Introduction
Primary urethral carcinoma (PUC) is an aggressive
and infrequent carcinoma, accounting for ≤1% of malignant tumors of
the genitourinary system (1). The
incidence of this cancer in male subjects is three times higher
than that of female subjects and an increased incidence has been
noted for the elderly (2).
Recurrent urinary tract infections, sexually transmitted diseases
and chronic irritation through catheterization, are important risk
factors for the development of PUC (3). According to the current World Health
Organization-based program, three main histological types have been
reported for PUC, including urothelial carcinoma (UCSD; 55%),
squamous cell carcinoma (21.5%) and adenocarcinoma (16.4%)
(4). The remaining cases are
extremely rare and involve clear cell and adenoid cystic carcinomas
(5).
The presence of tissue variations in the
pathological reports is very important due to their prognostic and
therapeutic significance (6).
Squamous differentiation of UCSD refers to the presence of both
urothelial and squamous differentiation in the same tumor, although
the ratio of the two is not clearly defined. Squamous
differentiation requires the presence of intercellular bridges
and/or keratinization (7). UCSD
accounts for 10-20% of bladder cancer cases and is the most common
variant of bladder cancer (8).
Muscle invasion is present in 60-70% of UCSD cases. The latter is
considered a more invasive cancer type that progresses more rapidly
than pure UCSD and is associated with a poor prognosis (7). However, in contrast to UCSD, PUC with
squamous differentiation is rarely reported. In the present study,
a rare case of a male patient is presented whose pathological
result was UCSD in PUC. The clinical challenges and management of
this condition were discussed.
Case report
A 68-year-old man who presented with the major
complaint of an extra-urethral mass and difficulty voiding urine
was treated at the Affiliated Hospital of Guizhou Medical
University (Guiyang, China). A hard mass with poor mobility and an
approximate size of 3x2 cm could be palpated in the overhanging
part of the penis approximately 1.5 cm from the distal end of the
penile bulb. The prostatic findings were normal and no enlarged
lymph nodes were found by bilateral inguinal palpation. No
abnormalities were found in the remaining laboratory tests except
for hematuria, which was indicated by urine analysis.
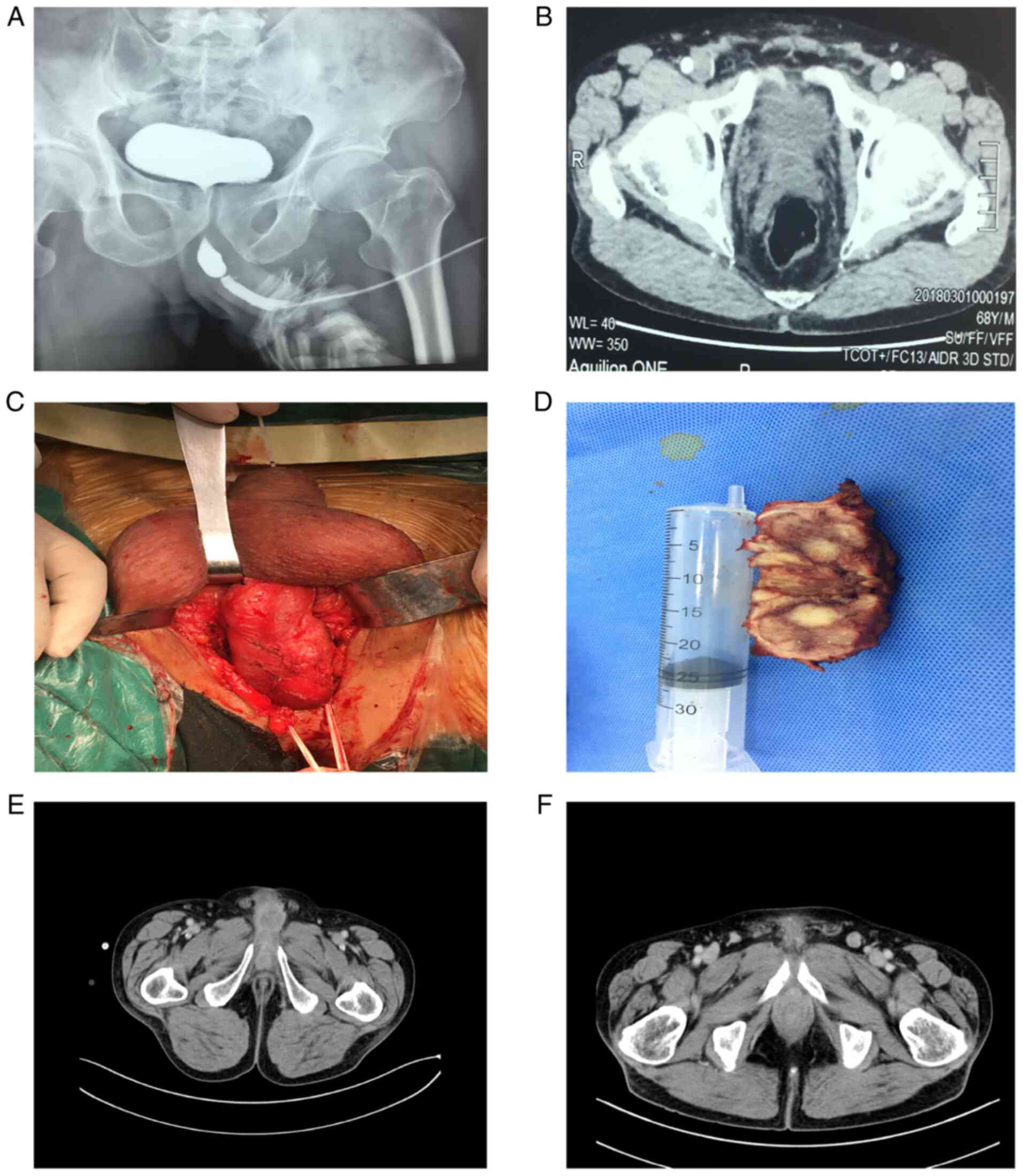
The patient had undergone several imaging
examinations. Urethrography indicated an apparent urethral
stricture (Fig. 1A). Abdominal
computed tomography (CT; Fig. 1B)
demonstrated the presence of a neoplastic lesion in the overhanging
part of the penis, with approximate dimensions of 4.0x2.0x2.0 cm.
Concomitantly, abdominal CT indicated that the inner wall of the
bladder was smooth in the absence of an apparent space-occupying
sign. The bladder was devoid of inguinal nodules, and organ or
other nodal metastases (cT3 cN0 cM0). Preoperative urethral
cystoscopy confirmed the presence of urethral stricture and the
space-occupying site was located approximately at a site 7.0 cm
from the external orifice of the urethra. After the replacement of
a thinner pediatric ureteroscope, it was still unable to pass
through the narrow segment. Therefore, a fine needle biopsy had to
be performed on the mass site of the patient. The results suggested
that the tumor was malignant. Subsequently, a partial penectomy was
performed. Notably, during the surgery, it was found that the mass
had invaded the corpus cavernosum (Fig. 1C and D).
Microscopic findings (Fig. 2A) and immunohistochemical results
combined with clinical data indicated the presence of high-grade
UCSD with focal areas of squamous differentiation. Fortunately, no
neoplastic involvement was present in the surgical resection
margins. Immunohistochemical analysis provides a semi-quantitative
assessment of the expression levels of specific markers in tumor
cells. These cells are considered to be positive or negative with
regard to the expression of these markers. The immunohistochemical
results in the present study were the following: Cytokeratin (CK)
5/6 (+) (Fig. 2B), P40 (nuclear
and cytoplasmic +) (Fig. 2C), CK7
(+), p63 (+), GATA binding protein 3 (nuclear focally and weakly +)
(Fig. 2D), CK20 (-), uroplakin III
(-), and prostate-specific antigen (-).
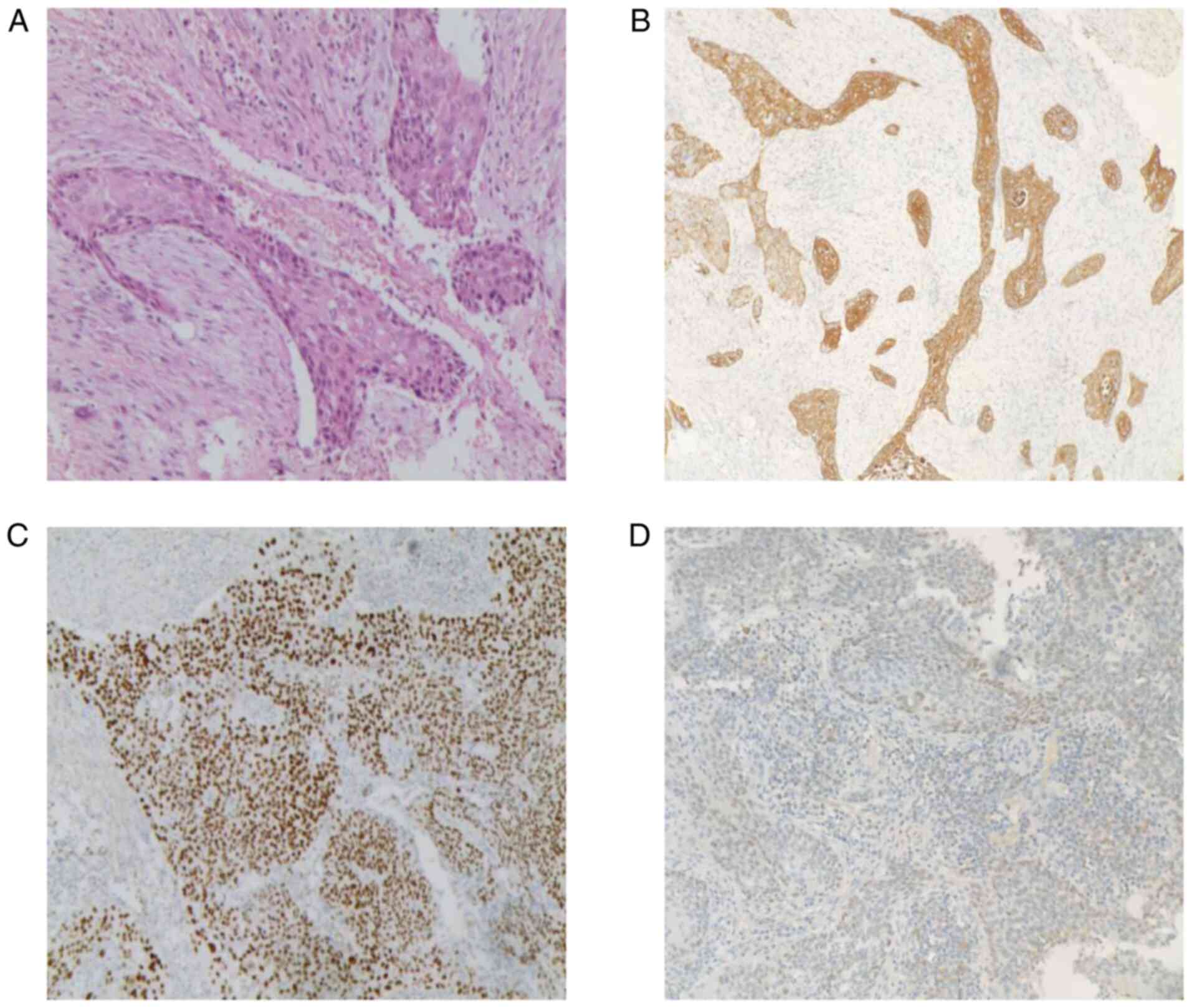 | Figure 2Histopathological examination of the
resected specimen. (A) H&E staining of tumor sections indicated
infiltrative growth of cancer nests in the stroma. Certain cells
had rich cytoplasm and were slightly eosinophilic with large and
deep stained nuclei and paving stone-like changes. The
differentiation of the squamous epithelium and the proliferation of
the surrounding fibrous tissue was also noted (original
magnification, x200). Immunohistochemical staining of the tumor
cells revealed positive expression for (B) CK5/6 (original
magnification, x40), (C) P40, (original magnification, x100), and
nuclear focal and weak positivity for (D) GATA-3 (original
magnification, x40). H&E, hematoxylin and eosin; CK5/6,
cytokeratin 5/6; GATA-3, GATA-3, GATA binding protein 3. |
Therefore, the patient was discharged. During the
3-month postoperative follow-up, no apparent abnormality was noted
according to the laboratory or imaging examination. However, 9
months later, the patient returned to the hospital due to recurrent
dysuria with hematuria and a re-examination of abdominal
enhancement CT indicated that the enhancement of the anterior part
of the cavernous body was decreased (Fig. 1E), the enhancement of the prostate
was uneven, and the bilateral inguinal lymph nodes were enlarged
with apparent enhancement (Fig.
1F). The final clinical diagnosis was tumor recurrence with
prostate invasion and bilateral inguinal lymph node metastasis. Due
to the poor medical condition of the patient, multimodal treatment
was declined, including salvage surgery and/or chemoradiotherapy,
and cystostomy was accepted to merely relieve urinary retention. As
a consequence of his progressive disease, the condition of the
patient significantly deteriorated. The patient succumbed to his
illness, 3 months after the recurrent presentation.
Discussion
PUC is defined by the European Association of
Urology as a tumor with its very first lesion located in the
urethra (9). Early diagnosis of
urethral cancer is difficult due to the absence of apparent
symptoms in the early stages and a lack of specific screening
indicators. The major role of the imaging examination of primary
urethral cancer is to detect the extent of the local lesions and to
evaluate the presence of metastatic diseases. Due to its improved
spatial resolution, superior soft tissue contrast, and lack of
ionizing radiation, magnetic resonance imaging (MRI) has emerged as
the most sensitive imaging modality for assessing the local staging
of urethral cancer (10). Although
MRI performs well in the local staging of the disease due to its
excellent representation of the soft tissue, CT imaging can
accurately depict adenopathy and distant metastatic disease in the
abdomen and pelvis (11). The CT
imaging of this patient indicated that the tumor invaded the corpus
cavernosum; however, no enlarged pelvic or celiac lymph nodes were
found. The disease was staged as T3N0M0 according to the tumor,
nodes, and metastases classification of the newly updated European
Association of Urology Guidelines for primary urethral carcinoma
(12).
As an invasive examination, diagnostic urinary
cystoscopy and biopsy can initially evaluate urinary tract tumors
based on the extent, location, and potential histology of the tumor
(13). Multiple methods, such as
cystoscopy with cold-cup biopsy forceps or a
transurethral/percutaneous approach using a 14-gauge Temno biopsy
needle, can aid the diagnosis of the proximal tumors (14). Although the present case report was
prepared to be examined by diagnostic urethrocystoscopy, a
successful diagnosis was not possible due to urinary tract
stricture, which was confirmed by urethrography.
Given the rarity and lack of level I evidence of
primary urethral cancer, a limited number of prospective
multi-agency studies have determined the optimal treatment for PUC.
For several years, partial or radical penectomy for distal tumors
and total penectomy with cystoprostatectomy for proximal tumors
were the standard treatments for urethral cancer. A retrospective
cohort study of 1,544 non-metastatic patients with PUC indicated
that the overall 5-year survival rate of patients who received
local treatment or radical surgery was considerably higher than
that noted in patients who did not undergo surgery at the primary
site (1). Penis preservation
surgery is recommended by the current guidelines for the treatment
of localized PUC. This method has become the preferred treatment
option while maintaining optimal local cancer control (12,15).
A retrospective series demonstrated that for patients with
pT1-3NO-2 anterior urethral cancer and clinically suspected nodular
diseases, penis-preserving surgery with <5 mm resection margins
combined with iliac/inguinal lymphadenectomy did not lead to local
recurrence of the disease (16).
In the present case report, for the patient in stage cT3N0M0,
according to the disease management of the European Association of
Urology Guidelines on Primary Urethral Carcinoma in Males with
Localised PUC (12), and combined
with the willingness of the patient and his family to retain the
penis, partial penectomy without inguinal lymphadenectomy was
finally performed.
Radiation therapy (RT) or chemotherapy are the two
standard treatment options for the treatment of patients with PUC
in addition to surgery. In localized PUC, the survival rate and
recurrence rate of RT are worse than those of surgery. In addition,
the patients experience a higher number of side effects, which
limit the application of RT in genital protection therapy (2). However, in locally advanced UC,
multimodal therapy is highly respected in both sexes due to the
monotherapies leading to lower disease recurrence and patient
survival rates. Multimodal therapy in PUC includes definite surgery
plus chemotherapy and additional RT can be selected (14). According to the National Cancer
Database, the overall survival rate of patients with locally
advanced PUC receiving well-defined multimodal treatment has
improved. A large multicentre cohort study demonstrated increased
overall survival rates in patients who received perioperative
chemotherapy plus surgery for advanced PUC (17). The patient reported in the present
study exhibited a postoperative recurrence. Due to this fact,
neoadjuvant chemotherapy with 5-fluorouracil and cisplatin combined
with salvage surgery was recommended. Unfortunately, due to his
poor performance status and expensive treatment, as well as the
fact that the tumor may have metastasized to the prostate and
inguinal lymph nodes, the patient refused the treatment.
In recent years, with the deepening of our
understanding of tumor immunology, systemic immunotherapy targeting
immune checkpoint inhibition has been explored and applied in the
field of urothelial cancer (18).
Despite the fact that a limited number of systematic reports or no
reports have been published on the use of immunotherapy for
urethral cancer, the latest literature has suggested that
programmed death-ligand 1 May be strongly expressed in certain
urethral adenocarcinomas. Therefore, it is possible to apply
immunotherapy in specific cases of advanced or recurrent
adenocarcinoma. Miyama et al (19) reported for the first time that
squamous differentiation is a potential and novel marker used for
the prediction of the treatment of progressive UCSD with
pembrolizumab therapy. The study further demonstrated that squamous
differentiation was significantly associated with tumor progression
and shorter overall survival.
In general terms, different histological results are
closely related to the presence of high-level and high-stage
diseases (8). Accurate
classification is important as the clinical behaviors, prognosis,
and therapeutic strategies differ between pure urothelial
carcinoma, UCSD, and pure squamous cell carcinoma (20). Liu et al (21) reported that UCSD was frequently
detected in patients with advanced tumor stage (pT3-4: 72.3%) and
nodal metastasis. A retrospective study proposed that compared with
pure UCSD, UCSD indicated a considerably higher pathological stage.
Moreover, it was reported that UCSD of the bladder may be
associated with a poor oncological outcome following radical
cystectomy and it could be used to predict lowered rates of overall
survival and recurrence-free survival (22). Although a limited number of studies
have been reported, it is generally accepted that squamous
differentiation is not confined to UCSD of the bladder as this
morphology has also been reported in UCSD of the PUC. Zhang et
al (23) focused on 130 cases
of primary urethral tumors, of which 106 were classified as ‘PUCs’.
The latter is a new entity proposed by the authors of that study
and refers to poorly differentiated tumors with mixed features of
urothelial and squamous differentiation. It is considered that this
type of cancer is different from the typical UCSD in the bladder
and from the squamous cell carcinoma in the male distal urethral
orifice, or balanus. It develops from the intraepithelial precursor
state of the urethral mucosa to the sequence of dysplasia/carcinoma
in situ. Clinically, it is highly invasive, with frequent
regional lymphatic metastasis and distant organ metastasis. In the
present case report, the microscopic findings indicated that the
papillary structures of urethral carcinomas were short and
irregular with an extensive fibrovascular core and cancer nest
formation; in addition, the intercellular bridge could be observed
in the squamous differentiation components. These morphological
characteristics are the basis for supporting the diagnosis of
UCSD.
Pathological diagnosis is primarily based on
morphology and immunohistochemistry, but when there are boundary
features and tissue artifacts or morphological overlap, it will
hinder the best evaluation of morphology, so the presence of
immunohistochemical biomarkers may help differentiate between UCSD
and traditional urothelial carcinoma. According to Gaisa et
al (24), primary bladder
squamous cell carcinomas were all positive for high molecular
weight keratin CK5/6, whereas pure urothelial carcinomas were
positive for 33%. In comparison with adenocarcinoma, a specific
subtype of p63 called delta-np63 (P40) is not only expressed in 95%
of urothelial cell carcinomas but also highly specific for squamous
cell carcinomas (8). GATA3, a
transcription factor located on chromosome 10p14, is a
trans-acting T-cell-specific transcription factor (25). Based on the morphology of
hematoxylin and eosin, GATA3 has a sensitivity of 88% and a
specificity of 100% for distinguishing urothelial carcinoma from
squamous cell carcinoma (26).
Although there is no one-to-one correspondence between these
biomarkers and a specific subtype of PUC, the immunohistochemical
results of the above three coexisting biomarkers coupled with the
morphological characteristics were highly consistent with those
reported by Zhang et al (23).
Squamous differentiation has important diagnostic,
prognostic, and therapeutic implications. This includes UCSDs or
the new entities termed ‘PUCs’. Ignoring this particular subtype
may lead to an increased risk of clinical understaging and occult
metastatic disease. However, the present case report has inherent
limitations, such as the inability to generalize the findings
reported, the inability to determine causality, and the risk of
overinterpretation. In addition, since the Department of Pathology
of our hospital did not report the lymphatic vessel, vein, or
perineural invasion of the tumor, unfortunately, the specific mode
of tumor invasion could not be elucidated. Despite these drawbacks,
in future studies, individualized, risk-based, sex-specific
treatment strategies for PUC are anticipated to be developed, based
on the following important risk factors: Tumor location, clinical
and pathological tumor stage, and histological classification.
Furthermore, additional similar cases and potential molecular
determinants will be of great significance for subsequent
investigations since UCSD in PUC appears to be generally
underrecognized and underreported.
In conclusion, the present study recommends that
additional caution should be paid in patients with pathological
findings suggestive of UCSD in PUC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KT contributed to the concept and design of the case
report. KT, ML, and SX participated in the surgery. ML produced the
first draft of the article. SX and JH obtained the raw data of the
patient, such as laboratory and imaging examinations and
preliminary examination results, and participated in the diagnosis
and treatment of the patient. YM and KC collected the postoperative
pathological results and advised on patient treatment. KT, BC, and
WZ critically revised the manuscript with regard to the content.
All authors confirm the authenticity of the data and have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
daughter of the patient for publication of this case report and of
the accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu J, Wang YC, Luo WJ, Bo D, Ye DW and Zhu
YP: Primary tumor surgery improves survival in non-metastatic
primary urethral carcinoma patients: A large population-based
investigation. BMC Cancer. 21(857)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Janisch F, Abufaraj M, Fajkovic H, Kimura
S, Iwata T, Nyirady P, Rink M and Shariat SF: Current disease
management of primary urethral carcinoma. Eur Urol Focus.
5:722–734. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wenzel M, Nocera L, Colla Ruvolo C,
Würnschimmel C, Tian Z, Shariat SF, Saad F, Briganti A, Tilki D,
Mandel P, et al: Incidence rates and contemporary trends in primary
urethral cancer. Cancer Causes Control. 32:627–634. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Williams C, Lamar M and Delgado P:
Urethral carcinoma: A compilation of case studies and research
findings. Urol Case Rep. 31(101169)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital Organs-Part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shah RB, Montgomery JS, Montie JE and
Kunju LP: Variant (divergent) histologic differentiation in
urothelial carcinoma is under-recognized in community practice:
Impact of mandatory central pathology review at a large referral
hospital. Urol Oncol. 31:1650–1655. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Minato A, Fujimoto N and Kubo T: Squamous
differentiation predicts poor response to cisplatin-based
chemotherapy and unfavorable prognosis in urothelial carcinoma of
the urinary bladder. Clin Genitourin Cancer. 15:e1063–e1067.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gellert LL, Warrick J and Al-Ahmadie HA:
Urothelial carcinoma with squamous differentiation-the pathologists
perspective. Urol Oncol. 33:437–443. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gakis G, Witjes JA, Compérat E, Cowan NC,
De Santis M, Lebret T, Ribal MJ and Sherif AM: European Association
of Urology. EAU guidelines on primary urethral carcinoma. Eur Urol.
64:823–830. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Stewart SB, Leder RA and Inman BA: Imaging
tumors of the penis and urethra. Urol Clin North Am. 37:353–367.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Galgano SJ, Sivils C, Selph JP, Sanyal R,
Lockhart ME and Zarzour JG: The male urethra: Imaging and surgical
approach for common pathologies. Curr Probl Diagn Radiol.
50:410–418. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gakis G, Bruins HM, Cathomas R, Compérat
EM, Cowan NC, van der Heijden AG, Hernández V, Linares Espinós EE,
Lorch A, Neuzillet Y, et al: European association of urology
guidelines on primary urethral carcinoma-2020 update. Eur Urol
Oncol. 3:424–432. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Karnes RJ, Breau RH and Lightner DJ:
Surgery for urethral cancer. Urol Clin North Am. 37:445–457.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zinman LN and Vanni AJ: Management of
proximal primary urethral cancer: Should multidisciplinary therapy
be the gold standard? Urol Clin North Am. 43:505–513.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Spiess PE, Agarwal N, Bangs R, Boorjian
SA, Buyyounouski MK, Clark PE, Downs TM, Efstathiou JA, Flaig TW,
Friedlander T, et al: Bladder cancer, version 5.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:1240–1267. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Smith Y, Hadway P, Ahmed S, Perry MJ,
Corbishley CM and Watkin NA: Penile-preserving surgery for male
distal urethral carcinoma. BJU Int. 100:82–87. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gakis G, Morgan TM, Daneshmand S, Keegan
KA, Todenhöfer T, Mischinger J, Schubert T, Zaid HB, Hrbacek J,
Ali-El-Dein B, et al: Impact of perioperative chemotherapy on
survival in patients with advanced primary urethral cancer: Results
of the international collaboration on primary urethral carcinoma.
Ann Oncol. 26:1754–1759. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim HS and Seo HK: Immune checkpoint
inhibitors for urothelial carcinoma. Investig Clin Urol.
59:285–296. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miyama Y, Morikawa T, Miyakawa J, Koyama
Y, Kawai T, Kume H and Ushiku T: Squamous differentiation is a
potential biomarker predicting tumor progression in patients
treated with pembrolizumab for urothelial carcinoma. Pathol Res
Pract. 219(153364)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gulmann C, Paner GP, Parakh RS, Hansel DE,
Shen SS, Ro JY, Annaiah C, Lopez-Beltran A, Rao P, Arora K, et al:
Immunohistochemical profile to distinguish urothelial from squamous
differentiation in carcinomas of urothelial tract. Hum Pathol.
44:164–172. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu Y, Bui MM and Xu B: Urothelial
carcinoma with squamous differentiation is associated with high
tumor stage and pelvic lymph-node metastasis. Cancer Control.
24:78–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Minato A, Noguchi H, Tomisaki I, Fukuda A,
Kubo T, Nakayama T and Fujimoto N: Clinical significance of
squamous differentiation in urothelial carcinoma of the bladder.
Cancer Control. 25(1073274818800269)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang M, Adeniran AJ, Vikram R, Tamboli P,
Pettaway C, Bondaruk J, Liu J, Baggerly K and Czerniak B: Carcinoma
of the urethra. Hum Pathol. 72:35–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gaisa NT, Braunschweig T, Reimer N,
Bornemann J, Eltze E, Siegert S, Toma M, Villa L, Hartmann A and
Knuechel R: Different immunohistochemical and ultrastructural
phenotypes of squamous differentiation in bladder cancer. Virchows
Arch. 458:301–312. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ko LJ, Yamamoto M, Leonard MW, George KM,
Ting P and Engel JD: Murine and human T-lymphocyte GATA-3 factors
mediate transcription through a cis-regulatory element within the
human T-cell receptor delta gene enhancer. Mol Cell Biol.
11:2778–2784. 1991.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chaux A, Han JS, Lee S, Gonzalez-Roibon N,
Sharma R, Burnett AL, Cubilla AL and Netto GJ: Immunohistochemical
profile of the penile urethra and differential expression of GATA3
in urothelial versus squamous cell carcinomas of the penile
urethra. Hum Pathol. 44:2760–2767. 2013.PubMed/NCBI View Article : Google Scholar
|