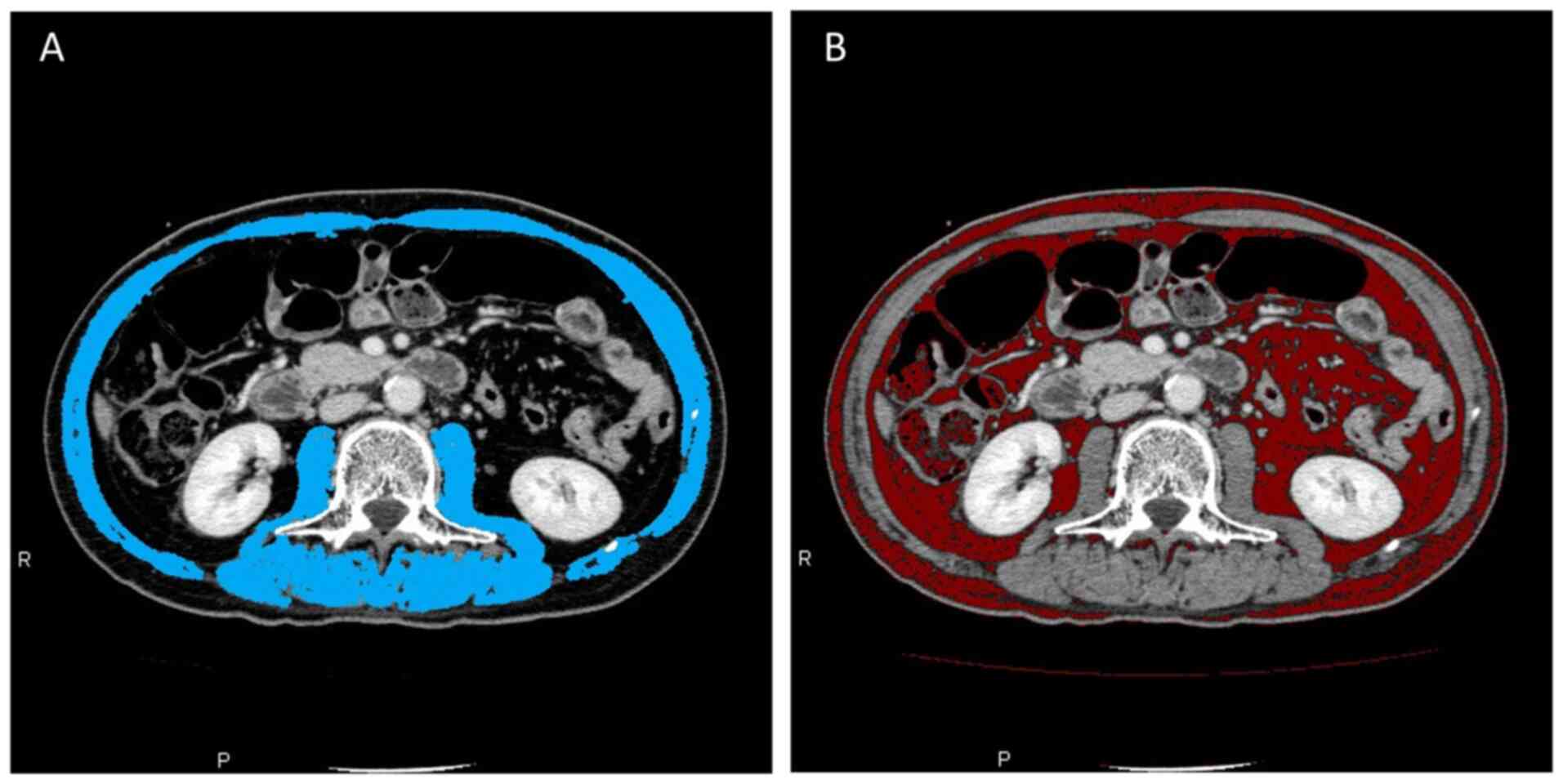
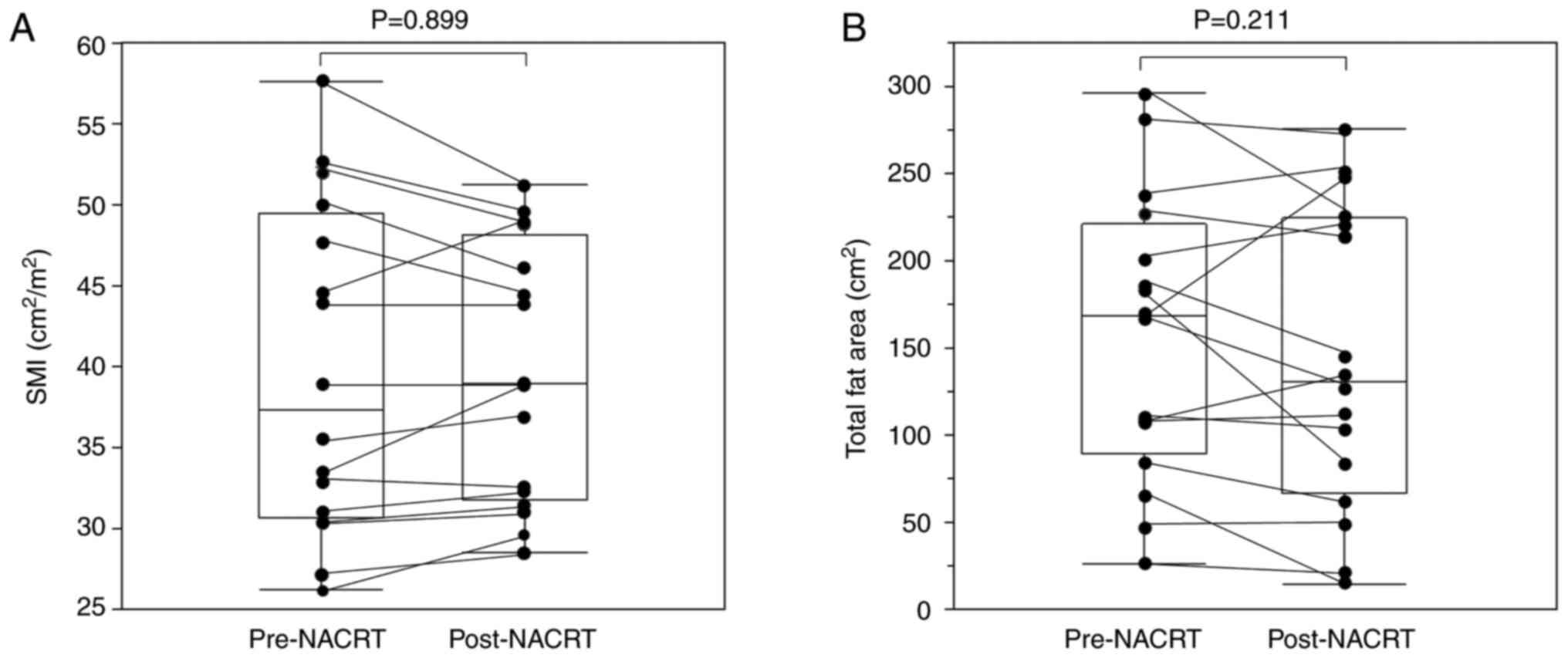
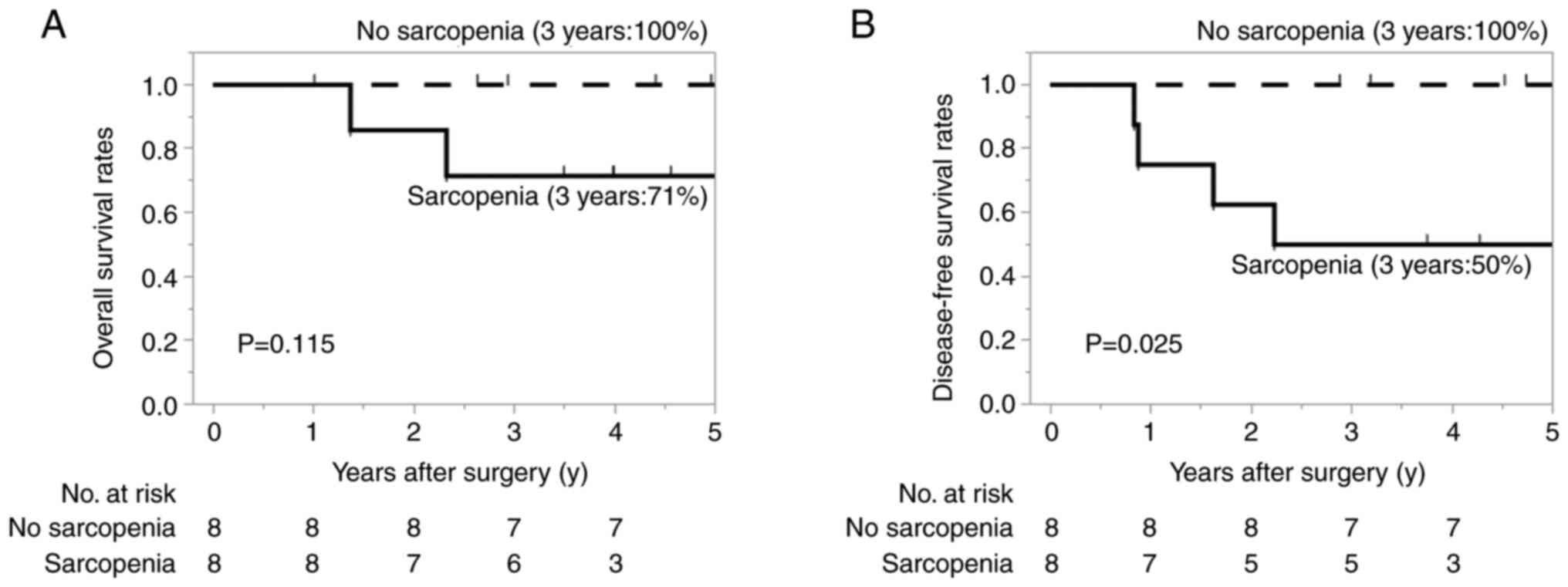
|
1
|
Miyakawa S, Ishihara S, Horiguchi A,
Takada T, Miyazaki M and Nagakawa T: Biliary tract cancer
treatment: 5,584 Results from the biliary tract cancer statistics
registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg.
16:1–7. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kobayashi S, Gotoh K, Takahashi H, Akita
H, Marubashi S, Yamada T, Teshima T, Nishiyama K, Yano M, Ohigashi
H, et al: Clinicopathological features of surgically-resected
biliary tract cancer following chemo-radiation therapy. Anticancer
Res. 36:335–342. 2016.PubMed/NCBI
|
|
3
|
Ohigashi H, Ishikawa O, Eguchi H,
Takahashi H, Gotoh K, Yamada T, Yano M, Nakaizumi A, Uehara H,
Tomita Y and Nishiyama K: Feasibility and efficacy of combination
therapy with preoperative full-dose gemcitabine, concurrent
three-dimensional conformal radiation, surgery, and postoperative
liver perfusion chemotherapy for T3-pancreatic cancer. Ann Surg.
250:88–95. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nishikawa H, Shiraki M, Hiramatsu A,
Moriya K, Hino K and Nishiguchi S: Japan society of hepatology
guidelines for sarcopenia in liver disease (1st edition):
Recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 46:951–963. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yip C, Goh V, Davies A, Gossage J,
Mitchell-Hay R, Hynes O, Maisey N, Ross P, Gaya A, Landau DB, et
al: Assessment of sarcopenia and changes in body composition after
neoadjuvant chemotherapy and associations with clinical outcomes in
oesophageal cancer. Eur Radiol. 24:998–1005. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Awad S, Tan BH, Cui H, Bhalla A, Fearon
KC, Parsons SL, Catton JA and Lobo DN: Marked changes in body
composition following neoadjuvant chemotherapy for oesophagogastric
cancer. Clin Nutr. 31:74–77. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reisinger KW, Bosmans JW, Uittenbogaart M,
Alsoumali A, Poeze M, Sosef MN and Derikx JP: Loss of skeletal
muscle mass during neoadjuvant chemoradiotherapy predicts
postoperative mortality in esophageal cancer surgery. Ann Surg
Oncol. 22:4445–4452. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mirkin KA, Luke FE, Gangi A, Pimiento JM,
Jeong D, Hollenbeak CS and Wong J: Sarcopenia related to
neoadjuvant chemotherapy and perioperative outcomes in resected
gastric cancer: A multi-institutional analysis. J Gastrointest
Oncol. 8:589–595. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Levolger S, Van Vledder MG, Alberda WJ,
Verhoef C, De Bruin RWF, Ijzermans JNM and Burger JW: Muscle
wasting and survival following pre-operative chemoradiotherapy for
locally advanced rectal carcinoma. Clin Nutr. 37:1728–1735.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: Cisplatin plus gemcitabine versus gemcitabine for
biliary tract cancer. N Engl J Med. 362:1273–1281. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Okusaka T, Ishii H, Funakoshi A, Yamao K,
Ohkawa S, Saito S, Saito H and Tsuyuguchi T: Phase II study of
single-agent gemcitabine in patients with advanced biliary tract
cancer. Cancer Chemother Pharmacol. 57:647–653. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Otsuji H, Yokoyama Y, Ebata T, Igami T,
Sugawara G, Mizuno T and Nagino M: Preoperative sarcopenia
negatively impacts postoperative outcomes following major
hepatectomy with extrahepatic bile duct resection. World J Surg.
39:1494–1500. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pamoukdjian F, Bouillet T, Levy V, Soussan
M, Zelek L and Paillaud E: Prevalence and predictive value of
pre-therapeutic sarcopenia in cancer patients: A systematic review.
Clin Nutr. 37:1101–1113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mathur A, Pitt HA, Marine M, Saxena R,
Schmidt CM, Howard TJ, Nakeeb A, Zyromski NJ and Lillemoe KD: Fatty
pancreas: A factor in postoperative pancreatic fistula. Ann Surg.
246:1058–1064. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sandini M, Bernasconi DP, Ippolito D,
Nespoli L, Baini M, Barbaro S, Fior D and Gianotti L: Preoperative
computed tomography to predict and stratify the risk of severe
pancreatic fistula after pancreatoduodenectomy. Medicine
(Baltimore). 94(e1152)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chakedis J, Spolverato G, Beal EW, Woelfel
I, Bagante F, Merath K, Sun SH, Chafitz A, Galo J, Dillhoff M, et
al: Pre-operative sarcopenia identifies patients at risk for poor
survival after resection of biliary tract cancers. J Gastrointest
Surg. 22:1697–1708. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cooper AB, Slack R, Fogelman D, Holmes HM,
Petzel M, Parker N, Balachandran A, Garg N, Ngo-Huang A,
Varadhachary G, et al: Characterization of anthropometric changes
that occur during neoadjuvant therapy for potentially resectable
pancreatic cancer. Ann Surg Oncol. 22:2416–2423. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shachar SS, Williams GR, Muss HB and
Nishijima TF: Prognostic value of sarcopenia in adults with solid
tumours: A meta-analysis and systematic review. Eur J Cancer.
57:58–67. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Okumura S, Kaido T, Hamaguchi Y, Fujimoto
Y, Kobayashi A, Iida T, Yagi S, Taura K, Hatano E and Uemoto S:
Impact of the preoperative quantity and quality of skeletal muscle
on outcomes after resection of extrahepatic biliary malignancies.
Surgery. 159:821–833. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Eriksson S, Nilsson JH, Strandberg Holka
P, Eberhard J, Keussen I and Sturesson C: The impact of neoadjuvant
chemotherapy on skeletal muscle depletion and preoperative
sarcopenia in patients with resectable colorectal liver metastases.
HPB (Oxford). 19:331–337. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pratesi A, Tarantini F and Di Bari M:
Skeletal muscle: An endocrine organ. Clin Cases Miner Bone Metab.
10:11–14. 2013.PubMed/NCBI View Article : Google Scholar
|