Introduction
Ovarian cancer (OC) is the third most common
gynecological cancer and the second leading cause of gynecological
cancer deaths globally (1). The
most prominent risk factor for OC is the familial accumulation of
breast and/or ovarian cancer cases. On average, germline mutations
in the high-risk genes BRCA1 and BRCA2 are detected
in 42% of affected families with OC (2). The cumulative OC risk in patients who
are 80 years old or older is 44% (95% CI, 36-53%) for BRCA1
and 17% (95% CI, 11-25%) for BRCA2 carriers (3). Patients with germline BRCA
mutations demonstrate different clinical characteristics from those
with non-BRCA-associated OCs, including younger age at
diagnosis, advanced stage at presentation, high-grade histology and
optimal response to platinum-based chemotherapy (4). Although BRCA1/2 mutation
carriers show improved prognosis compared with non-carriers
(5), approximately 70-85% of
patients relapse. The median survival for patients with recurrent
disease ranges from 12 to 24 months (6). The overall survival (OS) rates have
not significantly increased for these patients over the past 30
years. The use of carboplatin and paclitaxel as standard
chemotherapy was implemented in 2003. Moreover, surgical complete
resection is recognized as the most important prognostic factor
that can influence the outcome of the disease. Since 2003, no
significant improvements have been achieved (7,8), and
the initial attempts at targeted therapy did not achieve a
breakthrough. This was dramatically altered with the introduction
of poly(ADP-ribose) polymerase (PARP) inhibitors (PARPi), which are
a new class of antineoplastic targeted agents (9). Their function is based on the concept
of synthetic lethality with a defect of the homologous
recombination repair (HRR) (10).
Patients with germline mutations in BRCA1 or BRCA2
have profound deficits in homologous recombination (HRD) and,
therefore, benefit the most in clinical trials with PARPi (11-13,15).
It was recently demonstrated that patients benefit from PARPi
regardless of their BRCA status or HRD (16).
In the present study, the exceptional case of a
BRCA2 mutation carrier who was diagnosed in 2008 with
advanced OC (FIGO IIIC; Fédération Internationale de Gynécologie et
d'Obstétrique, version before 01.01.2014) is reported. Following
the second relapse in 10/2010, the patient was enrolled in one of
the first PARPi studies (ICEBERG 2, AZ D810C00042, AZD2281;
KU-0059436; https://clinicaltrials.gov/ct2/show/results/NCT00494442?term=olaparib&recrs=e&cond=ovarian+cancer&draw=1&rank=2)
and has remained recurrence-free for >10 years.
Case report
A 67-year-old Caucasian woman presented with an
extensive relapse of a high-grade serous ovarian carcinoma (HGSOC)
in December 2010 at the Centre of Familial Breast and Ovarian
Cancer Cologne. The patient had a serum cancer antigen 125 (CA-125)
level of 42 kU/l. In 07/2008, she was first diagnosed with HGSOC
(stage FIGOIIIc, M0) and she underwent primary debulking surgery
(median laparotomy with total abdominal hysterectomy, bilateral
salpingo-oopohorectomy, omentectomy, appendectomy, dissection of
pelvic lymph nodes, resection of the peritoneum) with complete
tumor resection followed by standard adjuvant chemotherapy with six
cycles of carboplatin (AUC5) and paclitaxel (175 mg/m²).
Following a platinum-free interval (PFI) of 12
months, the patient was diagnosed in 01/2010 with the first relapse
and was administered re-induction chemotherapy containing six
cycles of carboplatin (AUC5) and paclitaxel (175 mg/m²). After
three cycles of chemotherapy, she developed a tumor-associated
ischaemic covered colon perforation. She underwent a partial colon
resection with end-to-end anastomosis and local tumour debulking,
which showed histologically vital tumour tissue. She then completed
chemotherapy with three further cycles until 05/2010. Her second
relapse was diagnosed in 10/2010 following a 5-month PFI.
Specifically, the patient developed progressive subdiaphragmal
peritoneal metastases compared to 03/2010, newly diagnosed
peritoneal metastases on the liver, ascites and right-sided pelvic
nodular lesions, as well as metastastic spread below the abdominal
wall 1.7 cm above the umbilicus (Fig.
1A-H). Since clinically there was no doubt about the presence
of a second recurrence due to the tumour spread pattern,
furthermore there was still no possibility of operating on the
patient macroscopically completely tumour-free and as the patient
was to be saved expected complications, histological confirmation
of the second recurrence was not performed.
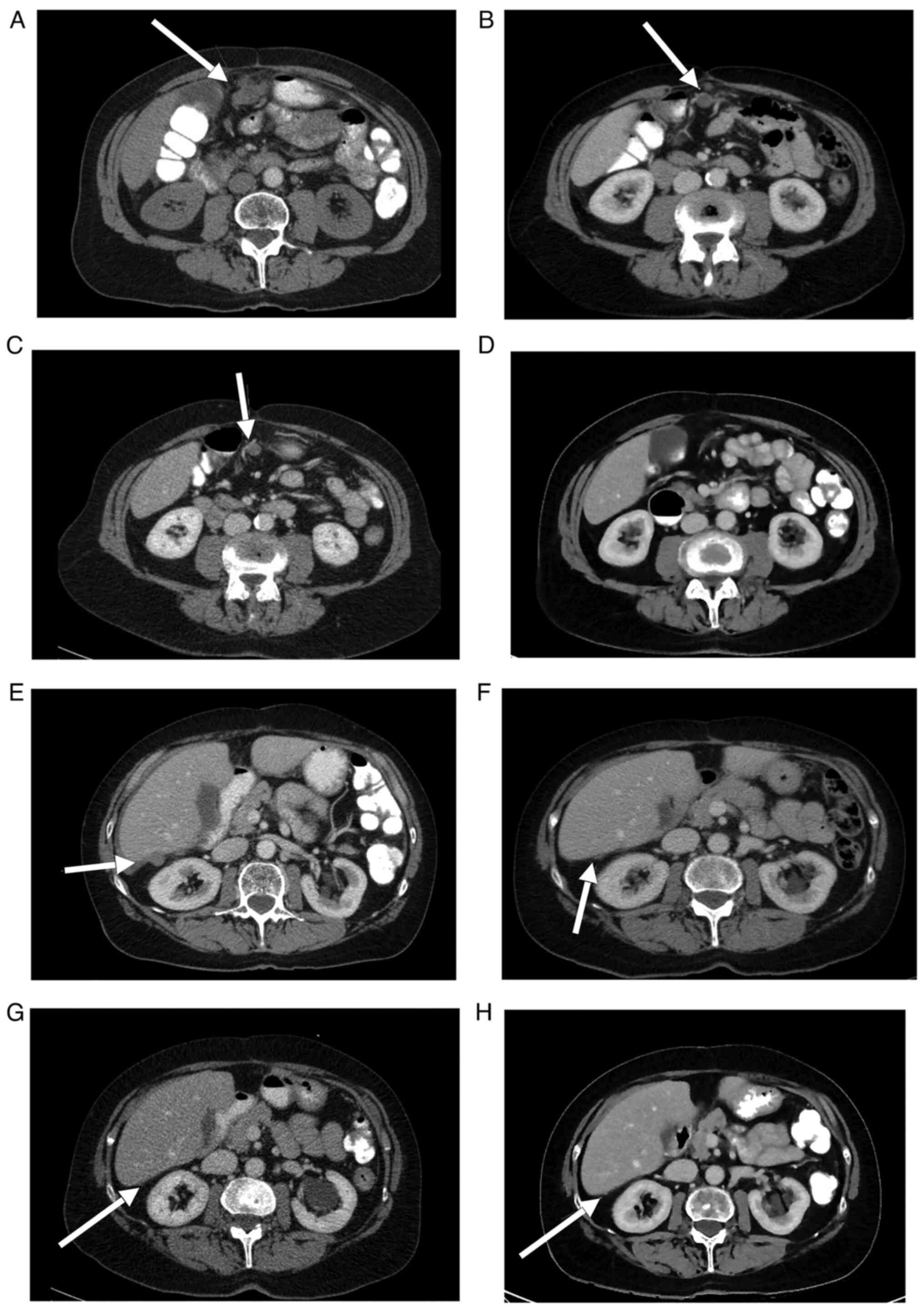 | Figure 1(A-D) Computed tomography imaging
demonstrating the regression of a lesion of peritoneal
carcinomatosis on the anterior abdominal wall (arrows) prior to the
initiation of PARPi therapy on January 13, 2011 and the patient
follow-up on March 2, 2011, November 7, 2012 and November 30, 2015.
(E-H) Computed tomography imaging indicating the regression of a
perihepatic lesion of peritoneal carcinomatosis (arrows) prior to
initiation of PARPi therapy on January 13, 2011 and the follow-up
on March 2, 2011, November 7, 2012 and November 30, 2015. |
Due to her cancer history, which was associated with
a significant familial cancer burden, a BRCA1/2 germline
analysis was recommended. The patient suffered from bilateral
breast cancer of the left side in 1987 and of the right side in
1999. She was treated with bilateral mastectomy and double CMF
chemotherapy followed by endocrine therapy from 1987 to 1992 (20 mg
tamoxifen/d) and from 1999 to 2002 (2.5 mg letrozol/d). Her family
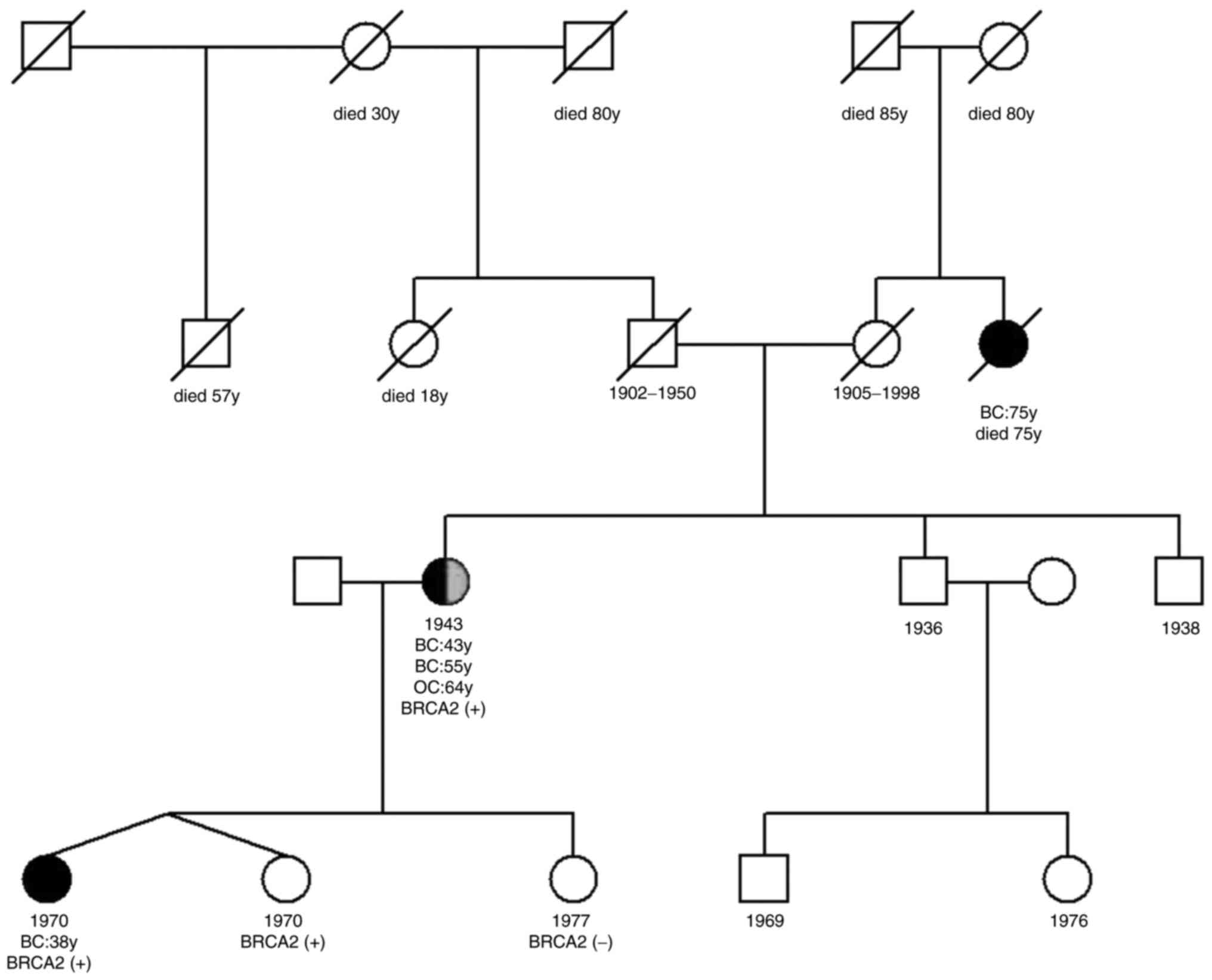
history indicated that one of her two dizygotic twin daughters,
born in 1970, was diagnosed with a triple-negative breast cancer at
the age of 38 years, and her maternal aunt was diagnosed with
breast cancer at the age of 75 years (Fig. 2). The patient demonstrated a
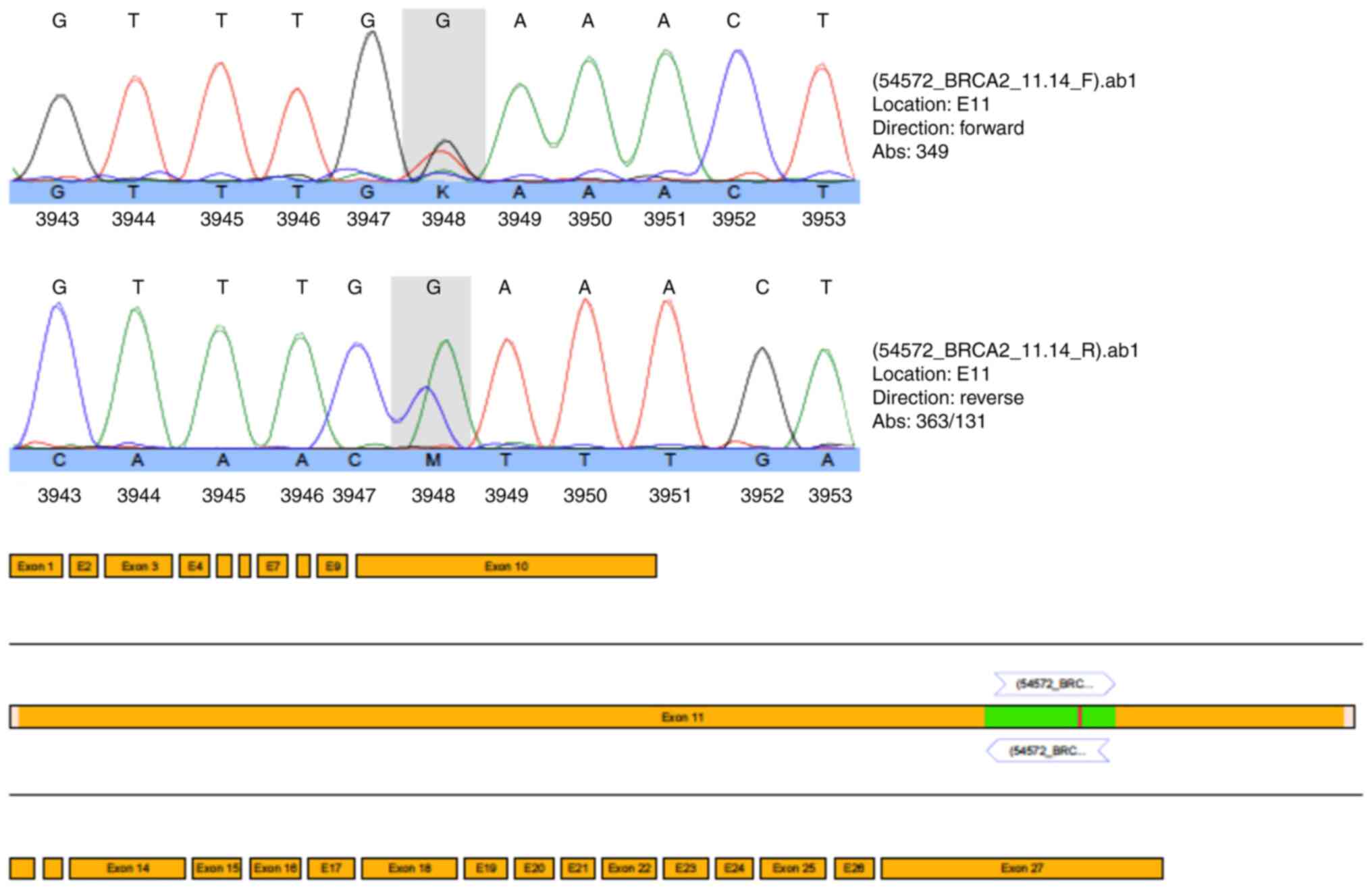
heterozygous pathogenic nonsense mutation (c.5857G>T
(p.Glu1953X) in exon 11 of the BRCA2 gene (Fig. 3). Due to the mutation status and
after obtaining her written informed consent, the patient was
included in the ICEBERG 2 study (phase 2 study to assess the
efficacy and safety of the PARPi olaparib for the treatment of
BRCA-positive advanced OC) in January 2011, with 400 mg
olaparib/2x per day prior to cytoreductive surgery or chemotherapy.
The ethical approval regarding the inclusion of patients in the
study was received from the Ethics Committee of the University
Hospital of Cologne (EudraCT-Nr. 2010-022278-15, Protokoll-Nr.
D0810C00042). During the first staging period, which occurred 8
weeks after enrollment, the patient exhibited a partial response as
evidenced by CT imaging (small residual findings) and decreased
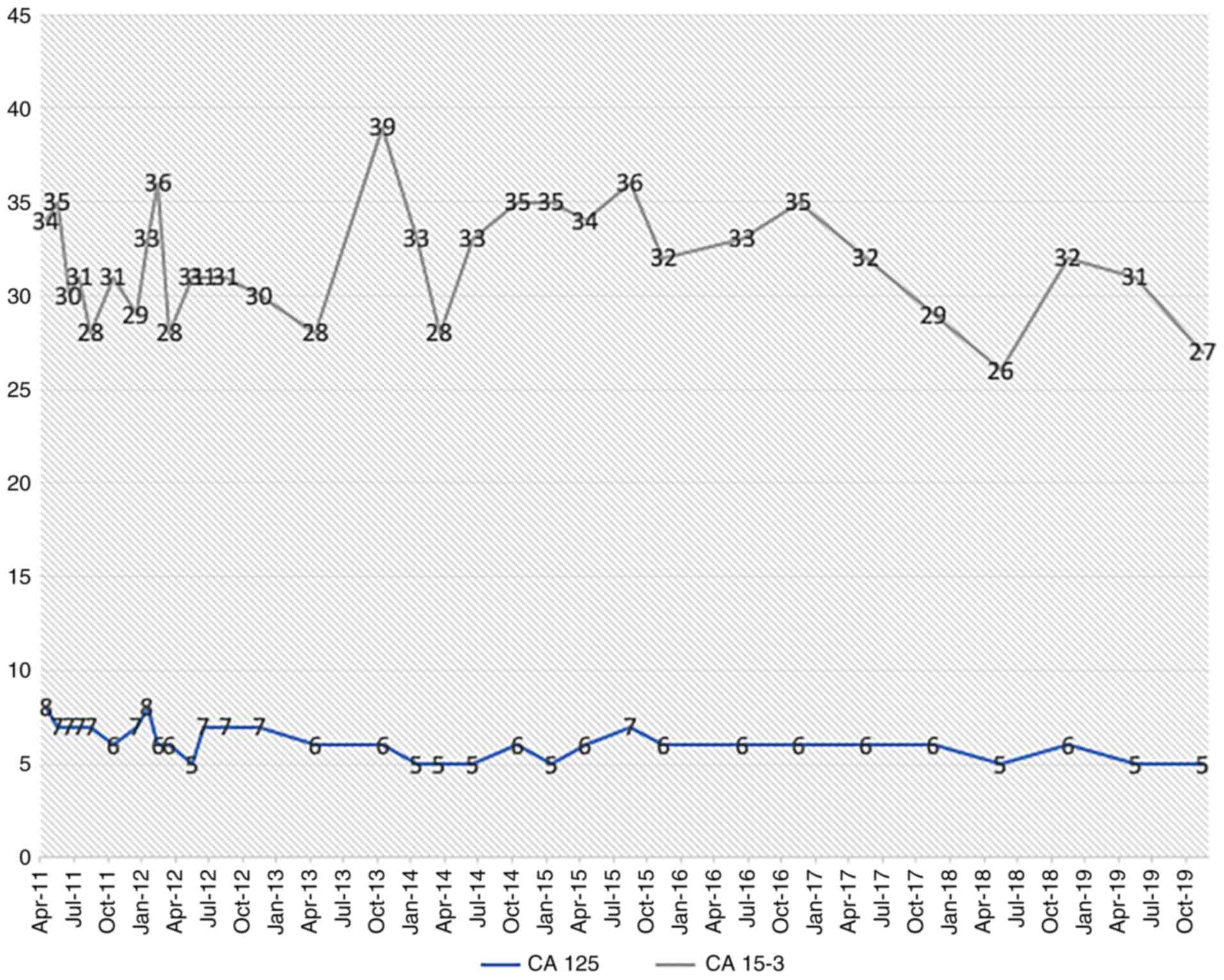
levels of the tumor marker CA-125 (from 142 kU/l in January 2010 to
7 kU/l). Re-staging was performed every 8 weeks within the first
6-month period and subsequently every 12 weeks until the end of the
study in November 2015. The assessment included CT of the
abdomen/thorax and tumor marker controls (CA-125 and CA-153)
without any sign of relapse (Fig.
1A-H). Since 2015, the patient has remained symptom-free and is
subjected to regular tumor marker level measurements (CA-125 and
CA-153, Fig. 4), which have been
within the normal range (<35 kU/l). The results of additional
examinations (haematology, clinical chemistry and urinanalysis)
have also been within the normal range. Gynecological and physical
examinations, including sonography, indicate no suspicion of
relapse. The patient has remained relapse-free for 10 years
(close-out visit: 12.07.2021, patient has given her written consent
tot he publication of this case report). Therefore, PARPi therapy
was associated with optimal efficacy and tolerability.
Discussion
The 10-year complete remission following a second
relapse of OC on PARPi monotherapy described in the present case
report is exceptional. Unlike the present case, long-term survivors
of OC usually display favourable clinical characteristics,
including optimal cytoreductive surgery at diagnosis and
platinum-sensitive disease, indicated by a recurrence-free interval
of >12 months following first-line chemotherapy (17). The patient reported in the present
study was diagnosed with second relapse 5 months after completion
of a re-induction of platinum-containing chemotherapy and received
neither surgery nor chemotherapy prior to the initiation of PARPi
therapy.
The favourable effect of a BRCA mutation on
the prognosis of patients with OC has been well documented. Several
studies have shown that OC patients with germline and somatic
BRCA mutations have short-term survival benefits compared to
OC patients without these mutations (18,19).
By including PARPi in the treatment regimens, the outcome can be
considerably improved.
PARPi provide maximum benefit in all clinical trials
to patients with relapsed and platinum-sensitive OC with
BRCA1 and BRCA2 germline mutations (gBRCAm)
(12-14,20).
Currently, PARPi therapy is indicated following complete or partial
response to platinum-based chemotherapy (PBC), whereas it has been
noted that, with each relapse, the response rates to PBC decrease.
Second-line PBC achieves a response of 50-65% (21,22)
compared with 11.9% of third-line chemotherapy (23).
In patients with advanced OC and germline
BRCA mutations who have received at least two lines of
previous chemotherapy, olaparib maintenance has been shown to
prolong PFS compared to the placebo (median, 19.1 vs. 5.5 months;
hazard ratio [HR], 0.30; 95% CI, 0.22-0.41) (14). An additional study on maintenance
therapy with olaparib was conducted in a similar population and
demonstrated a trend toward improved OS in patients receiving
olaparib compared with placebo (HR, 0.73; 95% CI, 0.45-1.17).
Moreover, the median OS was 34.9 months in patients with germline
BRCA mutations. In the present study, 15 patients (11%)
remained progression-free following 6 years on olaparib, although
the mechanism of such a durable response remain unclear (24). The patient in the present study
exceeded the median PFS reported in these trials, although she did
not fulfil the optimal conditions (platinum-resistant OC, no prior
surgery or platinum before PARPi). The BRCA2 mutation is a
predictive factor for the response to PARPi therapy (12-14,20)
and is most likely the explanation for the excellent therapeutic
response. BRCA2-deficient cells are acutely sensitive to
PARPi, as a result of their deficiency in homologous recombination.
This is likely attributed to the lack of collapsed replication fork
repair. Therefore, PARPi activity is essential in homologous
recombination-deficient BRCA2 mutant cells (10), and mutation analysis is a central
component in the treatment of OC patients and should be carried out
for each patient.
The patient discussed herein had a striking personal
and family history. However, approximately 50% of patients with OC,
who are carriers of gBRCAm, do not have family history of
ovarian or breast cancer, and may not be considered as candidates
for germline genetic testing (25).
The prevalence of deleterious germline variants in consecutive OC
patients was 15.5 and 5.5% in the BRCA1 and BRCA2
genes, respectively (26).
Moreover, the mutation prevalence in BRCA1 and BRCA2
depended on the age at onset (<60 y, 30.2% vs. >60y, 10.6%),
positive family history (31.6% vs. 11.4%) and the histological
subtype (HGSOC, 23.2% vs. other cancer types, 10.2%). However, a
relevant proportion of patients carry mutations without fulfilling
these factors, which renders genetic testing significant for all OC
patients. The prevalence of deleterious somatic BRCA1/2
variants is 2-5%, while the vast majority of the deleterious
variants found in the tumor was of germline origin (76.6%)
(27).
Previous clinical studies have demonstrated that
sensitivity to PARPi also occurs in tumor cells without
gBRCA1/2m, indicating that the HRR pathway may be
compromised by other mechanisms (e.g., mutation in other HRR genes
and promoter methylation in BRCA1, PALB2 or RAD51C)
(27). An estimated 50% of
high-grade serous OC cases, which is considered the most common
subtype of OC, has a deficiency in the HRR pathway (The Cancer
Genome Atlas). Therefore, PARPi are beneficial in a much wider
proportion of OC patients rather than only BRCA1/2 mutation
carriers (28). The present case
stresses the importance of genetic testing for patients with OC.
Several trials are in progress in order to assess PARPi in patients
with a wide variety of BRCA-associated tumors (e.g.,
prostate and pancreatic cancer), patients with tumors caused by
mutations in other genes involved in HRR, and those patients
without favourable clinical characteristics (e.g.,
platinum-resistance). The results of these studies may shed more
light on the selection of specific patient groups than may benefit
from targeted PARPi treatment.
References
|
1
|
World Health Organization: https://www.who.int/ionizing_radiation/research/iarc/en/.
March, 2020.
|
|
2
|
Kast K, Rhiem K, Wappenschmidt B, Hahnen
E, Hauke J, Bluemcke B, Zarghooni V, Herold N, Ditsch N, Kiechle M,
et al: Prevalence of BRCA1/2 germline mutations in 21 401 families
with breast and ovarian cancer. J Med Genet. 53:465–471.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kuchenbaecker KB, Neuhausen SL, Robson M,
Barrowdale D, McGuffog L, Mulligan AM, Andrulis IL, Spurdle AB,
Schmidt MK, Schmutzler RK, et al: Associations of common breast
cancer susceptibility alleles with risk of breast cancer subtypes
in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res.
16(3416)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gadducci A and Guerrieri ME: PARP
inhibitors alone and in combination with other biological agents in
homologous recombination deficient epithelial ovarian cancer: From
the basic research to the clinic. Crit Rev Oncol Hematol.
114:153–165. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bolton KL, Chenevix-Trench G, Goh C,
Sadetzki S, Ramus SJ, Karlan BY, Lambrechts D, Despierre E,
Barrowdale D, McGuffog L, et al: Association between BRCA1 and
BRCA2 mutations and survival in women with invasive epithelial
ovarian cancer. JAMA. 307:382–390. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chuang YT and Chang CL: Extending
platinum-free interval in partially platinum-sensitive recurrent
ovarian cancer by a non-platinum regimen: Its possible clinical
significance. Taiwan J Obstet Gynecol. 51:336–341. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Du Bois A, Lück HJ, Meier W, Adams HP,
Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, et
al: A randomized clinical trial of cisplatin/paclitaxel versus
carboplatin/paclitaxel as first-line treatment of ovarian cancer. J
Natl Cancer Inst. 95:1320–1329. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Du Bois A, Reuss A, Pujade-Lauraine E,
Harter P, Ray-Coquard I and Pfisterer J: Role of surgical outcome
as prognostic factor in advanced epithelial ovarian cancer: A
combined exploratory analysis of 3 prospectively randomized phase 3
multicenter trials: By the Arbeitsgemeinschaft Gynaekologische
Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe
d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire
(GINECO). Cancer. 115:1234–1244. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921.
2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Audeh MW, Carmichael J, Penson RT,
Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN,
Oaknin A, Loman N, et al: Oral poly(ADP-ribose) polymerase
inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and
recurrent ovarian cancer: A proof-of-concept trial. Lancet.
376:245–251. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott C, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
platinumsensitive relapsed ovarian cancer. N Engl J Med.
366:1382–1392. 2012.
|
|
13
|
Ledermann JA, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott C, Meier W,
Shapira-Frommer R, Safra T, et al: Overall survival in patients
with platinum-sensitive recurrent serous ovarian cancer receiving
olaparib maintenance monotherapy: An updated analysis from a
randomised, placebo-controlled, double-blind, phase 2 trial. Lancet
Oncol. 17:1579–1589. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pujade-Lauraine E, Ledermann JA, Selle F,
Gebski V, Penson RT, Oza AM, Korach J, Huzarski T, Poveda A,
Pignata S, et al: Olaparib tablets as maintenance therapy in
patients with platinum-sensitive, relapsed ovarian cancer and a
BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised,
placebocontrolled, phase 3 trial. Lancet Oncol. 18:1274–1284.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mirza MR, Åvall Lundqvist E, Birrer MJ,
dePont Christensen R, Nyvang GB, Malander S, Anttila M, Werner TL,
Lund B, Lindahl G, et al: Niraparib maintenance therapy in
platinumsensitive, recurrent ovarian Cancer. N Engl J Med.
375:2154–2164. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dao F, Schlappe BA, Tseng J, Lester J,
Nick AM, Lutgendorf SK, McMeekin S, Coleman RL, Moore KN, Karlan
BY, et al: Characteristics of 10-year survivors of high-grade
serous ovarian carcinoma. Gynecol Oncol. 141:260–263.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
McLaughlin JR, Rosen B and Moody J:
Long-term ovarian cancer survival associated with mutation in BRCA1
or BRCA2. J Natl Cancer Inst. 105:141–148. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu K, Yang S and Zhao Y: Prognostic
significance of BRCA mutations in ovarian cancer: An updated
systematic review with meta-analysis. Oncotarget. 8:285–302.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Drew Y, Ledermann J, Hall G, Rea D,
Glasspool R, Highley M, Jayson G, Sludden J, Murray J, Jamieson D,
et al: Phase 2 multicentre trial investigating intermittent and
continuous dosing schedules of the poly(ADP-ribose) polymerase
inhibitor rucaparib in germline BRCA mutation carriers with
advanced ovarian and breast cancer. Br J Cancer. 114:723–730.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Markman M, Rothman R, Hakes T, Reichman B,
Hoskins W, Rubin S, Jones W, Almadrones L and Lewis JL Jr:
Second-line platinum therapy in patients with ovarian cancer
previously treated with cisplatin. J Clin Oncol. 9:389–393.
1991.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Parmar MK, Ledermann JA, Colombo N, du
Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W,
Torri V, et al: Paclitaxel plus platinum-based chemotherapy versus
conventional platinum-based chemotherapy in women with relapsed
ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet.
361:2099–2106. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bruchim I, Jarchowsky-Dolberg O and
Fishman A: Advanced (>second) line chemotherapy in the treatment
of patients with recurrent epithelial ovarian cancer. Eur J Obstet
Gynecol Reprod Biol. 166:94–98. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Friedlander M, Matulonis U, Gourley C, du
Bois A, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R,
Safra T, et al: Long-term efficacy, tolerability and overall
survival in patients with platinum-sensitive, recurrent high-grade
serous ovarian cancer treated with maintenance olaparib capsules
following response to chemotherapy. Br J Cancer. 119:1075–1085.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Alsop K, Fereday S, Meldrum C, deFazio A,
Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, et
al: BRCA mutation frequency and patterns of treatment response in
BRCA mutation-positive women with ovarian cancer: A report from the
Australian Ovarian Cancer Study Group. J Clin Oncol. 30:2654–2663.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Harter P, Hauke J, Heitz F, Reuss A,
Kommoss S, Marmé F, Heimbach A, Prieske K, Richters L, Burges A, et
al: Prevalence of deleterious germline variants in risk genes
including BRCA1/2 in consecutive ovarian cancer patients
(AGO-TR-1). PLoS One. 12(e0186043)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hauke J, Hahnen E, Schneider S, Reuss A,
Richters L, Kommoss S, Heimbach A, Marmé F, Schmidt S, Prieske K,
et al: Deleterious somatic variants in 473 consecutive individuals
with ovarian cancer: Results of the observational AGO-TR1 study
(NCT02222883). J Med Genet. 56:574–580. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
González-Martín A, Pothuri B, Vergote I,
DePont Christensen R, Graybill W, Mirza MR, McCormick C, Lorusso D,
Hoskins P, Freyer G, et al: PRIMA/ENGOT-OV26/GOG-3012
investigators. niraparib in patients with newly diagnosed advanced
ovarian cancer. N Engl J Med. 381:2391–2402. 2019.PubMed/NCBI View Article : Google Scholar
|