Introduction
NTRK-rearranged spindle cell neoplasms (NTRK-RSCNs)
are soft tissue tumors that harbor mainly NTRK1 or
NTRK3 fusion genes, other than infantile fibrosarcoma
(1). NTRK-RSCNs comprise a broad
range of tumors, including morphological heterogeneity and
histological grade. Histologically, NTRK-RSCNs resemble
inflammatory myofibroblastic tumors, solitary fibrous tumors
(SFTs), and malignant peripheral nerve sheath tumors (2,3).
Recently, lipofibromatosis-like neural tumors were described,
characterized by local aggressiveness and co-expression of Pan-TRK,
CD34, and S100(4), which are
involved in NTRK-RSCNs in the presence of the NTRK1 fusion
gene. Regarding histological grade, NTRK-RSCNs range from benign to
low- and high-grade lesions (1).
The frequency of NTRK-RSCNs is reported to be 0.68%
in sarcoma cases, analyzing 1915 cases at Memorial Sloan Kettering
Cancer Center (5). Assays commonly
performed to identify NTRK fusions include Pan-TRK
immunohistochemistry, fluorescence in situ hybridization,
DNA sequencing using targeted cancer panels, and RNA sequencing. In
clinical settings, Pan-TRK immunohistochemistry and DNA sequencing
are commonly used; however, these assays can miss the detection of
NTRK fusions at a frequency of approximately 20% (5). These methods tend to miss the
detection of NTRK3 fusions, and the specificity and staining
of Pan-TRK are reported to be low and faint in sarcoma cases
(5). NTRK3 fusion-positive
mesenchymal tumors are more aggressive than NTRK1
fusion-positive mesenchymal tumors (2). Therefore, the clinical features and
characteristics of imaging findings are important to assess
NTRK-RSCNs and treat patients with advanced NTRK-RSCNs, because
NTRK inhibitors have shown dramatic and durable activity against
NTRK fusion-positive tumors (6).
Several histopathological studies have focused on
gene fusion and prognosis, but no previous case series has reported
the radiological features of NTRK-RSCNs. Therefore, the present
study aimed to clarify the radiological features of NTRK-RSCNs to
improve the diagnostic rate of this rare tumor and compare them
with histopathological features.
Patients and methods
Patients
A retrospective review of spindle cell tumors
morphologically resembling NTRK-RSCNs treated in our institutions
The University of Tokyo Hospital (Tokyo, Japan) and The Cancer
Institute Hospital of the Japanese Foundation for Cancer Research
(Tokyo, Japan) between January 2003 and December 2019 revealed that
six patients were diagnosed with NTRK-RSCNs and had NTRK gene
fusions, which was confirmed using next-generation sequencing. One
case has been published previously (Case 1) (7); we have included it here to enhance
our evidence of radiological and histopathological features. We
examined the patients' clinical information, including patient age,
sex, location of the tumor, clinical follow-up, and features of
magnetic resonance imaging (MRI) and compared the histopathological
features. This study was approved by the institutional review board
of The University of Tokyo Hospital (approval number 11019).
Written informed consent was obtained from the patients or the
parents of patients for all of the participants. All procedures
described in this study were performed according to the ethical
standards of the Declaration of Helsinki of 1975, revised in 2000,
and the national law.
Magnetic resonance imaging (MRI)
acquisition and analysis
MRI examinations were performed using 1.5-Tesla
magnets from different two centers. MRI evaluations included
T1-weighted fast spin-echo, short tau inversion recovery, and
T2-weighted fast spin-echo images in at least two planes (usually
the axial and coronal planes). All six patients were also studied
using dynamic contrast-enhanced sequences.
All MRI scans were reviewed by a senior radiologist
(YT) and orthopedist (HK) to assess inter-observer reproducibility.
Tumor location, size, local invasion, vascularity, MRI signal
intensities, and presence of necrosis, fatty or fibroblastic
component (defined as low signal intensity on T1- and T2-weighted
image and possible subtle enhancement by gadolinium
administration), and peritumoral edema were noted. When there was
disagreement between observers, they re-evaluated and discussed the
images to determine the existence of each characteristic. Tumor
growth patterns were classified into three types, as previously
reported (8) pushing type,
entirely well-defined tumor; focal type, tumor with irregular
borders and infiltration of surrounding tissues representing
<25% of tumor circumference; and diffuse type, tumor with
irregular borders and infiltration of surrounding tissues
representing 25% of tumor circumference.
Histopathological analysis
Surgical specimens stained with hematoxylin and
eosin were retrospectively reviewed by expert pathologists. Mitotic
count/10 high-power fields, cellularity, presence of necrosis,
infiltration into surrounding tissues, and surgical margin were
evaluated. Surgical margins were classified into three
categories-R0, microscopically negative margin; R1, macroscopically
complete with positive microscopic margin; and R2, macroscopically
incomplete margin. Immunohistochemistry of CD34, S100, and MIB1 was
also analyzed.
Results
Patient characteristics
Patient characteristics are presented in Table I. This study included three women
and three men, with a mean age of 22 (range, 2-43) years. The
tumors were in the knee (n=3), lower leg (n=1), buttock (n=1), and
perineum (n=1). One patient (Case 5) had a recurrent tumor after
treatment at another hospital, and the other five patients had
primary tumors. One patient was diagnosed with NTRK-RSCN because
the patient was treated in 2019, and the entity of NTRK-rearranged
mesenchymal tumors was suggested. However, three patients were
diagnosed with SFTs, one patient had dedifferentiated liposarcoma
because of the positivity of MDM2 immunohistochemical staining and
fatty component, and one patient had sarcoma with intermediate
malignancy. Four and two tumors harbored NTRK1 and
NTRK3 fusions, respectively. All patients underwent surgery,
and no recurrence was observed, except in one patient. Five
patients were disease free or had no evidence of disease, and one
patient with recurrence and distant metastasis (Case 3) was treated
with chemotherapy; however, the patient did not survive.
 | Table IPatients' clinical characteristics,
fusion gene and prognosis. |
Table I
Patients' clinical characteristics,
fusion gene and prognosis.
| Case | Age, years | Sex | Location of primary
tumor | Diagnosis by resected
tumor | Fusion gene | Treatment | Follow- up duration,
months | Prognosis |
|---|
| 1 | 23 | F | Lower leg | NTRK-rearranged
spindle cell neoplasm | LMNA-NTRK1 | Surgery | 16 | CDF |
| 2 | 2 | M | Knee | Sarcoma, intermediate
malignancy | TPM3-NTRK1 | Surgery | 125 | CDF |
| 3 | 16 | F | Buttock | SFT, grade 3 | YWHAE-NTRK3 | Surgery,
chemotherapy | 39 | DOD |
| 4 | 13 | M | Knee | SFT, grade 1 | TPR-NTRK1 | Surgery | 146 | CDF |
| 5 | 43 | F | Knee | SFT, grade 2 | PPFIBP-NTRK3 | Surgery | 125 | NED |
| 6 | 35 | M | Perineal | Dedifferentiated
liposarcoma, grade 2 | LMNA-NTRK1 | Surgery | 31 | CDF |
MRI findings
The imaging characteristics, including the location,
size, and properties of the tumors, are summarized in Tables II and III. Four tumors were located in the
deep area, and two were located in the subcutaneous tissue. The
mean diameter was 9.4 (range, 4-11.3) cm. These tumors were
isointense or slightly high in intensity compared to the skeletal
muscle on T1-weighted images and high intensity on T2-weighted
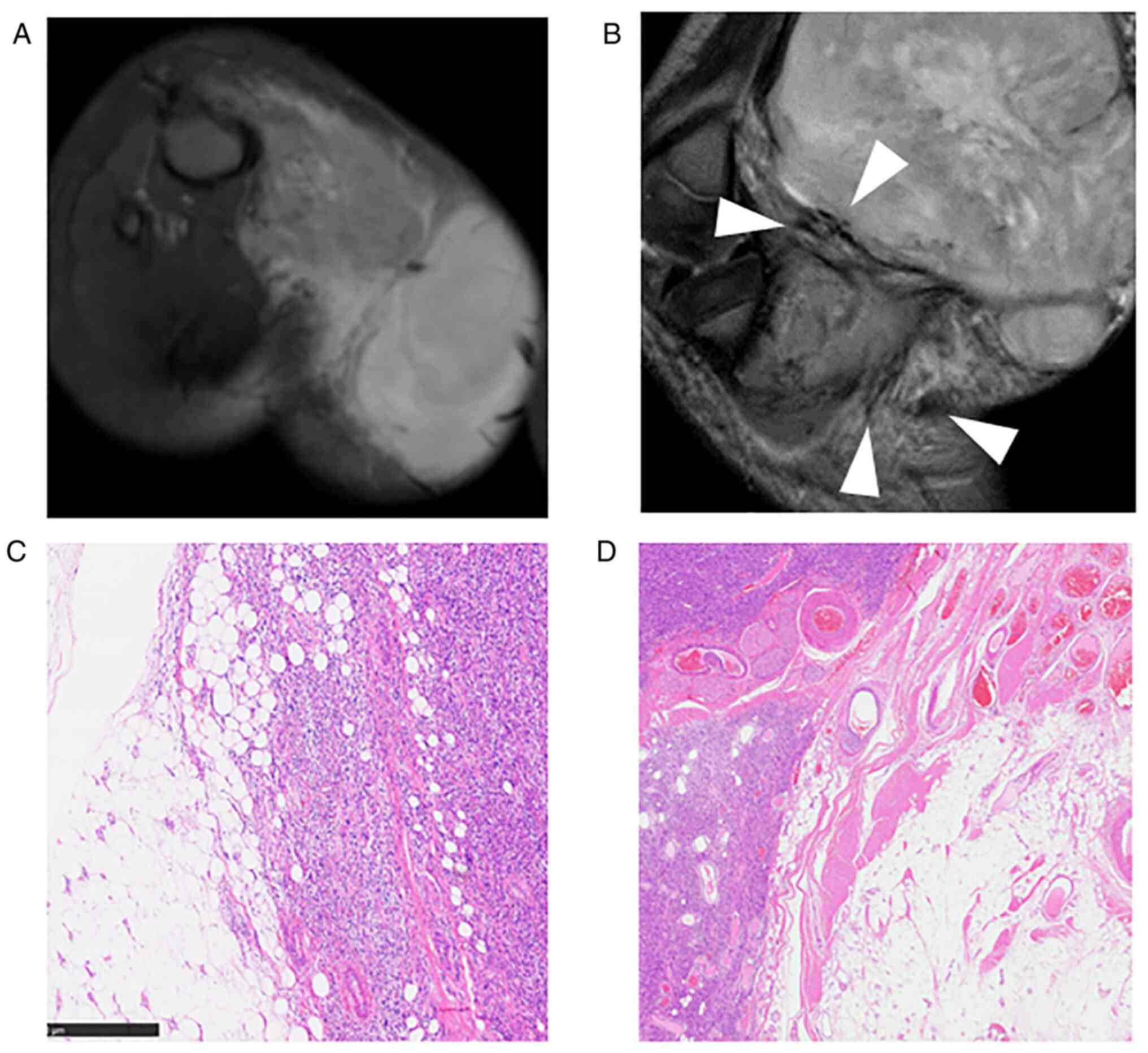
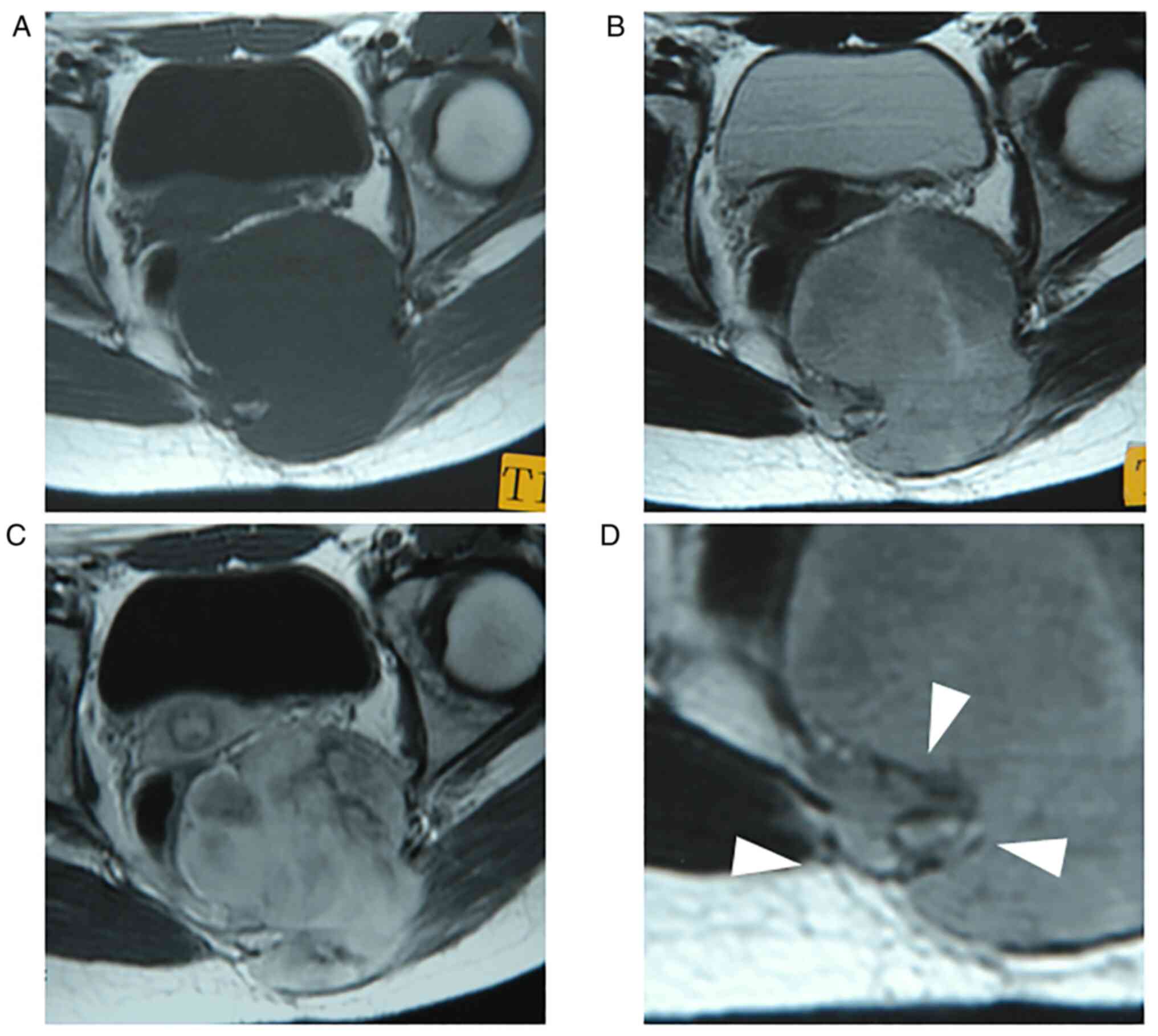
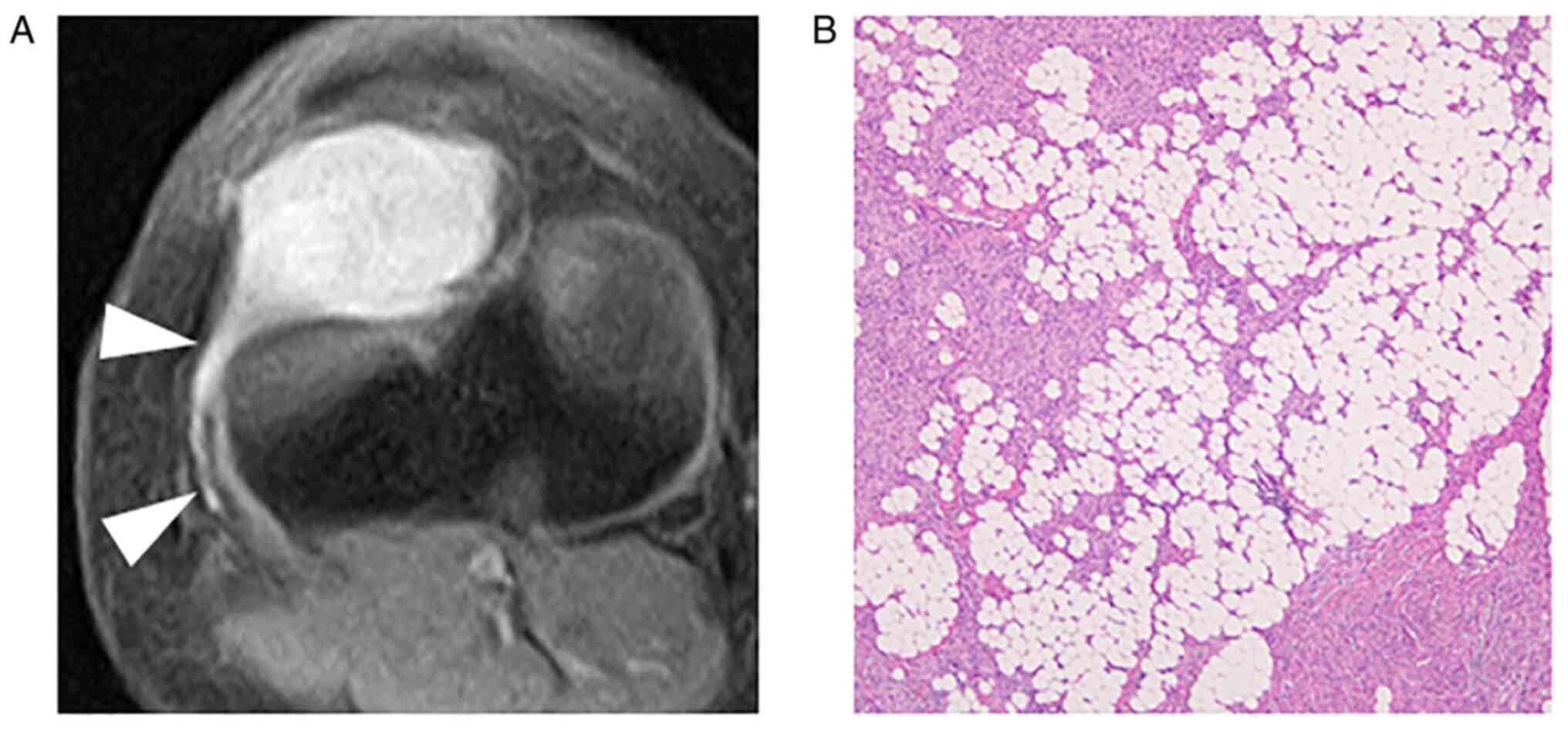
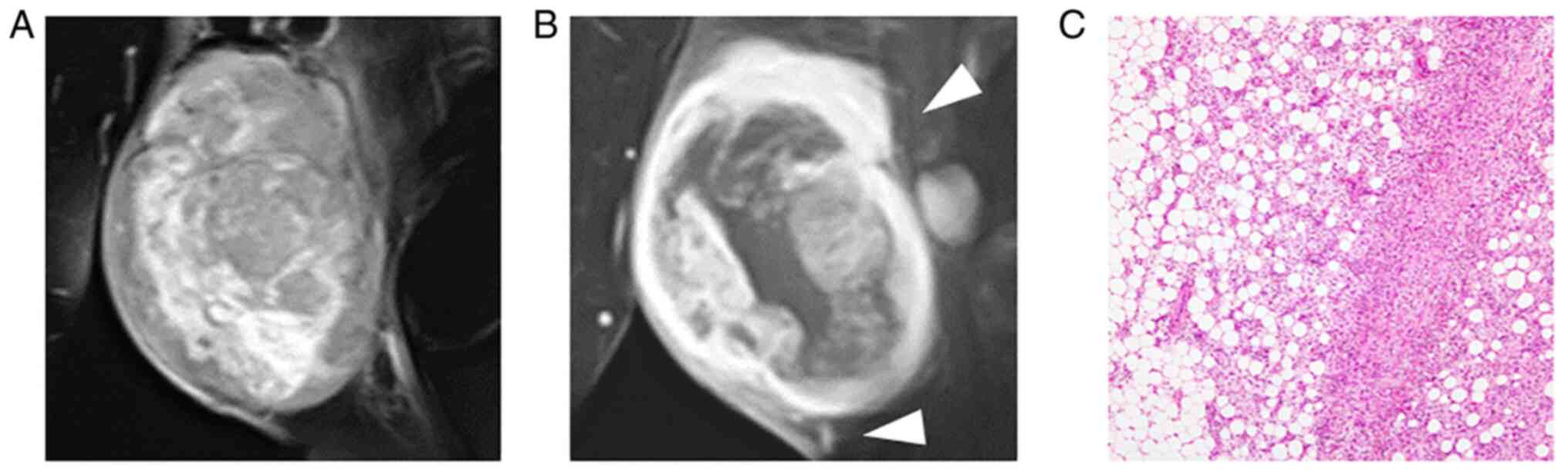
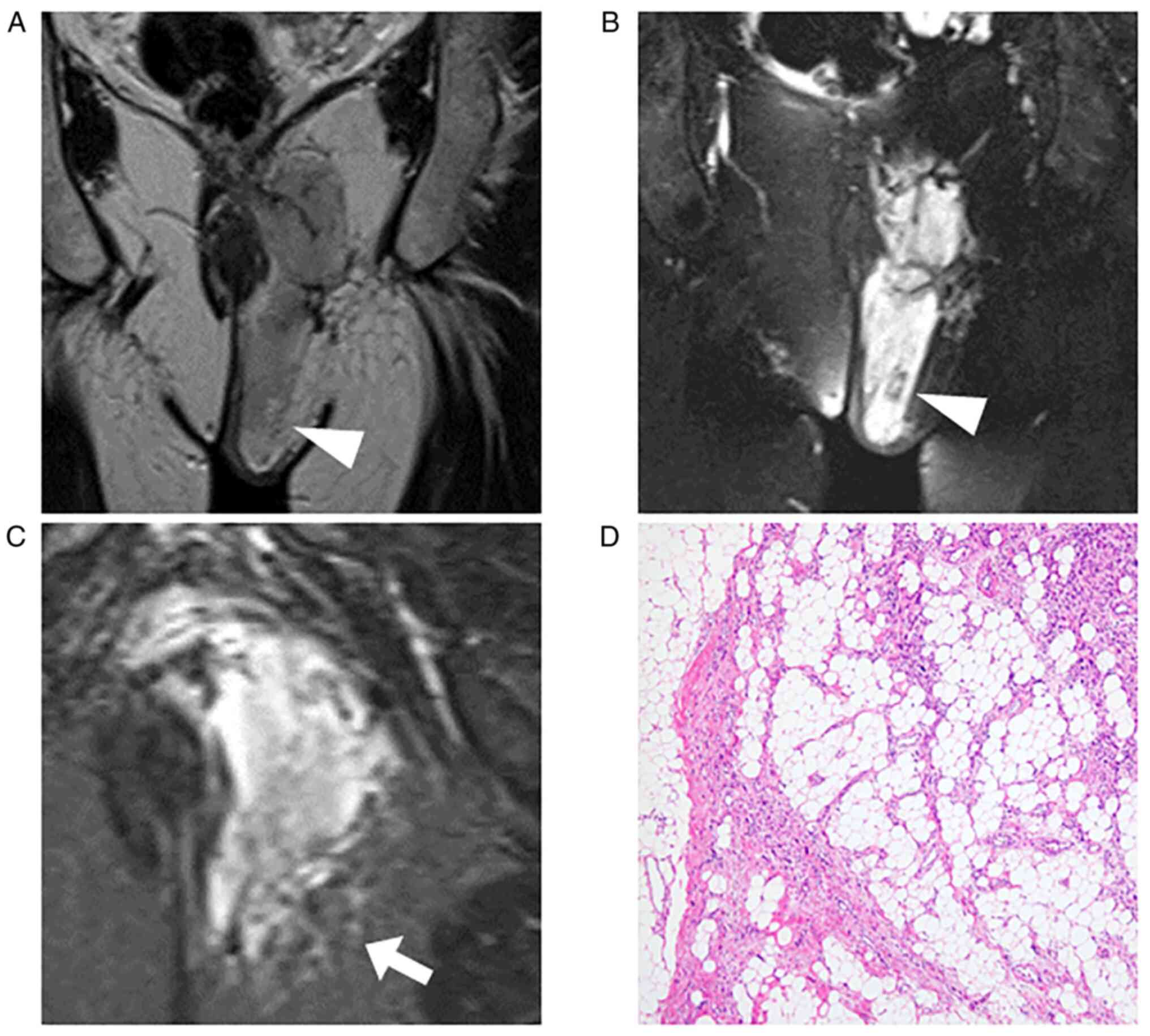
images. All tumors were intensely enhanced by gadolinium (Fig. 1, Fig.
2, Fig. 3, Fig. 4 and Fig. 5), and peritumoral enhancement was
observed in two patients. Regarding intratumoral features, one
tumor had a fatty component (Fig.
5), two fibrotic component, three central necrosis (Fig. 4), and one lobular architecture.
 | Table IIImaging findings of the tumor. |
Table II
Imaging findings of the tumor.
| Case | Depth | Maximal diameter,
cm | T1 | T2 | Degree of contrast
enhancement | Pattern of
enhancement | Peritumoral
enhancement | Fatty
component | Fibroblastic
component | Central
necrosis | Lobular
architecture |
|---|
| 1 | Deep | 11 | High | High | Intense | Homogeneous | Yes | No | Yes | No | No |
| 2 | Deep | 11.3 | Iso | High | Intense | Heterogeneous | No | No | No | Yes | Yes |
| 3 | Deep | 8.6 | Iso | High | Intense | Heterogeneous | No | No | No | Yes | No |
| 4 | Deep | 4 | Iso | High | Intense | Homogeneous | Yes | No | Yes | No | No |
| 5 | Superficial | 11 | Iso | High | Intense | Heterogeneous | No | No | No | Yes | No |
| 6 | Superficial | 10.5 | Iso | High | Intense | Homogeneous | No | Yes | No | No | No |
 | Table IIIImaging findings of the tumor growth
pattern and flow-voids. |
Table III
Imaging findings of the tumor growth
pattern and flow-voids.
| Case | Growth pattern | Edema | Infiltration into
fatty tissue | Tail sign | Distribution of
flow-voids | Number of
flow-voids | Maximal diameter of
flow-void, mm |
|---|
| 1 | Focal-type | Limited | Yes | No | Intra and
peripheral | ≥5 | 8 |
| 2 | Diffuse-type | Limited | Yes | No | Intra and
peripheral | ≥5 | 4 |
| 3 | Pushing-type | Absent | No | No | Peripheral | ≤5 | 1.5 |
| 4 | Focal-type | Absent | Yes | Yes | None | NA | NA |
| 5 | Focal-type | Limited | Yes | No | Peripheral | ≤5 | 1 |
| 6 | Focal-type | Absent | Yes | No | Intra and
peripheral | ≥5 | 4 |
The growth pattern of these tumors was focally and
globally infiltrative in five patients, especially infiltration
into the surrounding fat tissue (Figs.
1, 3, 4 and 5),
and one tumor had distinct tumor margin (Fig. 2). Five tumors had flow voids
(Figs. 1, 2 and 5)-three cases at the intra- and
peripheries (Figs. 1 and 5) and two cases at the periphery
(Fig. 2). Three tumors had more
than five flow voids, and these tumors had a relatively large
diameter of the flow voids.
Histopathology and correlation with
imaging characteristics
Representative histopathological findings and
surgical margin are presented in Tables IV and V. Five patients were both CD34 and S100
positive, and one patient was CD34 positive. Mitotic counts equal
to or greater than 10 were observed in two patients, whereas others
had few mitoses. Cellularity was high in all four patients. In all
patients, invasion of the surrounding tissues was observed,
especially in fat tissue compatible with MRI findings. Invasion
into the surrounding tissues from the main nodule was also observed
in histological evaluation (Figs.
1, 3, 4 and 5).
Local recurrence was observed in one patient (Case 3) after R1
resection, and no local recurrence was observed with marginal or
wide margin resection. Vascular invasion was observed in one
patient (Case 3) with distant metastasis.
 | Table IVHistopathological findings. |
Table IV
Histopathological findings.
| Case | Mitosis | Cellularity | Necrosis | Infiltration into
fat tissue | Vascular
invasion |
|---|
| 1 | 0-1 | High | No | + | - |
| 2 | 10 | High | No | + | - |
| 3 | 10-15 | High | <50% | + | + |
| 4 | 0-2 |
Intermediate-high | No | + | - |
| 5 | 5-10 | Intermediate | <50% | + | - |
| 6 | 0-5 | High | No | + | - |
 | Table VSurgical margin. |
Table V
Surgical margin.
| Case | Intended surgical
margin | Invasion from the
main nodule | Microscopic
surgical margin |
|---|
| 1 | Wide | Fat tissue,
muscle | R0 |
| 2 | Wide | Fat tissue | R1 |
| 3 | Marginal | Fat tissue, muscle,
intermuscular fat tissue | R1 |
| 4 | Wide | Fat tissue | R0 |
| 5 | Wide | Fat tissue | R0 |
| 6 | Wide | Fat tissue, muscle,
intermuscular fat tissue | R0 |
Discussion
NTRK-RSCNs are extremely rare tumors, and
identification of these tumors is important because NTRK inhibitors
are highly effective and durable (6). NTRK-RSCNs include various fusion
types, and their histological features and grades vary (1). According to a recent report,
DNA-sequencing target panel and Pan-TRK immunohistochemistry,
commonly available methods, could overlook these tumors (5); therefore, it is important to
understand the clinical features of these tumors. There are only
two case reports describing the imaging characteristics of these
tumors (7,9). In this study, we characterized the
MRI appearance of NTRK-RSCNs, which suggested that NTRK-RSCNs could
be one of the differential diagnoses of highly vascular-rich
mesenchymal tumors, such as SFT and alveolar soft part sarcoma
(ASPS). Furthermore, we observed that NTRK-RSCNs can infiltrate the
surrounding tissues, especially fat tissues, by comparing imaging
characteristics and histopathological features. These findings
could help surgeons plan the extent of resection of these
tumors.
Imaging features of NTRK-RSCNs have been mentioned
so far in only two case reports (7,9).
This case series of six patients with NTRK-RSCNs reveals that these
tumors have a highly vascular nature, presence of intra- or
peritumoral vessels is characteristic feature of these tumors, and
the findings are compatible with that of a previous case report
(7,9). The number of flow voids and the
diameter of flow-void vessels were different in each patient, and
five out of the six patients showed intra- or peritumoral flow
voids. As for other mesenchymal tumors with a highly vascular
nature, SFTs and ASPS have been reported. McCarville et al
reported that ASPS imaged with MRI in 19 patients had flow voids in
all patients and that 95% (18/19 patients) of patients had both
intra- and peripheral flow voids (10). Compared to the results of this
report, NTRK-RSCNs could be less vascular than ASPS, although they
tend to be highly vascular tumors. Crombé et al reported
that ASPS had eight characteristic imaging features-deep location,
high signal intensities on T1-weighted imaging, central area of
necrosis, absence of fibrotic component, infiltrative growth
pattern, absence of tail sign, presence of intra- and peritumoral
flow voids, and number of flow voids ≥5. Moreover, 80% of ASPS pose
at least seven out of the eight features (11). In our patients, the scores for
these eight characteristics were 6, 7, 4, 3, 5, and 6 points in
Cases 1-6, respectively. These results indicate that NTRK-RSCNs
resemble ASPS in terms of highly vascular tumors, but NTRK-RSCNs
tend to be less vascular than ASPS and have different imaging
characteristics. SFT is an important differential diagnosis for
NTRK-RSCNs because it shows monotonous spindle cell tumors and
vascular-rich lesions with a hemangiopericytomatous appearance,
which is also observed in NTRK-RSCNs (12). In our cases, three patients were
first diagnosed with SFTs. Garcia-Bennett et al reported
imaging findings of SFTs in nine patients and revealed that the
common findings were well-defined polylobulated masses and the
presence of vascular pedicles. However, invasion into adjacent
structures was reported to be only 22% (13), and other reports of imaging
findings of SFTs demonstrated that the ratio was 9% (14). In contrast, NTRK-RSCNs in our cases
showed infiltration into the adjacent tissues on MRI, and
infiltration into the surrounding tissues was confirmed by
histopathological analysis. The finding of infiltration into
adjacent tissues was consistent with previous reports (2,4,15).
This infiltrative feature can be a differential point of imaging
between SFTs and NTRK-RSCNs. SFT is histologically diagnosed using
its specific molecular marker, STAT6, because of the
NAB2-STAT6 fusion gene (16). In cases where imaging findings show
a highly vascular mass and histological findings show spindle cell
tumor without STAT6 immunostaining, the screening of NTRK
gene fusion, including Pan-TRK, fluorescence in situ hybridization,
DNA-sequencing targeted panel, and RNA sequencing, should be
considered to discover NTRK-RSCNs. Recently, spindle cell tumors
with CD34 or S100 immunostaining positive could pose other
RTK-related fusions, including ALK, BRAF,
ROS1, and RET (14,17).
It is unknown whether these tumors are vascular-rich lesions;
therefore, further investigation should be performed.
We recognized the clinically important feature, that
is, focal infiltration of the tumor into adjacent structures, in
imaging and histopathological findings, which was recently observed
in previous case reports (7,9). In
five of the six patients, infiltration into fatty tissue was
observed on MRI, which was confirmed by histological findings. In
Case 3, histological infiltration of the surrounding tissue,
including fat tissue, intermuscular fat, and muscle, was observed
despite imaging findings with the pushing type and no invasive
pattern into fat tissue. This patient had metastasis after surgery,
with relatively higher mitosis compared with other patients, and
vascular invasion was observed histologically. Although it is
difficult to conclude from our findings, due to limited cases, that
imaging findings could not predict the malignant potential of this
tumor, NTRK-RSCNs could have malignant potential although the
imaging findings were well circumscribed. Furthermore, histological
findings with high mitotic count should be considered a sign of
malignant potential of this tumor, as described by previous reports
(2,17), and vascular invasion should be
carefully investigated, which could be a poor prognostic factor,
similar to other soft tissue sarcomas (18). Intended wide resection with
negative histological margins resulted in no recurrence in four
patients, although one patient with a marginal margin had no
recurrence. Considering the infiltrative nature of this tumor, wide
resection is recommended. Effective chemotherapeutic NTRK
inhibitors, including entrectinib and larotrectinib, are usually
used in advanced cases, whereas the efficacy and safety of
neoadjuvant larotrectinib treatment have been reported in the
management of children with locally advanced TRK fusion sarcomas
(19). To reduce the loss of
function after extended tumor resection, neoadjuvant treatment with
NTRK inhibitors for NTRK-RSCNs is promising, and further analysis
is required.
The present study has some limitations. The has a
retrospective design and analyzed a small number of patients. The
entity of NTRK-RSCNs includes a broad range of histological
features and grades, and local aggressiveness and metastatic
potential differ depending on the tumor. However, our imaging
findings of highly vascular tumors might be a common feature of
this tumor driven by the same driver NTRK gene fusions.
Despite these limitations, to the best of our knowledge, this is
the first case series reporting the MRI findings of NTRK-RSCN
lesions in soft tissues.
In conclusion, this study mainly presented two
important imaging findings of NTRK-RSCNs-high vascularity and focal
invasiveness into the surrounding tissues, especially fat tissue.
NTRK-RSCNs could be candidates for the differential diagnosis of
highly vascular mesenchymal tumors, including SFT and ASPS.
Furthermore, the focal invasiveness of the tumor shown by MRI was
confirmed by histopathological analysis, and NTRK-RSCNs could be
resected with wide margin to avoid local recurrence. These findings
provide useful information for the diagnosis and treatment of
NTRK-RSCNs and would aid in improving the detection and curative
rates of these tumors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
HK wrote the paper and performed the literature
review. HK and KA confirmed the authenticity of all the raw data.
HK, YT, KA, KY, NM and ST contributed to the conception and design
of the manuscript, and critically revised the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the institutional review
board of The University of Tokyo Hospital (approval no. 11019).
Written informed consent was obtained from the patients or the
parents of patients for all individual participants included in the
study at The University of Tokyo Hospital and The Cancer Institute
Hospital of the Japanese Foundation for Cancer Research.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this report and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO Classification of Tumours Editorial
Board: Soft Tissue and Bone Tumours: WHO Classification of Tumors.
5th edition. IARC Press, Lyon, pp403-409, 2020.
|
|
2
|
Suurmeijer AJ, Dickson BC, Swanson D,
Zhang L, Sung YS, Huang HY, Fletcher CD and Antonescu CR: The
histologic spectrum of soft tissue spindle cell tumors with NTRK3
gene rearrangements. Genes Chromosomes Cancer. 58:739–746.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Baranov E and Hornick JL: Soft tissue
special issue: Fibroblastic and myofibroblastic neoplasms of the
head and neck. Head Neck Pathol. 14:43–58. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Agaram NP, Zhang L, Sung YS, Chen CL,
Chung CT, Antonescu CR and Fletcher CD: Recurrent NTRK1 gene
fusions define a novel subset of locally aggressive
lipofibromatosis-like neural tumors. Am J Surg Pathol.
40:1407–1416. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Solomon JP, Linkov I, Rosado A, Mullaney
K, Rosen EY, Frosina D, Jungbluth AA, Zehir A, Benayed R, Drilon A,
et al: NTRK fusion detection across multiple assays and 33,997
cases: Diagnostic implications and pitfalls. Mod Pathol. 33:38–46.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cocco E, Scaltriti M and Drilon A: NTRK
fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin
Oncol. 15:731–747. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nakamura T, Matsumine A, Matsubara T,
Asanuma K, Yada Y, Hagi T and Sudo A: Infiltrative tumor growth
patterns on magnetic resonance imaging associated with systemic
inflammation and oncological outcome in patients with high-grade
soft-tissue sarcoma. PLoS One. 12(e0181787)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Takamiya A, Ishibashi Y, Makise N, Hirata
M, Ushiku T, Tanaka S and Kobayashi H: Imaging characteristics of
NTRK-rearranged spindle cell neoplasm of the soft tissue: A case
report. J Orthop Sci. S0949-2658(00367-5)2022.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
9
|
Overfield CJ, Edgar MA, Wessell DE, Wilke
BK and Garner HW: NTRK-rearranged spindle cell neoplasm of the
lower extremity: Radiologic-pathologic correlation. Skeletal
Radiol. 51:1707–1713. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McCarville MB, Muzzafar S, Kao SC, Coffin
CM, Parham DM, Anderson JR and Spunt SL: Imaging features of
alveolar soft-part sarcoma: A report from children's oncology group
study ARST0332. AJR Am J Roentgenol. 203:1345–1352. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Crombé A, Brisse HJ, Ledoux P,
Haddag-Miliani L, Bouhamama A, Taieb S, Le Loarer F and Kind M:
Alveolar soft-part sarcoma: Can MRI help discriminating from other
soft-tissue tumors? A study of the French Sarcoma Group. Eur
Radiol. 29:3170–3182. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Haller F, Knopf J, Ackermann A, Bieg M,
Kleinheinz K, Schlesner M, Moskalev EA, Will R, Satir AA,
Abdelmagid IE, et al: Paediatric and adult soft tissue sarcomas
with NTRK1 gene fusions: A subset of spindle cell sarcomas unified
by a prominent myopericytic/haemangiopericytic pattern. J Pathol.
238:700–710. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Garcia-Bennett J, Olivé CS, Rivas A,
Domínguez-Oronoz R and Huguet P: Soft tissue solitary fibrous
tumor. Imaging findings in a series of nine cases. Skelet Radiol.
41:1427–1433. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wignall OJ, Moskovic EC, Thway K and
Thomas JM: Solitary fibrous tumors of the soft tissues: Review of
the imaging and clinical features with histopathologic correlation.
AJR Am J Roentgenol. 195:W55–W62. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kao YC, Suurmeijer AJH, Argani P, Dickson
BC, Zhang L, Sung YS, Agaram NP, Fletcher CDM and Antonescu CR:
Soft tissue tumors characterized by a wide spectrum of kinase
fusions share a lipofibromatosis-like neural tumor pattern. Genes
Chromosomes Cancer. 59:575–583. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Doyle LA, Vivero M, Fletcher CD, Mertens F
and Hornick JL: Nuclear expression of STAT6 distinguishes solitary
fibrous tumor from histologic mimics. Mod Pathol. 27:390–395.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Suurmeijer AJH, Dickson BC, Swanson D,
Zhang L, Sung YS, Cotzia P, Fletcher CDM and Antonescu CR: A novel
group of spindle cell tumors defined by S100 and CD34 co-expression
shows recurrent fusions involving RAF1, BRAF, and NTRK1/2 genes.
Genes Chromosomes Cancer. 57:611–621. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gustafson P, Akerman M, Alvegård TA,
Coindre JM, Fletcher CD, Rydholm A and Willén H: Prognostic
information in soft tissue sarcoma using tumour size, vascular
invasion and microscopic tumour necrosis-the SIN-system. Eur J
Cancer. 39:1568–1576. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
DuBois SG, Laetsch TW, Federman N, Turpin
BK, Albert CM, Nagasubramanian R, Anderson ME, Davis JL, Qamoos HE,
Reynolds ME, et al: The use of neoadjuvant larotrectinib in the
management of children with locally advanced TRK fusion sarcomas.
Cancer. 124:4241–4247. 2018.PubMed/NCBI View Article : Google Scholar
|