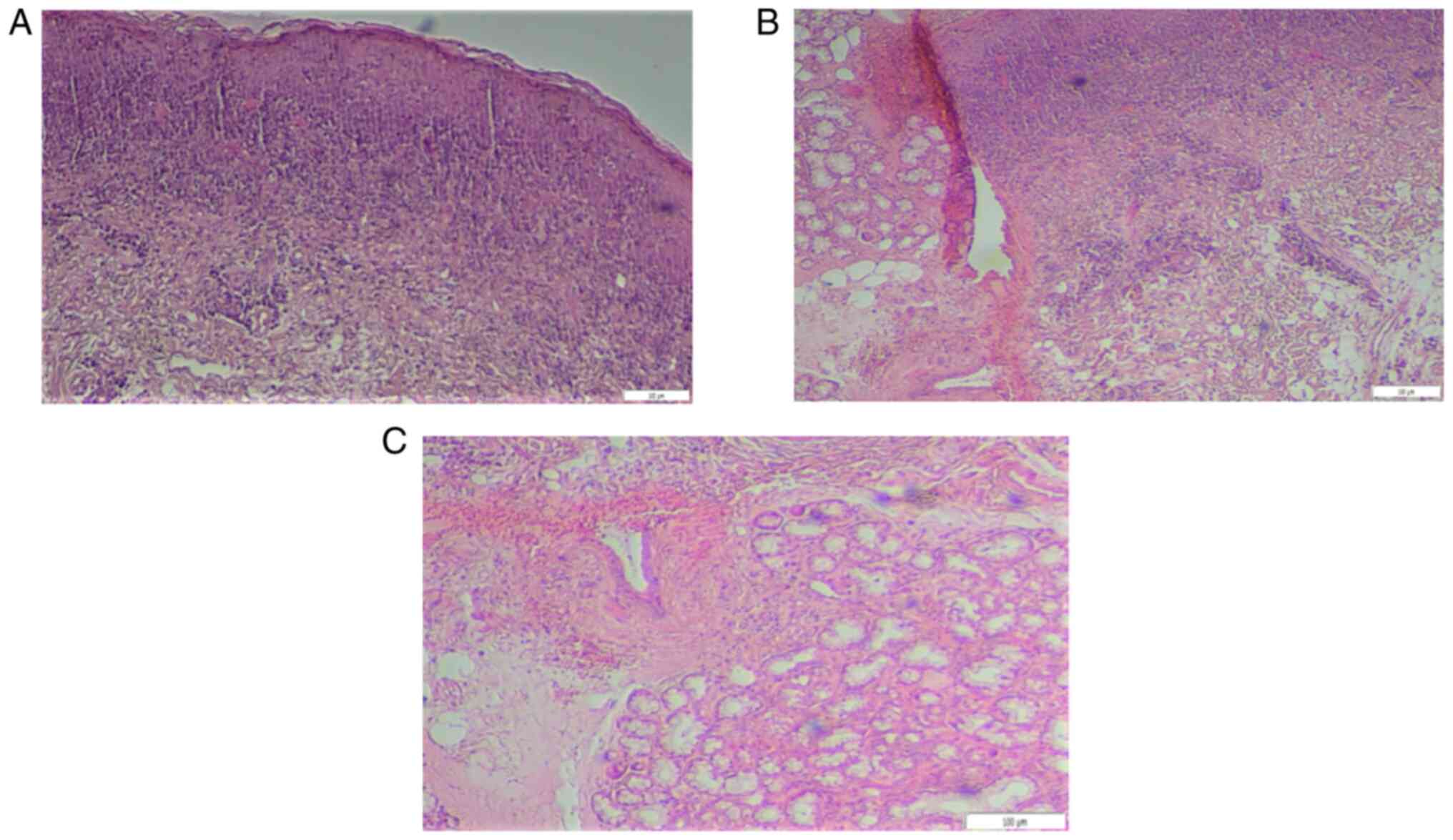
Biopsies were taken from the mucosa of the upper lip
for histological examination and definite diagnosis. Histologic
evaluation revealed mild local hyperkeratosis, mild spongiosis,
formation of colloid bodies, degeneration of the basal layer and a
band- like lymphocyte infiltration, also containing numbers of
histiocytes, neutrophils and eosinophils in the superficial lamina
propria (Fig. 2A). Deeper in the
corium, increased desmoplastic reaction was observed as well as
aggregates of granulomatous inflammation consisting of lymphocytes
and histiocytes (Fig. 2B),
clustering around scattered vessels leading to mild vascular
occlusions (Fig. 2C). Further
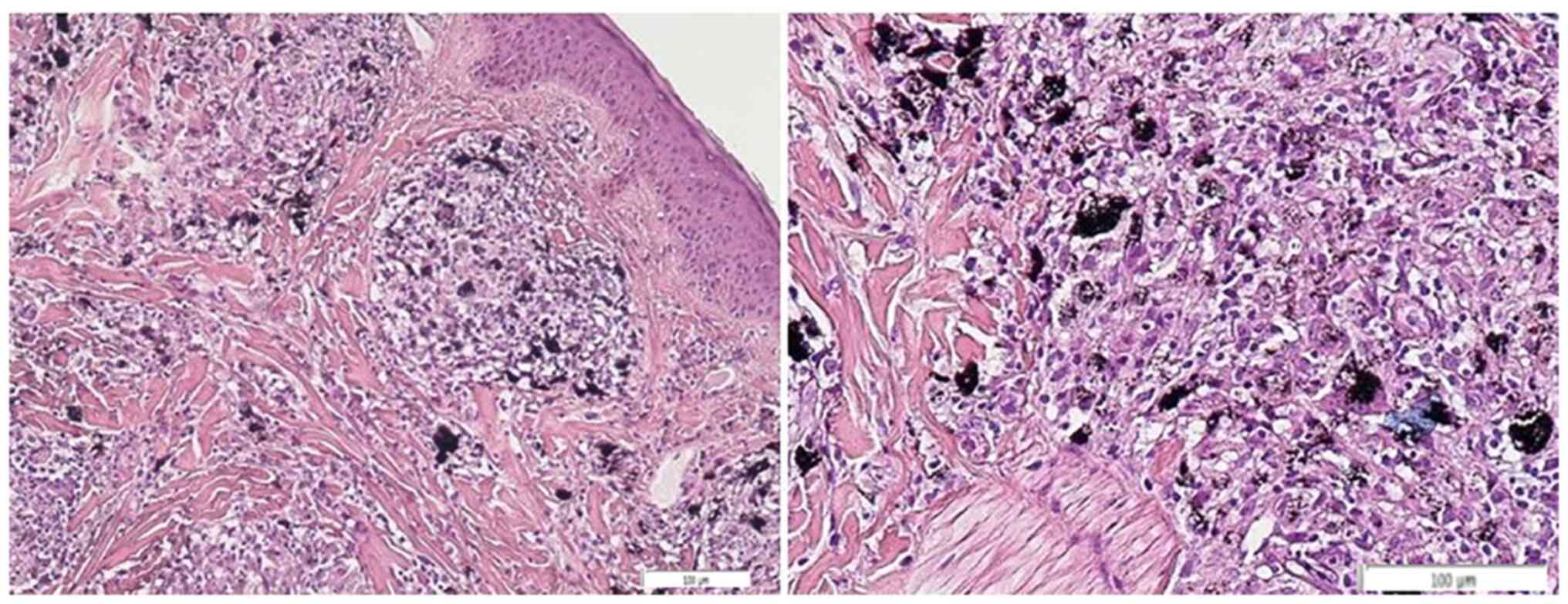
immunohistochemical staining demonstrated CD68-positive cells and
prevalence of CD4 vs. CD8 lymphocytes. Special staining for
microorganisms including acid-fast bacilli (AFB), grocott
methenamine-silver (GMS), and periodic acid-Schiff (PAS) was also
conducted. Positivity was noticed only in GMS staining representing
numbers of bacteria inside the corium and phagocytosised by
histiocytes (Fig. 3).
Following clinical and histopathologic evaluation, a
final diagnosis of LGS was given. The patient was treated with
topical corticosteroids in addition to doxycycline 40 mg twice a
day. After 2 weeks of treatment lesions totally resolved. However,
the patient was so distressed by the whole situation that she
refused to go on with immunotherapy. There was a permanent
discontinuation of nivolumab treatment. Eight months after
treatment discontinuation, the patient is free from malignant
melanoma recurrence.
Discussion
Patients treated with immune checkpoint inhibitors
targeting PD-1 or PD-L1, frequently present specific dermatologic
toxicities including cutaneous lichenoid reactions (8,9,19,20).
Interestingly, oral mucous involvement may often occur while oral
lichenoid reactions (OLR) have only anecdotally been reported
(8,9,20).
Indeed, 7 cases of OLR following anti-PD-1 therapy have already
been described in the English literature (5-9).
The majority of patients suffered from multiple erosions or ulcers
and clinically presented stomatitis of variable grading.
Histopathological examination of the lesions was compatible with
oral lichen planus, demonstrating epithelial necrosis and
spongiosis with a dense band-like layer of inflammatory infiltrate
within the upper mucosa, consisting of lymphocytes and histiocytes
(5-9).
Further examinations like direct immunofluorescence excluded other
related mucocutaneous entities in all cases (5-9).
As a reminding notice, oral lichen planus is characterized by six
clinical variants including reticular, papular, plaque-like,
erosive, atrophic and bullous types. Nevertheless, 6 of the 7
aforementioned cases presented with multiple painful erosions and
ulcers mainly located in soft/hard palate as well as lip and buccal
mucosa (5-7,9).
Subsequent rash treatment included topical or systemic
corticosteroid administration depending on existence of adjacent
cutaneous lesions, while OLR presence variably affected normal
antitumor therapy continuation (5-9).
Although our case appeared clinically as an oral
lichenoid reaction, it was histologically accompanied by
granulomatous infiltration. Therefore, it was finally diagnosed in
the context of a different morphologic spectrum of lichenoid
reaction which is named lichenoid granulomatous reaction. The later
could be divided into LGD and LGS (14). Treatment with nivolumab is the
cause that triggered the lichenoid granulomatous reaction in our
patient, as the first symptoms of stomatitis occurred one week
after the first cycle of therapy, and diagnosis was established a
few weeks later, with symptoms persisting and getting worse despite
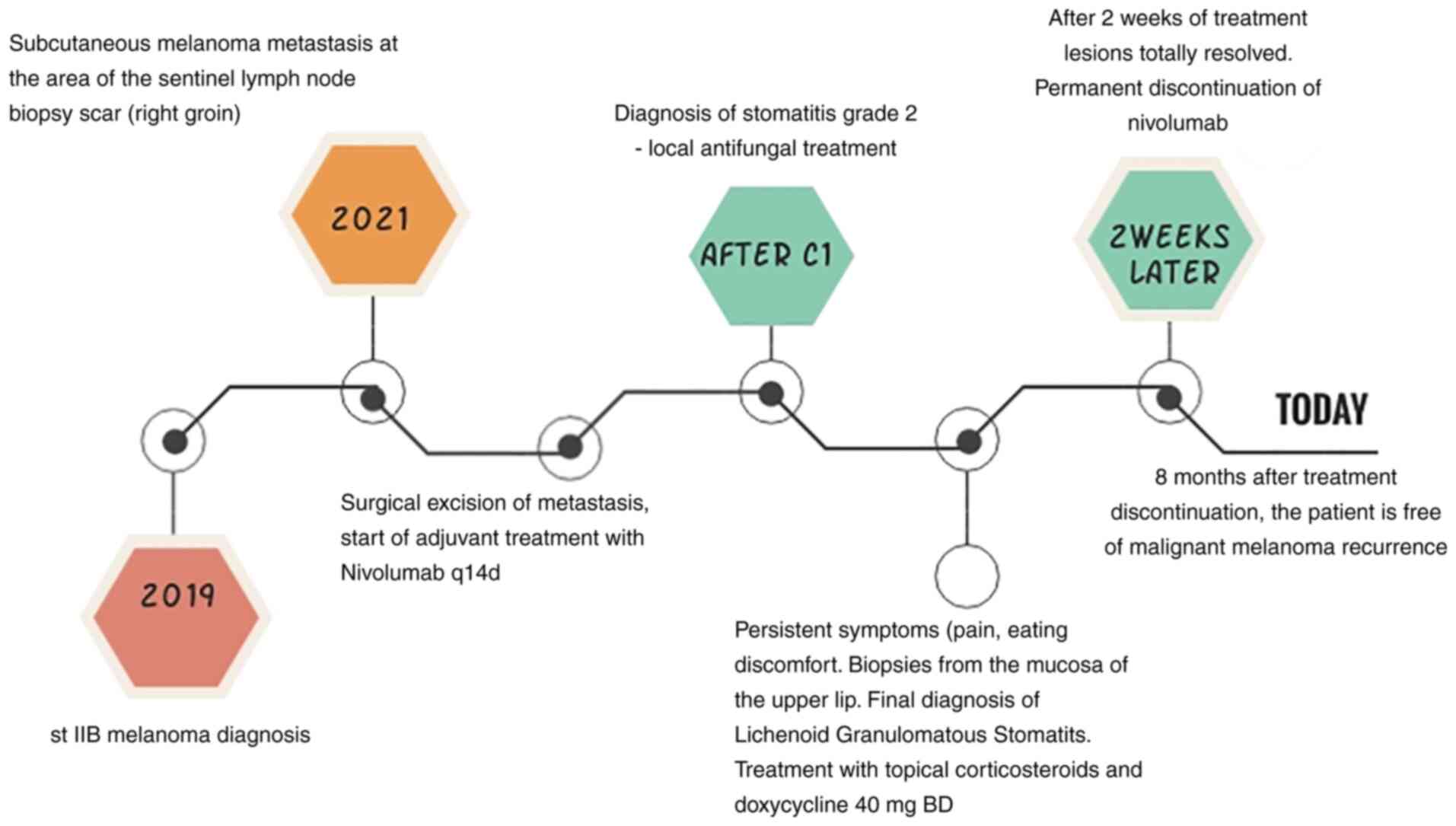
the local antifungal treatment. Fig.
4 represents the patient's course from melanoma diagnosis till
the subsequent initiation of nivolumab as well as the onset and
resolution of stomatitis. This is the first LGS case ever reported
as a side effect of an immune checkpoint inhibitor, while only one
nivolumab-induced LGD case has been also described in 2018(21). Gonzalez et al (16) initially presented this entity in
1986 while Magro and Crowson, in 2000, examined it in 40 patients
(17). Lichenoid granulomatous
reaction shows a histologic profile of band-like lymphocytic
infiltrates in close opposition to the epidermis or mucosa and
clusters of histiocytic or granuloma formation in the dermis or
corium. In addition, Magro and Crowson proposed 5 histologic
patterns according to histiocytic infiltration that included: i)
superficially disposed loose small histiocytic aggregates amidst a
band-like lymphocytic infiltrate; ii) small epithelioid granulomata
within and subjacent to the areas of band-like lymphocytic
infiltration; iii) an interstitial array between collagen fibers
reminiscent of interstitial granuloma annulare; iv) scattered
singly disposed Langhans and/or foreign body type giant cells; and
v) granulomatous vasculitis (17).
Following this classification, the present case may be classified
as type II LGS presenting band-like lymphocytic infiltration with
loose histiocyte aggregates, showing also signs of initiating
perivasculitis.
LGS incidence shows a female predilection (64%)
whereas the most common sites of lesion occurrence include gingiva,
buccal mucosa, vestibule, tongue, lip and palate. Initial clinical
diagnosis may refer to lichen planus, vesiculobullous disease,
leukoplakia, dysplasia, carcinoma in situ and allergy
(14). Furthermore, both LGD and
LGS may evolve as a result of drug eruptions (14,18,22),
cutaneous T-cell lymphoma, immune response to preceding viral
infections or active infections, hepatobiliary disorders and
rheumatoid arthritis (1).
Regarding nivolumab involvement, the specific mechanism that drives
anti-PD-1 mediated drug eruptions is still obscure. However, the
role of PD-1 pathway in the induction and maintenance of immune
tolerance has already been identified (7). On the other hand, anti-PD-1 treatment
enhances T-cell activation by inhibiting the PD-1 suppressive
effect on T cells, thus provoking anti-tumor activity in certain
cancers (23). In addition,
anti-PD-1 therapy enforces antigen recognition and T-cell
proliferation in lymph nodes that induces systemic cytotoxic T-cell
effects that finally involve both normal tissues and cancer cells
(23,24). Nonetheless, it has already been
reported that PD-L1/PD-1 pathways appear to be compromised in oral
lichen planus (25) and therefore
anti-PD-1 administration may further enhance lichenoid reactions.
More recent studies imply that nivolumab treatment is involved in
certain granulomatous eruptions including sarcoid-like
granulomatous reaction, granuloma annulare and granulomatous
inflammation of the pleura (11-13).
In current case report, local bacterial infection or excessive
immune response against local oral microbiome may be the cause of
granuloma formation in addition to the nivolumab-induced T-cell
proliferation that drives the lichenoid reaction. In agreement,
Magro and Crowson also report that lichenoid and granulomatous
infiltrates could occur as a result of active infection, or
idiopathic response to nonviable microbial antigen like microbial
proteins from viruses, mycobacteria, treponema and streptococci
(17).
There are no preventative drugs for stomatitis.
Close follow up of a patient with drug-induced stomatitis is
essential. The patient must be referred to an Oral Medicine
specialist when the problem persists. Topical steroid
administration is the most common LGS therapeutic approach
(14), while in present case
systemic doxycycline was also given due to the microbial
involvement. Doxycycline also has anti-inflammatory action in
granulomatous inflammation (25).
In conclusion, clinicians should be aware that LGD
and LGS are two rare but severe muco-cutaneous lesions that may
occur in patients under anti-PD-1 medication. Since this discomfort
could lead to therapy discontinuation, early diagnosis and proper
management would be of great benefit.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PG and IG conceptualized the study. PG, EAG and AG
curated the data. DNZ and PG designed the methodology and
interpretated the data. PG, EAG, VEK and SI wrote the original
draft and obtained/designed the figures. DT, IG, SD made
substantial contribution to the acquisition of data, reviewed and
edited this manuscript and confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript and
agree to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable. This case report was conducted in
accordance with the 1964 Helsinki Declaration and its later
amendments or comparable ethical standards. Written informed
consent was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
The ORCID iD for Dr Dionysia N. Zouki is
0000-0002-0623-1323.
References
|
1
|
Mishra S, Bajoria AA, Parihar AS, Kochhar
AS, Bhasin R and Bharadwaj A: Evaluation of lichenoid granulomatous
stomatitis cases: A Retrospective Study. J Nat Rem. 21:55–59.
2020.
|
|
2
|
Vigarios E, Epstein JB and Sibaud V: Oral
mucosal changes induced by anticancer targeted therapies and immune
checkpoint inhibitors. Support Care Cancer. 25:1713–1739.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Motzer RJ, Escudier B, George S, Hammers
HJ, Srinivas S, Tykodi SS, Sosman JA, Plimack ER, Procopio G,
McDermott DF, et al: Nivolumab versus everolimus in patients with
advanced renal cell carcinoma: Updated results with long-term
follow-up of the randomized, open-label, phase 3 CheckMate 025
trial. Cancer. 126:4156–4167. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dika E, Lambertini M, Gouveia B, Mussi M,
Marcelli E, Campione E, Gurioli C, Melotti B, Alessandrini A and
Ribero S: Oral manifestations in melanoma patients treated with
target or immunomodulatory therapies. J Clin Med.
10(1283)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Enomoto Y, Nakatani H, Kondo S, Kasai T
and Tsuchiya Y: Drug-induced oral lichenoid reaction during
nivolumab therapy. Int J Oral Maxillofac Surg. 48:488–491.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Namiki T, Hanafusa T, Ueno M, Miura K and
Yokozeki H: Severe oral ulcers associated with nivolumab treatment.
JAMA Dermatol. 153:235–237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Obara K, Masuzawa M and Amoh Y: Oral
lichenoid reaction showing multiple ulcers associated with
anti-programmed death cell receptor-1 treatment: A report of two
cases and published work review. J Dermatol. 45:587–591.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schaberg KB, Novoa RA, Wakelee HA, Kim J,
Cheung C, Srinivas S and Kwong BY: Immunohistochemical analysis of
lichenoid reactions in patients treated with anti-PD-L1 and
anti-PD-1 therapy. J Cutan Pathol. 43:339–346. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shi VJ, Rodic N, Gettinger S, Leventhal
JS, Neckman JP, Girardi M, Bosenberg M and Choi JN: Clinical and
histologic features of lichenoid mucocutaneous eruptions due to
anti-programmed cell death 1 and anti-programmed cell death ligand
1 immunotherapy. JAMA Dermatol. 152:1128–1136. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
James DG: A clinicopathological
classification of granulomatous disorders. Postgrad Med J.
76:457–465. 2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Benn BS, Lombard CM and Krishna G:
Nivolumab-induced granulomatous inflammation of the Pleura. J
Thorac Oncol. 12:e100–e101. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Danlos FX, Pagès C, Baroudjian B,
Vercellino L, Battistella M, Mimoun M, Jebali M, Bagot M, Tazi A
and Lebbé C: Nivolumab-induced sarcoid-like granulomatous reaction
in a patient with advanced melanoma. Chest. 149:e133–e136.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu J, Kwong BY, Martires KJ, Rieger KE,
Chung WH, Iyer GV and Lacouture ME: Granuloma annulare associated
with immune checkpoint inhibitors. J Eur Acad Dermatol Venereol.
32:e124–e126. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hakeem A, Bhattacharyya I, Aljabri M,
Bindakhil M, Pachigar K, Islam MN, Cohen DM and Fitzpatrick SG:
Lichenoid reaction with granulomatous stomatitis: A retrospective
histologic study of 47 patients. J Oral Pathol Med. 48:846–854.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ferguson A, Golden S and Morrison L:
New-onset oral lichen planus and granulomatous cheilitis in a
66-year-old woman. JAAD Case Rep. 2:177–180. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gonzalez JG, Marcus MD and Cruz DJ: Giant
cell lichenoid dermatitis. J Am Acad Dermatol. 15:87–92.
1986.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Magro CM and Crowson AN: Lichenoid and
granulomatous dermatitis. Int J Dermatol. 39:126–133.
2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Robinson CM, Oxley JD, Weir J and Eveson
JW: Lichenoid and granulomatous stomatitis: An entity or a
non-specific inflammatory process? J Oral Pathol Med. 35:262–267.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hofmann L, Forschner A, Loquai C,
Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal
JS, Göppner D, et al: Cutaneous, gastrointestinal, hepatic,
endocrine, and renal side-effects of anti-PD-1 therapy. Eur J
Cancer. 60:190–209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sibaud V, Eid C, Belum VR, Combemale P,
Barres B, Lamant L, Mourey L, Gomez-Roca C, Estilo CL, Motzer R, et
al: Oral lichenoid reactions associated with anti-PD-1/PD-L1
therapies: Clinicopathological findings. J Eur Acad Dermatol
Venereol. 31:e464–e469. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Diaz-Perez JA, Beveridge MG, Victor TA and
Cibull TL: Granulomatous and lichenoid dermatitis after IgG4
anti-PD-1 monoclonal antibody therapy for advanced cancer. J Cutan
Pathol. 45:434–438. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Khelifa-Hamdani E, Touati-Serraj M,
Perriard J, Chavaz P, Saurat JH and Kaya G: Giant cell lichenoid
dermatitis in a patient with baboon syndrome. J Cutan Pathol. 35
(Suppl 1):S17–S19. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Errico A: Immunotherapy: PD-1-PD-L1 axis:
Efficient checkpoint blockade against cancer. Nat Rev Clin Oncol.
12(63)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Villadolid J and Amin A: Immune checkpoint
inhibitors in clinical practice: Update on management of
immune-related toxicities. Transl Lung Cancer Res. 4:560–575.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Costa NL, Gonçalves JAM, de Lima SLG, de
Arruda JAA, Miranda ACC, Mesquita RA, da Silveira ÉJD and Batista
AC: Evaluation of PD-L1, PD-L2, PD-1 and cytotoxic immune response
in oral lichen planus. Oral Diseases. 26:1246–1254. 2020.PubMed/NCBI View Article : Google Scholar
|