Introduction
Glioma is a central nervous system tumor derived
from glial cells. Malignant primary brain tumor remains one of the
most difficult cerebral neoplasms to treat. In recent years,
despite significant progress in treatments such as surgery or
combined chemoradiotherapy, the prognosis of patients with glioma
is still discouraging and the 5-year survival rate is <35%
(1). Glioma, particularly
glioblastoma, has a high degree of malignancy and the survival time
of patients after diagnosis is <15 months. New drugs, delivery
systems, immunotherapy and other technical means are being studied
to identify suitable therapeutic targets in abnormal molecular
pathways (2).
Endoplasmic reticulum (ER) is a network structure of
cytoplasmic parts. Its main functions include protein synthesis,
modification and processing. When cells receive a variety of strong
stimuli, such as nutrient deficiency, Ca2+ metabolism
imbalance, toxin stimulation or persistent oxidative stress, the
homeostasis of cells is broken and ER stress (ERS) is then
activated to restore homeostasis. However, when ERS persists, the
damaged ER is engulfed and degraded (3). Thapsigargin (TG) is a cytotoxic drug
that is able to block the ER Ca2+ ATPase pump, disrupt
Ca2+ homeostasis and initiate ERS (4).
Cyclic adenosine monophosphate responsive element
binding protein 3 like 1 (CREB3L1) protein is a BZIP-type
transcription factor in the CREB/ATF family of cyclic adenylate
response element binding proteins. ERS is a necessary condition for
the activation of CREB3L1, which is highly expressed in osteoblasts
of bone tissue and astrocytes of the central nervous system
(5). CREB3L1 has been indicated to
be abnormally expressed in a variety of tumors and has an important
role (6-8).
In the present study, the expression of CREB3L1 in
glioma tissues of different grades was investigated and the effect
of ERS on the expression of CREB3L1 on the activity and apoptosis
of glioma cells was then investigated in order to provide a
theoretical basis for the diagnosis and treatment of glioma.
Materials and methods
Glioma samples
Formalin-fixed and paraffin-embedded tissue blocks
were collected from 30 patients diagnosed at the Department of
Pathology of Guizhou Medical University (Guiyang, China) between
January 2018 and January 2019, including 8 cases of World Health
Organization (WHO) grade I, 7 cases of WHO grade Ⅱ, 7 cases of WHO
grade Ⅲ and 8 cases of WHO grade Ⅳ. The experimental protocol was
established according to the ethical guidelines of the Helsinki
Declaration and approved by the Human Ethics Committee of Guizhou
Medical University (Guiyang, China) and written informed consent
was obtained from each patient. The demographic and
clinicopathological data of the patients are provided in Table I.
 | Table IDemographic data of the patients by
WHO grade. |
Table I
Demographic data of the patients by
WHO grade.
| Characteristic | Total (n=30) | WHO I (n=8) | WHO Ⅱ (n=7) | WHO Ⅲ (n=7) | WHO Ⅳ (n=8) |
|---|
| Age, years | 46.5 (1.2-85) | 46.5 (20-73) | 47 (1.2-75) | 46 (3.8-66) | 48.5 (3-77) |
| Sex, M/F | 12/18 | 2/6 | 2/5 | 2/5 | 6/2 |
Cell culture and main materials
U87-MG cells (cat. no. CL-0238) were obtained from
Procell Life Science & Technology Co., Ltd. In addition, the
identity of the cell line was verified by short tandem repeat
profiling. Genomic DNA was extracted from U87-MG cells using a
PureLink® Genomic DNA Mini Kit (cat. no. K182001; Thermo
Fisher Scientific, Inc.). A PowerPlex®18D kit (cat. no.
DC1802; Promega Corporation) was used for amplification and an
ABI3500 Genetic Analyzer (cat. no. 3500; Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used for detection. The results
indicated that no cross-contamination of human cells was present in
the U87-MG cell line. U87-MG glioma cells were cultured in DMEM
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and antibiotics (100 µg/ml penicillin and 100 µg/ml streptomycin)
in a humidified atmosphere with 5% CO2 at 37˚C. TG was
purchased from Beyotime Institute of Biotechnology, a Cell Counting
Kit-8 (CCK-8) assay kit was purchased from APeXBIO Technology LLC
and an Annexin Ⅴ-FITC/PI double staining cell apoptosis kit was
purchased from Nanjing KeyGen Biotech Co., Ltd. After 24 h of
incubation, samples were collected for flow cytometry (FCM) and
western blot analysis.
Immunohistochemistry (IHC)
Samples were sectioned at 3 µm thickness with a
conventional pathological microtome, and then the slices were
roasted, dewaxed and hydrated. Antigen retrieval was performed in a
pressure cooker using Tris-EDTA (pH 9.0). Sections were washed in
PBS and treated with 0.3% H2O2 at room
temperature for 15 min. Sections were then blocked in 5% goat serum
(cat. no. C0265; Beyotime Institute of Biotechnology) in PBS at
room temperature for 30 min, followed by incubation with primary
antibody against CREB3L1 (rabbit; cat. no. 11235-2-AP; 1:100
dilution; ProteinTech Group), glucose-regulated protein, 78 kDa
[GRP78, also known as heat shock protein family A (Hsp70) member 5]
(rabbit; Bs-1219R; 1:200 dilution; BIOSS) and C/EBP-homologous
protein (CHOP) (rabbit; Bs-20669R; 1:300 dilution; BIOSS) in
working solution overnight at 4˚C. Subsequently, the samples were
incubated with goat anti-rabbit IgG H&L/HRP (cat. no.
bs-40295G-HRP; dilution, 1:3000; BIOSS) at 37˚C for 30 min.
Colorimetric signals were developed using diaminobenzidine
substrate (Vector Laboratories, Inc.) followed by counterstaining
with hematoxylin, dehydration and sealing with gum. The images,
captured by Olympus BX53 biological microscope (Olympus
Corporation), were analyzed with the ImagePro Plus 6.0 software
package (Media Cybernetics, Inc.) and the expression level of
target protein was calculated as the integrated optical density
(sum)/area (sum).
CCK-8 assay
U87-MG glioma cells in the logarithmic growth phase
were collected and the cell concentration was adjusted to
5x104/ml. The cell suspension (100 µl/well) was
inoculated into 96-well plates and cultured in a 5% CO2
cell incubator at 37˚C for 24 h. Subsequently, 10 µl TG was added
into the wells to result in different final concentrations (0.025,
0.05 or 0.1 µmol/l), while DMSO was added to the negative control
group (final concentration of DMSO, <0.1%). The culture was
continued in the incubator for 6, 12, 24 and 48 h. A total of six
wells were selected from each group at each time-point and CCK-8
reagent (10 µl/well) was added to the wells. After 2 h of
incubation, the absorbance value of each well at 450 nm was
detected by a microplate reader.
FCM
An appropriate amount of U87-MG glioma cells in the
logarithmic growth phase were inoculated into 6-well plates and
treated under the corresponding conditions (0, 0.025, 0.05 or 0.1
µmol/l) for 24 h. The cells were then digested with trypsin without
EDTA, washed twice with 1 ml PBS (with centrifugation at 754.65 x g
for 5 min at room temperature and 1-5x105 cells were
collected. The cells were suspended with 500 µl Binding Buffer,
mixed with 5 µl Annexin Ⅴ-FITC and 5 µl propidium iodide (PI) was
then added. Following mixing, cells were incubated at room
temperature in the dark for 5-15 min. The cells were then analyzed
with a flow cytometer (B47905; Beckman Coulter) within 1 h.
Western blot analysis
Glioma cells treated with different concentrations
of TG for 24 h were collected and then were lysed with RIPA lysis
buffer and the total protein was extracted. The protein
concentration was determined using the BCA method. Total protein
(20 µg per lane) was separated by 10% SDS-PAGE and then transferred
to 0.45 µm PVDF membranes (EMD Millipore), followed by blocking
with 5% skimmed milk (Yili) at room temperature for 1 h. The
membranes were then incubated with primary antibody [GRP78 (cat.
no. bs-1219R; dilution, 1:1,000; BIOSS); CREB3L1 (cat. no.
11235-1-AP; dilution, 1:1,000; ProteinTech Group); Bcl-2 (cat. no.
AB112; dilution, 1:1,000; Beyotime Institute of Biotechnology); and
CHOP (cat. no. Bs-20669R; dilution, 1:1,000; BIOSS)] at the
dilution recommended by the supplier at 4˚C overnight. The next
day, the excess primary antibody was washed off with TBST and the
secondary antibody (goat anti-rabbit IgG (H+L); cat. no.
PMK-014-090M; dilution, 1:5,000; BIOPRIMACY) was added, followed by
incubation at room temperature for 2 h. The bands were visualized
using enhanced chemiluminescence (ChemiScope; Clinx) and the gray
value of the protein was determined with ImageJ v. 1.52 (National
Institutes of Health) for quantification.
Statistical analysis
All data were analyzed using SPSS 25.0 statistical
software (IBM Corporation). The measurement data conforming to a
normal distribution were represented as the mean ± standard
deviation. Multigroup comparisons of the means were performed by
one-way ANOVA followed by a Student-Newman-Keuls post-hoc test and
the GraphPad Prism 8 software package (GraphPad Software, Inc.) was
used to plot the data. P<0.05 was considered to indicate a
statistically significant difference.
Results
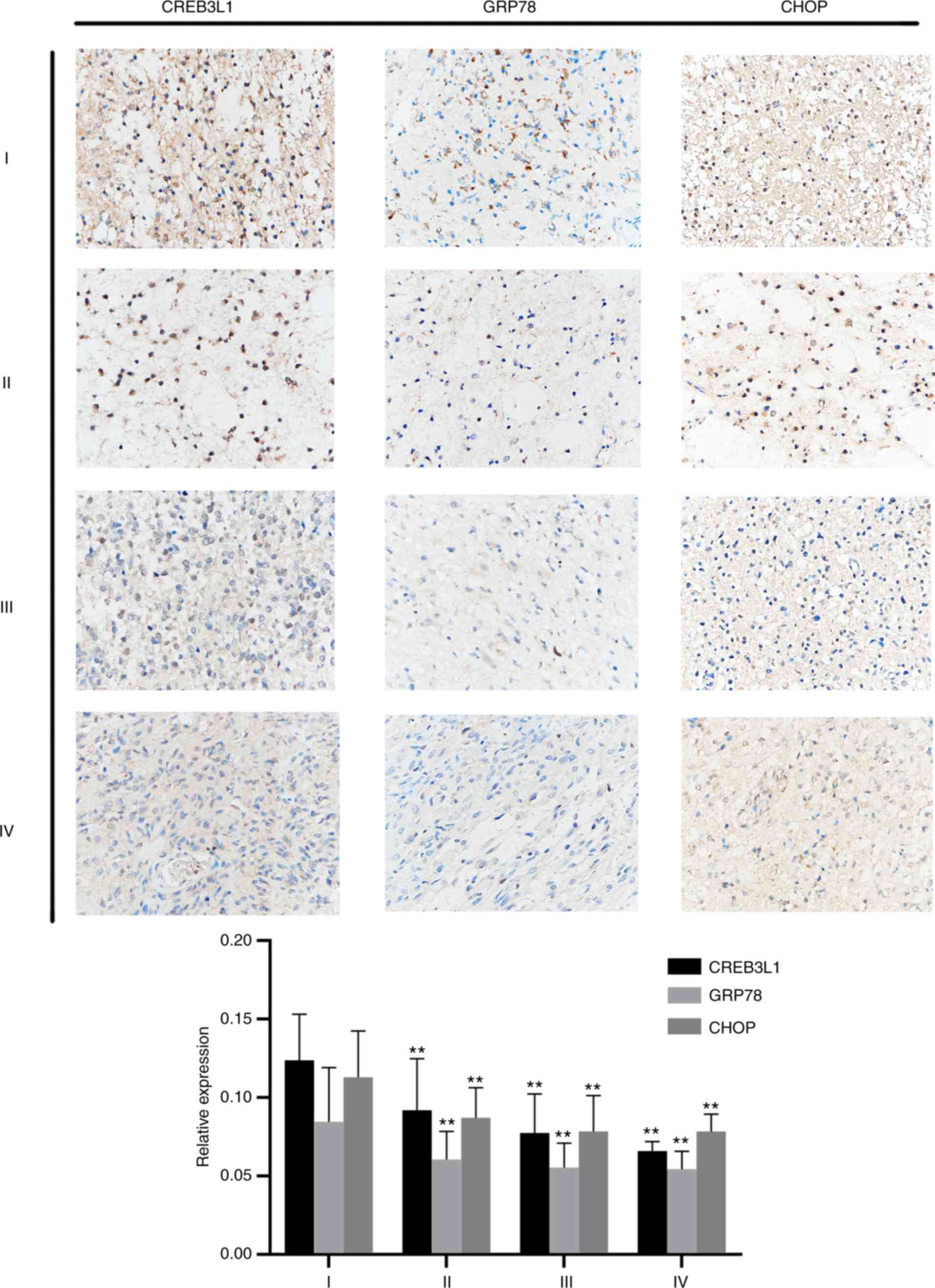
CREB3L1 is associated with the grade
of glioma
The cohort included 12 males and 18 females with a
mean age of 46.5 years (interquartile range, 1.2-85 years).
Positive staining for CREB3L1, GRP78 and CHOP in glioma tissue
sections was mainly present in the cytoplasm and appeared as
brown-yellow granules. As presented in Fig. 1, the expression level of CREB3L1 in
WHO I grade glioma was higher than that in WHO Grade Ⅱ, WHO Grade Ⅲ
and WHO Grade Ⅳ glioma (all P<0.01). The expression level of
GRP78 in WHO I grade was higher than that in WHO grade Ⅱ, WHO grade
Ⅲ and WHO grade Ⅳ glioma (all P<0.01); The expression level of
CHOP in WHO I grade was higher than that in WHO grade Ⅱ, WHO grade
Ⅲ and WHO grade Ⅳ (all P<0.01). These results suggested that
CREB3L1 may be related to the development of glioma (Fig. 1).
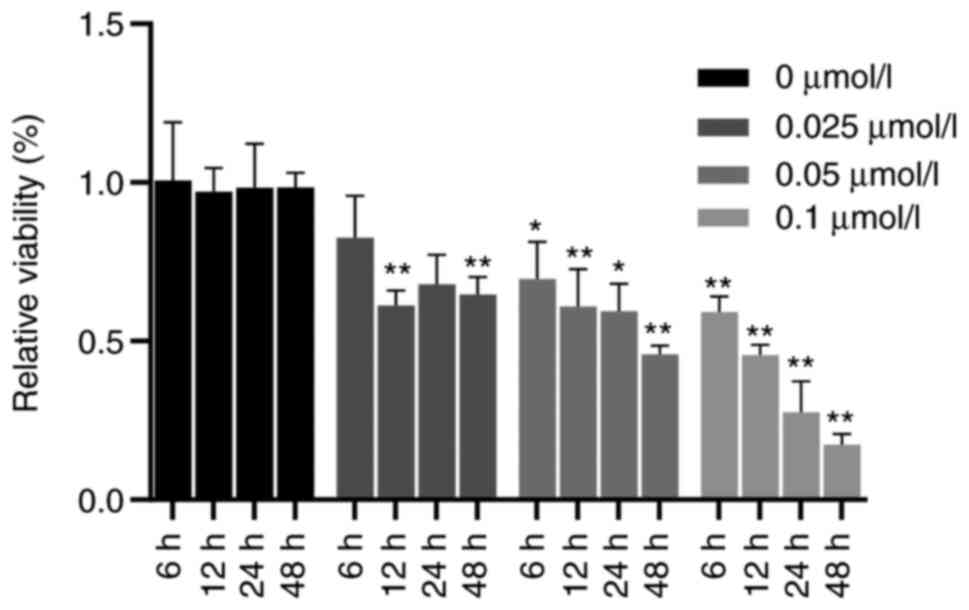
TG reduces the proliferative activity
of glioma cells
The results of the CCK-8 assay indicated that the
proliferative ability of cells treated with TG was significantly
lower than that of the blank group. Compared with that in the
control group, the activity of U87-MG glioma cells was
significantly decreased with the increase of the concentration at
12, 24 and 48 h (all P<0.05). When the concentration of TG was
0.1 µmol/ml, compared with that at 6 h, a longer treatment time led
to a significantly lower growth activity of U87-MG glioma cells
(all P<0.01; Fig. 2). These
results indicated that TG inhibited the proliferation of glioma in
a concentration- and time-dependent manner.
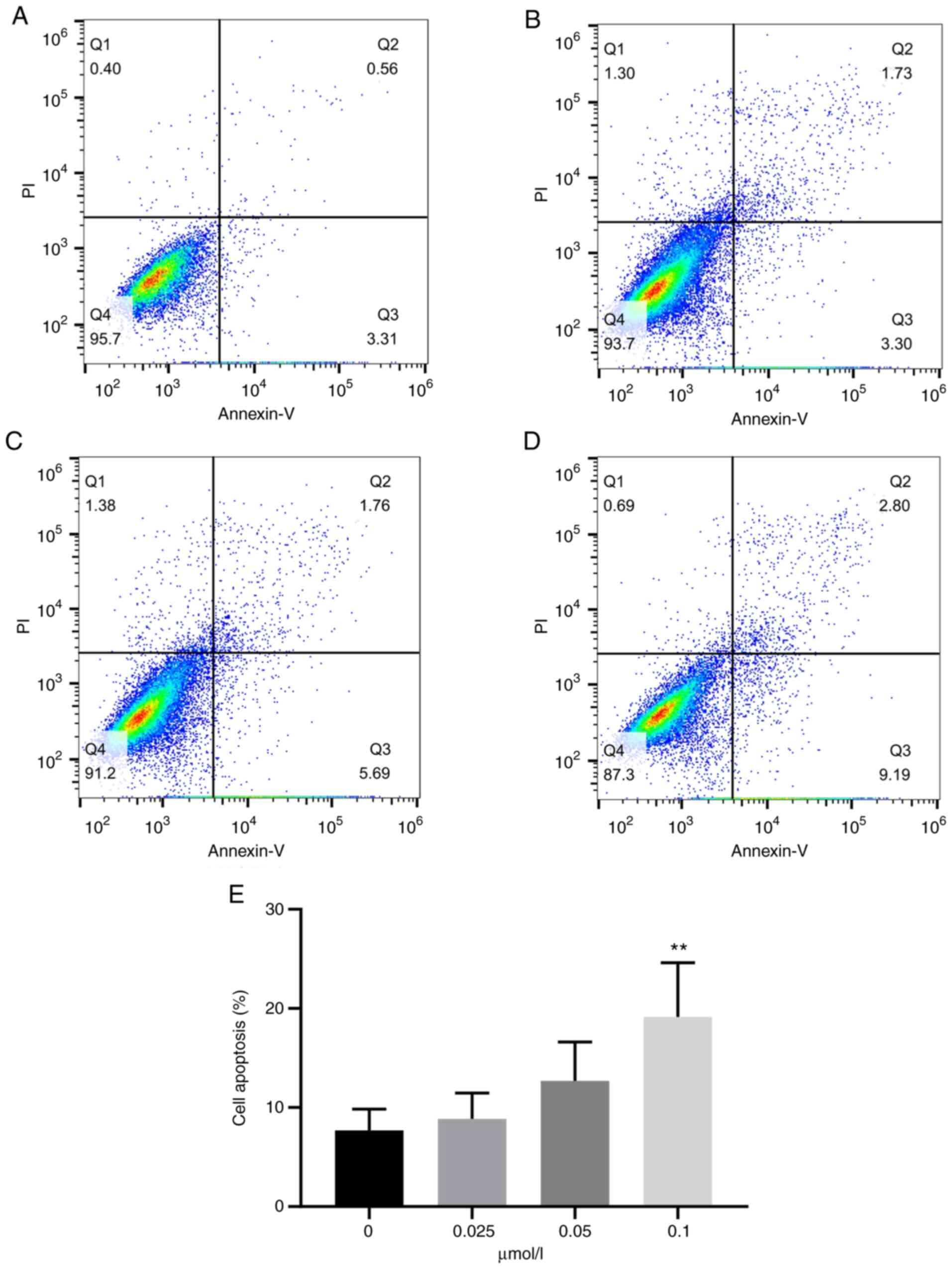
TG promotes apoptosis of U87-MG glioma
cells
In order to further detect the effect of TG on the
apoptosis of U87-MG glioma cells, the apoptotic rate of different
groups of cells was measured by FCM. An Annexin Ⅴ-FITC/PI double
staining kit was used for the experiment. TG was able to obviously
induce apoptosis of glioma U87-MG cells. The apoptosis rate of the
control group was 6.69±2.59% (Fig.
3A). The apoptosis rate of glioma U87-MG cells increased in a
dose-dependent manner with the increase of TG levels (8.87±2.32,
12.68±3.51 and 19.15±4.88%; Fig.
3B-D). When the drug concentration reached 0.1 µmol/l, the
difference was statistically significant (P<0.01). As indicated
in Fig. 3E, the apoptotic rate
rose with increasing doses of TG.
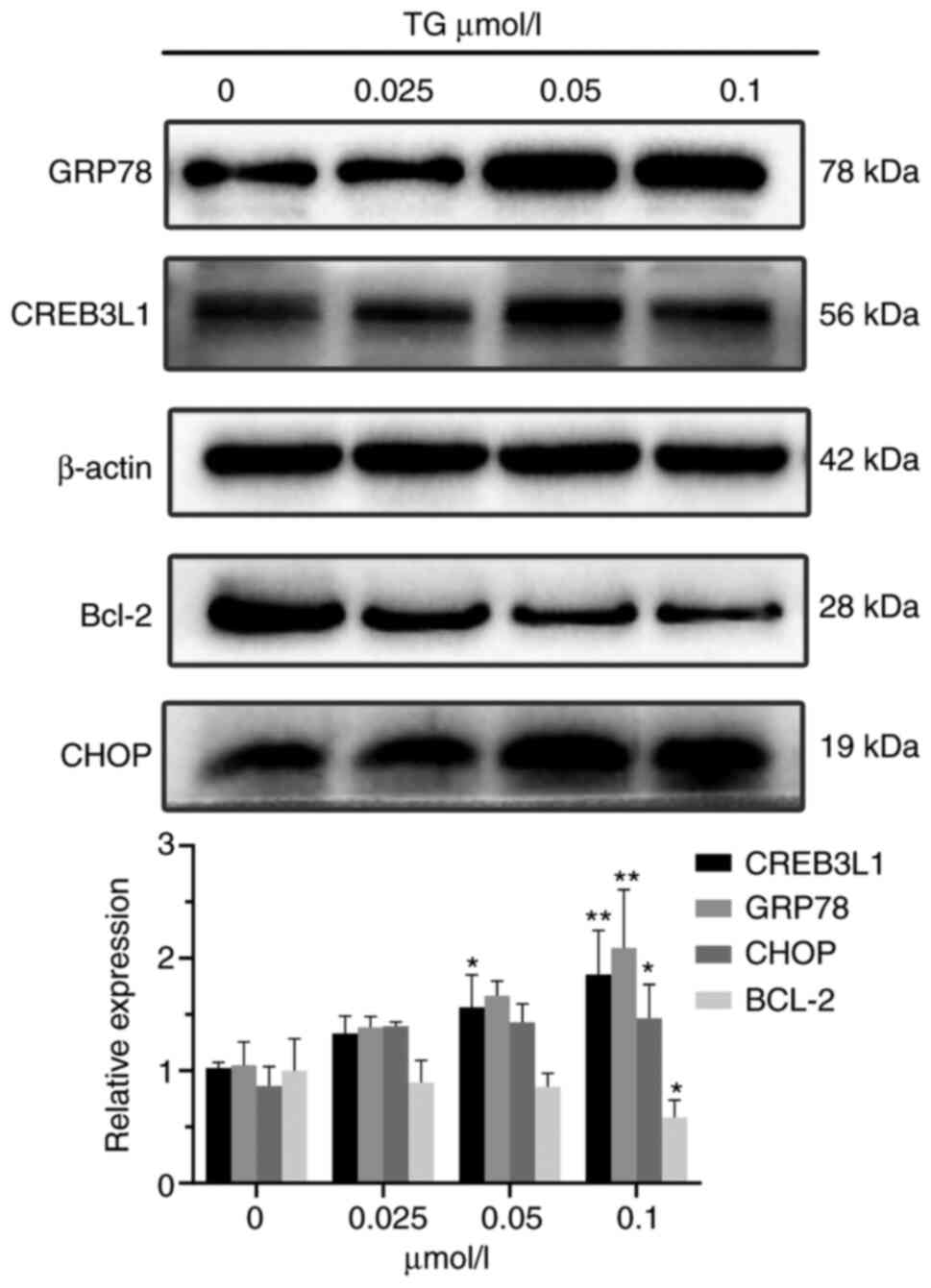
Effects of ERS on apoptosis-related
proteins in glioma cells
Western blot analysis indicated that the expression
levels of GRP78, CREB3L1 and CHOP in glioma cells increased after
ERS, while the expression level of Bcl-2 decreased. When the drug
concentration was 0.1 µmol/l, the differences from the control
group were statistically significant (P<0.05; Fig. 4).
Discussion
Glioma is a highly complex and malignant tumor type
of the central nervous system with a high mortality rate. Due to
factors such as the blood-brain barrier and multi-drug resistance,
the efficacy of chemotherapy for glioma is limited (9). It is of great significance to further
study the molecular mechanisms associated with glioma and its
therapeutic drug targets.
The ERS response is the self-protection mechanism of
stressed cells. When ERS is induced, tumor cells activate the
adaptive mechanism of the unfolded protein response, which is a
comprehensive signaling system that may restore ER homeostasis and
induce cell apoptosis (10).
Apoptosis is a process of programmed cell death under the
stimulation of the internal and external environment. It is also an
important cause of cell death induced by anti-tumor drugs (11). Apoptosis has a negative regulatory
role in the occurrence and development of tumors. The apoptosis
genes known to be related to the occurrence and development of
tumors mainly include the Bcl-2 family, Caspase family and the P53
gene. Bcl-2 is an anti-apoptotic protein in the Bcl-2 family.
Downregulation of Bcl-2 may promote apoptosis of tumor cells, while
overexpression of Bcl-2 may inhibit it (12). It has been indicated that when the
external stimulation was too strong, the ER induces cell apoptosis
through activation of the PKR-like ER kinase (PERK),
inositol-requiring enzyme 1 and activating transcription factor 6
(ATF6) pathways (13). ATF6 is a
transcription factor that is transferred to the Golgi body in
response to ERS, where it is cleaved by site-1 proteases (S1P) and
S2P, activates target gene GRP78 and then indirectly modulates cell
apoptosis through CHOP (14). It
is well known that CHOP is a key pro-apoptotic molecule that may
lead to bodily damage by causing cell cycle arrest and inducing
cell death. CHOP is involved in the PERK-ATF4-CHOP signaling
pathway during cell apoptosis. Bcl-2 family members are also
involved in the process. In addition to the above three signaling
pathways, the CREB3L1 transcription factor, similar to
ATF6(15), is also a type Ⅱ
membrane protein with cytoplasmic N-terminal transcription
(16). As a member of the CREB3
family, ERS is necessary to activate CREB3L1. Kondo et al
(17) indicated that in the
absence of ERS, CREB3L1 (also named OASIS) degraded rapidly in C6
glioma cells and its expression level dropped to 10% within 2 h;
however, the degradation rate of CREB3L1 was significantly
inhibited by ERS and the expression level of CREB3L1 remained at
40-90% within 2 h. This suggests that, although CREB3L1 is unstable
and easily degraded by proteasomes under normal conditions, they
are stabilized by ERS.
TG was originally in public use for the treatment of
rheumatic pain, lung disease and female infertility. Recently, it
was indicated that it is an effective cytotoxin that may induce
cell apoptosis by inhibiting the sarcoplasmic/ER Ca2+
ATPase pump and may be used as a novel type of anti-tumor drug
(18). The results of the present
study suggested that TG was able to inhibit the cell activity of
glioma U87-MG cells in a dose-dependent manner. In order to further
verify the promoting effect of TG on the apoptosis of glioma U87-MG
cells, FCM was used in the present study to determine the apoptotic
rate of cells and the results suggested that TG was able to promote
the apoptosis of glioma cells. In a previous study on rheumatoid
arthritis, TG induced MH7A cells in a time- and dose-dependent
manner (19). TG was able to
effectively impair the cell proliferation and survival of MH7A
cells. It may be assumed that TG is not only able to cause cell
apoptosis when the endoplasmic reticulum stress is high to affect
cell activity but also inhibits cell proliferation through
non-apoptotic pathways. This mechanism may be worthy of further
exploration.
In view of the regulatory effect of ERS on the
biological function of glioma cells, the possible regulatory
mechanism was discussed in the present study. First, according to
IHC analysis, the expression of proteins closely associated with
malignant progression of glioma indicated that certain factors may
induce CREB3L1 gene mutations, reduce CREB3L1 transcription factor
expression, block the pathway of apoptosis and cause excessive
proliferation, eventually leading to an increase in the degree of
malignancy of glioma (7). These
results suggest that CREB3L1 may act as a tumor suppressor gene to
promote apoptosis. Next, western blot analysis further confirmed
that the expression of ERS-related proteins GRP78 and CHOP
increased after TG treatment, indicating that TG may cause ERS,
significantly increase the expression level of CREB3L1 and markedly
decrease the level of Bcl-2. These results suggested that the
apoptosis induced by ERS may be related to CREB3L1. In their study
on breast cancer, Raiter et al (20) indicated that CREB3L1 enhanced the
expression of GRP78 on the surface of triple-negative breast cells
and reduced their ability to migrate and metastasize. Xiao et
al (21) reported that among
patients with soft-tissue sarcoma, the survival rate of patients
with high CREB3L1 expression was significantly higher than that of
patients with low CREB3L1 expression and the expression level of
CREB3L1 was determined as an independent prognostic factor for
survival. In a study on endothelial angiogenesis, CREB3L1 was
indicated to be a bona fide functional target of microRNA-146a,
regulating angiogenesis (22). In
addition, in a study on prostate cancer, inhibition of CREB3L1
expression was observed to promote the proliferation of cancer
cells, which may provide novel possibilities for potential
therapeutic targets for prostate cancer (23).
In conclusion, the present study indicated that
CREB3L1 was highly expressed in glioma tissues and correlated with
the degree of malignancy of the tumor. ERS may affect cell
proliferation and promote cell apoptosis through mediating CREB3L1
expression. However, certain limitations should be noted. First, it
was not possible to evaluate the extent of apoptosis by a second
independent method due to a lack of relevant antibodies.
Furthermore, the effect estimates in the experiments are based on
conventional and prospective observational studies; the
experimental methods are relatively traditional and the results are
mostly in the form of changes in cell phenotypes. Therefore, as for
the specific mechanism through which CREB3L1 affects the occurrence
and development of glioma, additional studies are required to
further clarify the role of CREB3L1 in glioma and provide a new
direction for the diagnosis and treatment of glioma.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the National Natural Science
Foundation of China (grant no. 81560409) and the Science Foundation
of Guizhou Province of China [grant nos. (2014) 6008 and QianKeHe
(2016) support 2905] and the Science and Technology Foundation
approved by Guizhou Provincial Health Commission [no. gzwkj
(2022-090)].
Availability of data and materials
The data generated and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization and methodology: LC, JL and YH.
Validation and investigation (including clinical sample collection
and other related cell experiments): ZY, YZ and QP.
Writing-original draft preparation and writing-review and editing:
JL, LC and ZY. Reading and approval of final manuscript: All
authors. ZY, JL and YZ checked and confirmed the authenticity of
the raw data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Guizhou Medical University (Guiyang, China). Written informed
consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lapointe S, Perry A and Butowski NA:
Primary brain tumours in adults. Lancet. 392:432–446.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chavda V, Patel V, Yadav D, Shah J, Patel
S and Jin JO: Therapeutics and Research Related to Glioblastoma:
Advancements and future targets. Curr Drug Metab. 21:186–198.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Qi Z and Chen L: Endoplasmic reticulum
stress and autophagy. Adv Exp Med Biol. 1206:167–177.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sehgal P, Szalai P, Olesen C, Praetorius
HA, Nissen P, Christensen SB, Engedal N and Møller JV: Inhibition
of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by
thapsigargin analogs induces cell death via ER Ca2+
depletion and the unfolded protein response. J Biol Chem.
292:19656–19673. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chan CP, Kok KH and Jin DY: CREB3
subfamily transcription factors are not created equal: Recent
insights from global analyses and animal models. Cell Biosci.
1(6)2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Denard B, Jiang S, Peng Y and Ye J:
CREB3L1 as a potential biomarker predicting response of triple
negative breast cancer to doxorubicin-based chemotherapy. BMC
Cancer. 18(813)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu LQ, Feng LF, Nan CR and Zhao ZM:
CREB3L1 and PTN expressions correlate with prognosis of brain
glioma patients. Biosci Rep: May 22, 2018 (Epub ahead of
print).
|
|
8
|
Morishita S, Yasuda H, Yamawaki S, Kawaji
H, Itoh M, Edahiro Y, Imai M, Kogo Y, Tsuneda S, Ohsaka A, et al:
CREB3L1 overexpression as a potential diagnostic marker of
Philadelphia chromosome-negative myeloproliferative neoplasms.
Cancer Sci. 112:884–892. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li XT, Tang W, Xie HJ, Liu S, Song XL,
Xiao Y, Wang X, Cheng L and Chen GR: The efficacy of RGD modified
liposomes loaded with vinorelbine plus tetrandrine in treating
resistant brain glioma. J Liposome Res. 29:21–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Markouli M, Strepkos D, Papavassiliou AG
and Piperi C: Targeting of endoplasmic reticulum (ER) stress in
gliomas. Pharmacol Res. 157(104823)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Y, Ma H, Lu Y, Tan BJ, Xu L, Lawal TO,
Mahady GB and Liu D: Menoprogen, a TCM herbal formula for
menopause, increases endogenous E2 in an aged rat model of
menopause by reducing ovarian granulosa cell apoptosis. Biomed Res
Int. 2016(2574637)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014(150845)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Penaranda Fajardo NM, Meijer C and Kruyt
FA: The endoplasmic reticulum stress/unfolded protein response in
gliomagenesis, tumor progression and as a therapeutic target in
glioblastoma. Biochem Pharmacol. 118:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Song S, Tan J, Miao Y, Li M and Zhang Q:
Crosstalk of autophagy and apoptosis: Involvement of the dual role
of autophagy under ER stress. J Cell Physiol. 232:2977–2984.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kondo S, Saito A, Asada R, Kanemoto S and
Imaizumi K: Physiological unfolded protein response regulated by
OASIS family members, transmembrane bZIP transcription factors.
IUBMB Life. 63:233–239. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Vellanki RN, Zhang L and Volchuk A:
OASIS/CREB3L1 is induced by endoplasmic reticulum stress in human
glioma cell lines and contributes to the unfolded protein response,
extracellular matrix production and cell migration. PLoS One.
8(e54060)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kondo S, Hino SI, Saito A, Kanemoto S,
Kawasaki N, Asada R, Izumi S, Iwamoto H, Oki M, Miyagi H, et al:
Activation of OASIS family, ER stress transducers, is dependent on
its stabilization. Cell Death Differ. 19:1939–1949. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jaskulska A, Janecka AE and Gach-Janczak
K: Thapsigargin-from traditional medicine to anticancer drug. Int J
Mol Sci. 22(4)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang H, Jia XZ, Sui CJ, Zhao YP, Mei YF,
Zheng YN and Zhang ZY: Effects of thapsigargin on the proliferation
and survival of human rheumatoid arthritis synovial cells.
ScientificWorldJournal. 2014(605416)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Raiter A, Lipovetsky J, Hyman L, Mugami S,
Ben-Zur T and Yerushalmi R: Chemotherapy controls metastasis
through stimulatory effects on GRP78 and its transcription factor
CREB3L1. Front Oncol. 10(1500)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiao W, Liang Y, Que Y, Li J, Peng R, Xu
B, Wen X, Zhao J, Guan Y and Zhang X: Comparison of the MAID (AI)
and CAV/IE regimens with the predictive value of cyclic
AMP-responsive element-binding protein 3 like protein 1 (CREB3L1)
in palliative chemotherapy for advanced soft-tissue sarcoma
patients. J Cancer. 10:3517–3525. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhu HY, Bai WD, Liu JQ, Zheng Z, Guan H,
Zhou Q, Su LL, Xie ST, Wang YC, Li J, et al: Up-regulation of
FGFBP1 signaling contributes to miR-146a-induced angiogenesis in
human umbilical vein endothelial cells. Sci Rep.
6(25272)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cui X, Cui M, Asada R, Kanemoto S, Saito
A, Matsuhisa K, Kaneko M and Imaizumi K: The androgen-induced
protein AIbZIP facilitates proliferation of prostate cancer cells
through downregulation of p21 expression. Sci Rep.
6(37310)2016.PubMed/NCBI View Article : Google Scholar
|