Introduction
The liver is a parenchymatous organ that is
essential for intermediate metabolism and detoxication. Hepatocytes
are the main cells in the liver, representing almost 80% of the
whole liver mass (1). Despite the
strong regenerative potential of the liver, the continuous or
repeated intake of several drugs can result in liver injury. This
phenomenon is commonly known as drug-induced liver injury (DILI).
As with any other drugs used for other purposes, the liver is the
first site of biotransformation during the systemic treatment of
cancer. The mechanisms of liver damage vary with particular
cytostatics and their combinations, and detailed data on the
epidemiology of chemotherapy-induced liver injury (CILI) are
insufficient (2).
There are several strategies used for the management
of CILI. Dose reduction or a delay in administering chemotherapy
are common strategies used to combat an impaired hepatic function.
However, both strategies may lead to a decrease in the efficacy of
systemic treatment in terms of lowering the survival rates
(3,4). Therefore, there is an urgent need for
the development of an active drug that can accelerate liver
regeneration.
Silymarin is an extract of milk thistle (Silybum
marianum), which consists of a mixture of flavonolignans,
flavonoids and polyphenols. Silybinin is the dominant and most
biologically active compound present in silymarin. The effect of
silymarin on the liver is pleiotropic. The excessive intake of
toxins or intensive oxidation as a part of free fatty acid
metabolism results in the elevated production of reactive oxygen
species. Silymarin mitigates this oxidative stress by scavenging
reactive oxygen species (5). The
anti-inflammatory effects mediated by the inhibition of a number of
pro-inflammatory cytokines (such as IL-1, IL-6 and TNF-α) have been
previously documented by Federico et al (6). The downregulation of oxidative and
inflammatory activity results in a decreased level of parenchymal
damage. Silymarin also inhibits the conversion of stellate cells
into myofibroblasts and reduces the production of procollagen III,
α-SMA and TGF-β. Along with the anti-apoptotic effect of silymarin,
the inhibition of the fibrotic transformation of liver tissue is
also observed (6).
In general, there are limited data available on the
efficacy of silymarin in DILI. The majority of trials focus on
liver toxicity induced by antituberculotic drugs. Luangchosiri
et al (7) reported that
silymarin at a dose of 420 mg daily decreased the incidence of DILI
associated with antituberculotic treatment. However, in another
randomized controlled trial by Marjani et al (8), silymarin failed to exhibit similar
activity in alleviating already developed DILI following the intake
of antituberculotic drugs (8).
Even less data are available regarding the efficacy
of silymarin in the management of CILI. The randomized prospective
trial by Moezian et al (9)
investigated the role of silymarin in 30 patients with early-stage
breast cancer treated with the chemotherapy regimen, AC/T
(doxorubicin, cyclophosphamide followed by paclitaxel) who
developed radiologically-confirmed CILI during/after chemotherapy.
Silymarin at a dose of 140 mg daily failed to lead to a
statistically significant improvement in the levels of biochemical
and radiological markers of liver injury in the experimental arm.
Nevertheless, they concluded that there was a trend in favor of the
use of silymarin. Acknowledging the limitations of this trial,
Moezian et al (9) called
for further investigations in this matter.
Another trial by Mohaghegh et al (10) focused on the effects of silymarin
on taxane-based CILI. In their study, 99 patients with invasive
breast cancer treated with anthracyclines followed by taxanes
(docetaxel or paclitaxel) were randomly divided into two study
arms. The patients in the experimental arm were administered
silymarin at a dose of 70 mg three times a day during chemotherapy.
The control arm was administered a placebo. The levels of two liver
enzymes [alanine aminotransferase (ALT) and aspartate
aminotransferase (AST)] and serum bilirubin (Bil) levels were
measured after each dose of taxane (10). Although there was initially only a
small difference in the levels of liver enzymes and Bil between the
groups, a statistically significant difference between the groups
was observed in the levels of liver enzymes after 1 month of
treatment. They thus concluded that silymarin can revoke the
increase in the levels of liver enzymes when administered to
patients treated with taxanes, and stated that this effect may be
enhanced with higher doses of silymarin (10).
Inconsistent results may originate from the various
doses of silymarin used in the cited trials. The optimal dosage of
silymarin is a matter of debate. Fathalah et al (11) reported improved outcomes in
patients treatment for liver injury with high-dose silymarin (1,050
mg daily) compared to a ‘standard’ dose of silymarin (420 mg daily)
in patients with decompensated liver cirrhosis. That trial suggests
that the effectivity of silymarin on liver injury may be
dose-dependent (11).
The present study retrospectively examined 180
patients treated with systemic oncological treatment (chemotherapy
and/or targeted therapy) who were also treated with silymarin due
to an elevation in the levels of liver enzymes and/or Bil. The
present study aimed to assess the association between the dose of
silymarin and a decrease in the levels of selected liver function
parameters. The goal was to determine whether an increased dose of
silymarin is associated with improved liver function parameters and
subsequently, to assess the optimal dose of silymarin for the
treatment and prevention of CILI.
Patients and methods
Patient information
Adult patients with solid malignancies, including
lymphomas treated with systemic oncological therapy and silymarin
between January, 2015 and November, 2021 were included in the
present retrospective study. Both male and female patients were
included. Patients with normal and elevated levels of liver
markers, such as ALT, AST and Bil in liver function tests (LFTs)
were enrolled in the present study. No patients with known viral
hepatitis were included in the study. There were no other selection
criteria regarding diagnosis, stage, age, systemic treatment or the
dose of silymarin. The characteristics of the included patients are
presented in Table I. The present
study was approved by the Ethics Committee of Faculty Hospital
Trencin (Trencin, Slovakia). Since the present study was
retrospective and non-interventional in nature, the requirement to
obtain patient informed consent for participation was waived by the
Ethics Committee.
 | Table IClinicopathological characteristics of
the 180 patients included in the present study. |
Table I
Clinicopathological characteristics of
the 180 patients included in the present study.
| Clinicopathological
characteristics | Values |
|---|
| Age, years | |
|
Average | 63.3 |
|
Median | 64.55 |
|
Range | 22.07-93.05 |
| Sex, n (%) | |
|
Male | 97 (53.89) |
|
Female | 83 (46.11) |
| Cancer subtypes, n
(%) | |
|
Adenocarcinoma | 162(90) |
|
Squamous
cell cancer | 6 (3.33) |
|
Non-Hodgkin
lymphoma | 4 (2.22) |
|
Neuroendocrine
tumor | 3 (1.67) |
|
GIST | 2 (1.11) |
|
Small cell
cancer | 1 (0.56) |
|
High-grade
glioma | 1 (0.56) |
|
Non-seminoma | 1 (0.56) |
| Stage at the time of
diagnosis, n (%) | |
|
I | 11 (6.11) |
|
II | 22 (12.22) |
|
III | 60 (33.33) |
|
III | 87 (48.33) |
| Stage at the time the
initiation of silymarin treatment, n (%) | |
|
I | 5 (2.79) |
|
II | 12 (6.67) |
|
III | 37 (20.55) |
|
IV | 126(70) |
| Grade, n (%) | |
|
1 | 43 (23.89) |
|
2 | 68 (37.78) |
|
3 | 65 (36.11) |
|
4 | 2 (1.11) |
| Non-applicable, n
(%) | 2 (1.11) |
|
Liver
tumor | |
|
Any | 77 (42.78) |
|
Liver
metastases | 73 (40.56) |
|
Primary
liver tumor | 4 (2.22) |
| Initial elevation of
liver function test, n (%) | |
|
ALT | 111 (61.67) |
|
AST | 116 (64.44) |
|
Bilirubin | 86 (47.78) |
Assessment of transaminases and
Bil
The levels of transaminases (ALT and AST) and total
serum Bil levels were assessed prior to the initiation of silymarin
use, and at 3-6 weeks (1st assessment; ALT1, AST1 and Bil1) and at
6-12 weeks (2nd assessment; ALT2, AST2 and Bil2) following the
initiation of treatment. Concurrently, the dose of silymarin was
determined at the initiation of treatment, and at the 1st and 2nd
assessment. In the case of incomplete data for AST, ALT or Bil
levels (missing 1st or 2nd assessment), the patient was censored
and LFTs with incomplete data were not evaluated. The reference
values for ALT, AST and Bil were 0.05-0.75, 0.05-0.63 µkat/l and
3.0-17.0 µmol/l, respectively.
An Architect® ci16200 analyzer (Abbott
Pharmaceutical Co. Ltd.) was used for the determination of the
serum ALT, AST and Bil levels. The International Federation of
Clinical Chemistry and Laboratory Medicine (IFCC)-approved method
with nicotinamide adenine dinucleotide hydrogen and pyridoxal
5'-phosphate was used for the evaluation of the ALT and AST levels.
The serum Bil level was determined using the IFCC-approved
diazonium salt method. The reagents were supplied by Abbott
Pharmaceutical Co. Ltd.
A >20% elevation from the baseline values in the
LFTs was considered as an increase. A decrease >20% of baseline
value was considered as a decrease. Any change in LFT values that
was <20% of the baseline value was regarded as stabilization.
Any change that was within the normal (reference) range of values
for a particular LFT was considered a stabilization.
Determination of the association
between the dose of silymarin and the levels of transaminases and
Bil
Pearson's correlation analysis was performed to
evaluate the association between the initial dose of silymarin, and
the levels of transaminases and Bil. Subsequently, three
independent variables were established based on collected data and
common medical practice when dealing with liver toxicity. These
variables were named ‘initial dose reduction of systemic treatment’
(IDR), ‘systemic treatment dose reduction at first assessment’
(DR1M) and ‘increase of the silymarin dose at first assessment’
(SDE).
The IDR was defined as the absolute difference
between the standard dose of systemic treatment (either calculated
according to body surface area/weight or flat-fixed dose) and the
actual dose of systemic treatment at the start of silymarin use.
DR1M was defined as the absolute difference between the standard
dose of systemic treatment (either calculated according to body
surface area/weight or flat-fixed dose) and the actual dose of
systemic treatment at 1st assessment (mentioned above). The SDE was
defined as the absolute difference between the initial dose of
silymarin and the actual dose of silymarin at 1st assessment.
Finally, the effects of the initial dose of
silymarin (IDoS), IDR, DR1M and SDE (independent variables) on the
levels of ALT2, AST2 and Bil2 (dependent variables) were evaluated.
These effects were evaluated in a population of patients with
livers tumor and without liver tumors.
Statistical analysis
The initial evaluation of the correlation between
the IDoS, and the values of ALT, AST and Bil was performed using
Pearson's correlation analysis. Subsequently, in order to detect
potential confounding factors in the association between the IDoS
and the results of the LFTs, regression analysis was performed.
Regression analysis was performed in a subpopulation of patients
with liver lesions and without liver lesions. Since the independent
variables were non-parametric (continuous data), multiple linear
regression was used to evaluate the effects of IDoS, IDR, DR1M and
SDE on the levels of ALT2, AST2 and Bil2. Python version 3.9.5
(Python Software Foundation, 2021) and IBM SPSS Statistic v 28.0
(IBM, 2021) were used for data processing.
Results
A total of 654 patients were screened in the initial
research. Subsequently, 180 patients (83 females and 97 males) were
included in the retrospective study. The median age of the patients
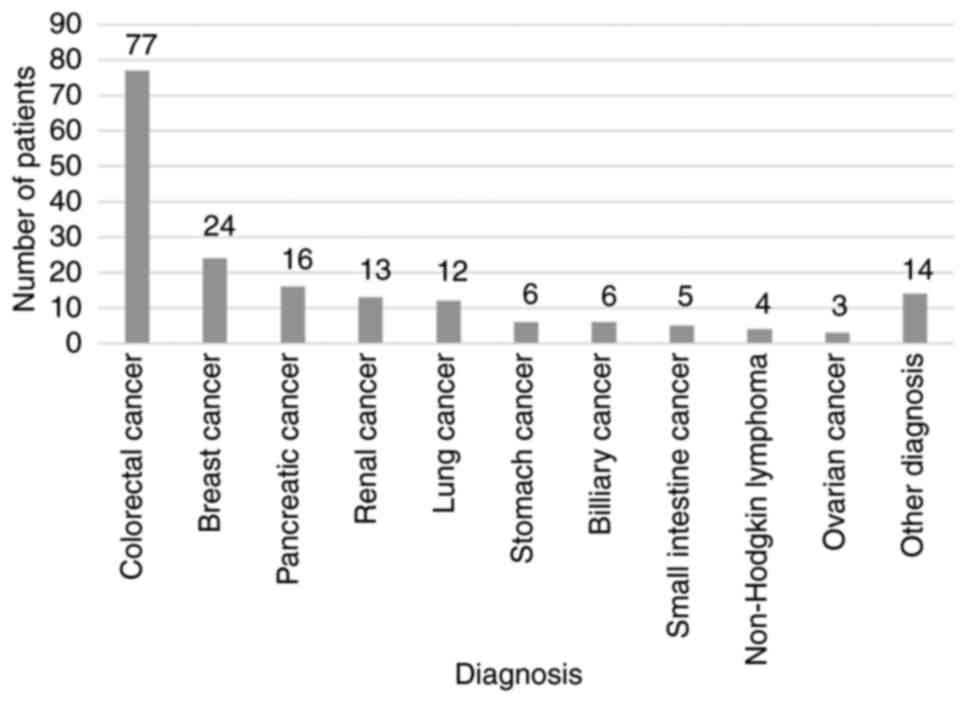
was 64.55 years. The most frequent diagnosis was that of colorectal
cancer (n=77, 42.78%) followed by breast (n=24, 13.33%) and
pancreatic cancer (n=16, 8.89%). The baseline characteristics of
the patients according to diagnosis are presented in Fig. 1.
At the time of diagnosis, 87 (48.33%) patients had
metastatic cancer, 60 (33.33%) patients had stage III cancer, stage
II was present in 22 (12.22%) patients and 11 (6.11%) patients had
stage I cancer. At the time of the initiation of silymarin
treatment, 126 (70%) patients had stage IV cancer, 37 (20.55%) had
stage III cancer, 12 (6.67%) patients presented with stage II
cancer and 5 (2.79%) patients had stage I cancer. Liver metastases
were present in 73 (40.56%) patients and 4 (2.22%) patients were
treated for primary liver tumors. The clinicopathological
characteristics of the included patients are presented in Table I.
An initial elevation in ALT levels was documented in
111 (61.67%) patients and an elevation in AST levels was found in
116 (64.44%) patients. On the other hand, less than half of the
patients presented with an elevation in total serum Bil levels (86,
47.78%) (Table I). In total, 111
(61.67%) patients were treated with chemotherapy, 38 (21.11%)
patients were treated with combined chemotherapy and targeted
therapy, and 31 (17.22%) patients were treated with targeted
therapy only. The characteristics of the patients according to the
systemic therapy used are presented in Fig. 2. The median initial dose of
chemotherapy was 75%; thus, the median dose reduction was 25%.
Since no clear recommendation regarding the dosage of silymarin is
available, the initial dose of silymarin was at the discretion of
the physician and ranged from 150-900 mg. However, the majority of
the patients were treated with a dose of 450 mg (n=101, 56%) and
300 mg (n=63, 35%). The median initial dose of silymarin was 450
mg. The sum of patients who achieved stabilization or a decrease in
the levels of transaminases and Bil is presented in Table II.
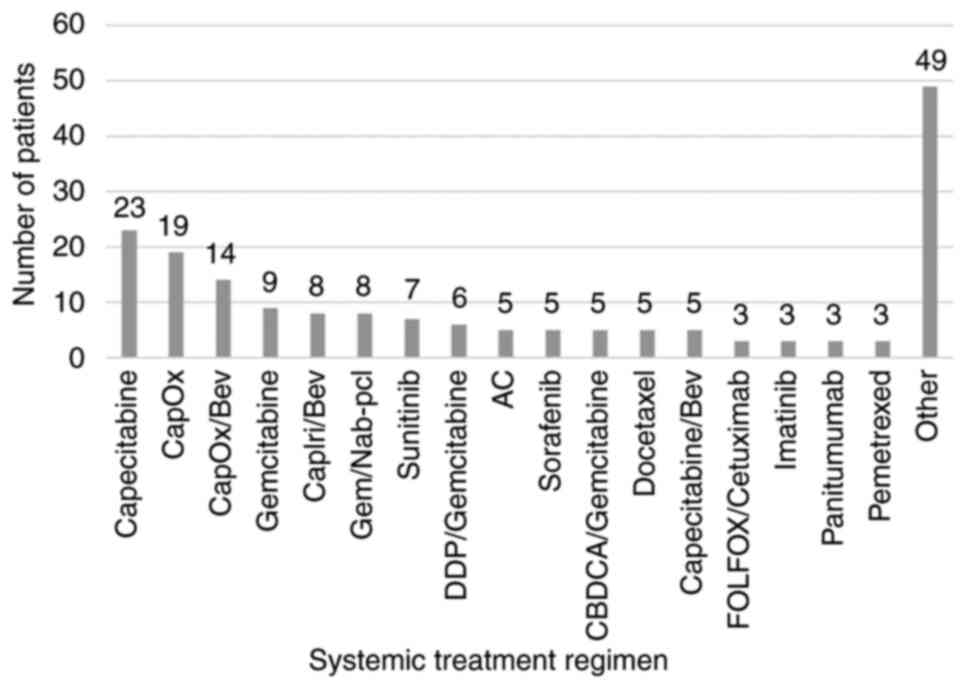 | Figure 2Characteristics of patients according
to the chemotherapy used. CapOx, capecitabine/oxaliplatin; Bev,
bevacizumab; CapIri, capecitabine/irinotecan; Gem/Nab-pcl,
gemcitabine/nab-paclitaxel; DDP, cisplatin; AC,
adriamycin/cyclophosphamide; CBDCA, carboplatin; FOLFOX, folinic
acid, 5-fluoruracil, oxaliplatin. Regimens marked as ‘Other’
contributed with <3 cases to the present study. |
 | Table IICharacteristics of the patients who
achieved a decrease, stabilization or an increase in parameters in
LFTs during treatment with silymarin. |
Table II
Characteristics of the patients who
achieved a decrease, stabilization or an increase in parameters in
LFTs during treatment with silymarin.
| LFT | Decrease, n (%) | Stabilization, n
(%) | Increase, n (%) | Censored, no. of
patients |
|---|
| ALT | 114 (67.46) | 2 (1.18) | 53 (31.37) | 11 |
| AST | 103 (62.8) | 5 (3.05) | 56 (34.15) | 16 |
| Bil | 86 (50.89) | 4 (2.37) | 79 (46.75) | 11 |
A weak to moderate correlation was observed between
the IDoS and a decrease in the levels of transaminases. The results
of Pearson's correlation analysis are presented in Table III. This correlation was,
however, statistically significant in both cases (P<0.001). No
statistically significant correlation was observed between the
Bilirubin levels and the IDoS. Regression analysis confirmed that
the IDoS was a statistically significant positive predictor for a
decrease in ALT2 levels either in patients with liver lesions or
without liver lesions. This effect appears to be stronger in
patients with liver lesions.
 | Table IIIOutcomes of Pearson's correlation
analysis between the initial dose of silymarin and the parameters
from LFTs. |
Table III
Outcomes of Pearson's correlation
analysis between the initial dose of silymarin and the parameters
from LFTs.
| Predictive
factor | LFT | Pearson's correlation
coefficient (R value; LFT) | Significance
(two-tailed) | No. of patients |
|---|
| Initial dose of
silymarin | ALT (1st
assessment) | 0.333 | <0.001 | 179 |
| Initial dose of
silymarin | ALT (2nd
assessment) | 0.361 | <0.001 | 169 |
| Initial dose of
silymarin | AST (1st
assessment) | 0,276 | <0.001 | 175 |
| Initial dose of
silymarin | AST (2nd
assessment) | 0.277 | <0.001 | 164 |
| Initial dose of
silymarin | Bil (1st
assessment) | -0.015 | 0.842 | 180 |
| Initial dose of
silymarin | Bil (2nd
assessment) | -0.009 | 0.910 | 169 |
Similarly, IDoS was a positive predictor for a
decrease in AST2 levels in patients with and without liver lesions.
Again, the effect was more pronounced in the population of patients
with liver lesions. Notably, the DR1M appeared to be a negative
predictor for a decrease in AST2 levels in patients without liver
lesions with borderline statistical significance. Finally, IDoS was
not observed to be a positive predictive factor for a decrease in
Bil levels. However, similar to AST2, the DR1M was a negative
predictive factor of a decrease in Bil levels in patients with
liver lesions. The complete results of the regression analyses for
the association between IDoS, DR1M and SDE, and the levels of
transaminases and Bil are presented in Table IV, Table V and Table VI.
 | Table IVOutcomes of the regression analysis on
the effects of IDoS, SDE, IDR and DR1M on a decrease in the ALT
level at the 2nd assessment. |
Table IV
Outcomes of the regression analysis on
the effects of IDoS, SDE, IDR and DR1M on a decrease in the ALT
level at the 2nd assessment.
| Dependent
variable | Liver lesion | Independent
variable | Std Beta | t-value | P-value | R2
value |
|---|
| ALT (2nd
assessment) | N | IDoS | 0.221 | 2.111 | 0.037 | 0.057 |
| ALT (2nd
assessment) | N | SDE | -0.030 | -0.290 | 0.772 | 0.057 |
| ALT (2nd
assessment) | N | IDR | -0.006 | -0.052 | 0.959 | 0.057 |
| ALT (2nd
assessment) | N | DR1M | -0.089 | -0.818 | 0.416 | 0.057 |
| ALT (2nd
assessment) | Y | IDoS | 0.453 | 4.196 | 0.001 | 0.215 |
| ALT (2nd
assessment) | Y | SDE | -0.105 | -0,972 | 0.334 | 0.215 |
| ALT (2nd
assessment) | Y | IDR | -0.107 | -0.883 | 0.380 | 0.215 |
| ALT (2nd
assessment) | Y | DR1M | 0.073 | 0.597 | 0.552 | 0.215 |
 | Table VOutcomes of the regression analysis
of the effects of IDoS, SDE, IDR and DR1M on a decrease in the AST
level at the 2nd assessment. |
Table V
Outcomes of the regression analysis
of the effects of IDoS, SDE, IDR and DR1M on a decrease in the AST
level at the 2nd assessment.
| Dependent
variable | Liver lesion | Independent
variable | Std Beta | t-value | P-value |
R2-value |
|---|
| AST (2nd
assessment) | N | IDoS | 0.279 | 2,684 | 0.009 | 0.110 |
| AST (2nd
assessment) | N | SDE | -0.008 | -0.080 | 0.936 | 0.110 |
| AST (2nd
assessment) | N | IDR | -0.107 | -0.980 | 0.330 | 0.110 |
| AST (2nd
assessment) | N | DR1M | -0.221 | -2.015 | 0.047 | 0.110 |
| AST (2nd
assessment) | Y | IDoS | 0.312 | 2.690 | 0.009 | 0.107 |
| AST (2nd
assessment) | Y | SDE | -0.110 | -0.948 | 0.347 | 0.107 |
| AST (2nd
assessment) | Y | IDR | -0.046 | -0.355 | 0.724 | 0.107 |
| AST (2nd
assessment) | Y | DR1M | 0.065 | 0.496 | 0.621 | 0.107 |
 | Table VIOutcomes of the regression analysis
on the effects of of IDoS, SDE, IDR and DR1M on Bil levels at the
2nd assessment. |
Table VI
Outcomes of the regression analysis
on the effects of of IDoS, SDE, IDR and DR1M on Bil levels at the
2nd assessment.
| Dependent
variable | Liver lesion | Independent
variable | Std Beta | t-value | P-value |
R2-value |
|---|
| Bil (2nd
assessment) | N | IDoS | 0.148 | 1.440 | 0.153 | 0.086 |
| Bil (2nd
assessment) | N | SDE | -0.203 | -1.979 | 0.051 | 0.086 |
| Bil (2nd
assessment) | N | IDR | 0.112 | 1.049 | 0.297 | 0.086 |
| Bil (2nd
assessment) | N | DR1M | -0.039 | -0.368 | 0.714 | 0.086 |
| Bil (2nd
assessment) | Y | IDoS | 0.016 | -0.142 | 0.887 | 0.141 |
| Bil (2nd
assessment) | Y | SDE | -0.093 | -0.816 | 0.417 | 0.141 |
| Bil (2nd
assessment) | Y | IDR | -0.237 | -1.872 | 0.065 | 0.141 |
| Bil (2nd
assessment) | Y | DR1M | -0.380 | -2.980 | 0.004 | 0.141 |
Discussion
The present retrospective study demonstrated that
silymarin was effective in reducing or stabilizing the ALT, AST and
Bil levels in more than half of the patients included (67.46, 62.8
and 50.89%, respectively). Although an increase was observed in the
levels of transaminases (31.37% for ALT and 34.15% for AST) and Bil
(46.75%) in a large portion of patients treated with silymarin, it
could not be concluded that silymarin was not effective in these
patients. Silymarin could have mitigated the detrimental effects on
LFTs caused by other factors (e.g., liver lesion progression, other
medication, unidentified underlying liver conditions, etc.). These
factors need to be identified and evaluated in future prospective
trials in selected populations.
The present study observed some association between
the IDoS and a decrease in the levels of transaminases. Correlation
coefficients expressing the correlation between increasing the
initial dose of silymarin and a decrease in ALT and AST levels were
low (R=0.333 and 0.276, respectively at the 1st assessment; and
R=0.361 and 0.277, respectively at the 2nd assessment). Although
the P-values of correlation models for both transaminases suggest
statistical significance, the impact of the IDoS on the levels of
transaminases appears to be low. These results were confirmed in
the regression analysis. Despite the fact that the present study
was retrospective in nature and only a limited number of medical
records were available, three independent variables were
established based on the available collected data and common
medical practice when dealing with liver toxicity (initial dose
reduction of systemic treatment, systemic treatment dose reduction
at first control, the elevation of silymarin dose at first
assessment). It was hypothesized that these three independent
variables may have affected the association between the IDoS and
the decrease in the values of parameters in the LFTs. This
presumption appears to have been mostly wrong.
Neither the IDR nor the SDE had any significant
effect on the ALT2 and AST2 levels. However, it should be taken
into consideration that the elevation in the silymarin dose was
done due to an unsatisfactory decrease in the levels of
transaminases and/or Bil after first period of treatment. While
there is only a weak association between increasing the IDoS and
its effect on the levels of transaminases, there may be other
factors diminishing the effect of silymarin e.g., a patient's
unresponsiveness to silymarin or a higher level of systemic therapy
toxicity.
The impact of DR1M on AST2 is questionable. A
borderline statistically significant association between DR1M and
AST2 was observed. This result suggests that the dose reduction of
systemic treatment would diminish the effect of silymarin on the
reduction of the AST level. However, this was observed in a
subpopulation of patients with no primary or secondary liver
tumors. There was no significant association between DR1M and AST2
in patients with liver tumors. The mechanism behind this finding
remains unknown.
No any association was observed between increasing
the IDoS and a decrease in Bil levels. However, in the regression
analysis, the DR1M had a significantly negative impact on the
decrease in the Bil level. This negative impact was observed only
in patients with primary liver tumors or liver metastases. It was
hypothesized that this effect may have been caused by the decreased
efficiency of systemic treatment delivered at a reduced dose. This
may have resulted in growth of liver tumors and in the promotion of
liver damage in some patients. The levels of serum bilirubin may
have been under the influence of other confounding factors not
covered by the present regression analysis. Moreover, serum Bil was
used as a marker of liver tissue function. However, due to
insufficient data, the present study did not evaluate conjugated
Bil in the patients. Thus, there could be a substantial portion of
patients with cholestasis of various etiologies. Although there is
limited evidence available on the positive effect of silymarin on
drug-induced cholestasis in mouse models (12), the authors were not able to assess
the efficacy of silymarin in the case of cholestasis caused by
extrahepatic factors e.g., bile duct obstruction.
The present study has several limitations which
should be mentioned. Firstly, patients with a wide variety of
underlying liver conditions may have been included in the study.
Given the fact that the patients were evaluated retrospectively,
only incomplete health records were available for consideration.
Moreover, patients with initially elevated results in LFTs were
enrolled. The present study focused on the dynamics of LFTs during
silymarin treatment regardless of the initial value. Finally,
malignant liver lesions (primary or metastatic liver tumors) can
considerably affect the outcomes of hepatoprotective treatment.
Therefore, the results presented in Table III, Table IV and Table V confront two subpopulations of the
patients: Those with liver lesions and those without liver
lesions.
Of note, in the present study, the R2
value of the regression model was low. This was caused by a high
variability in the data, despite a considerably large set of
patients. The dataset was derived from patients with various
diagnoses, at various stages of disease and, most importantly,
treated with a wide variety of systemic treatment.
In conclusion, the findings of the present study
suggest that the most prevalent initial dose of silymarin (300-450
mg) appears to be sufficient for the treatment of CILI and a higher
initial dose of silymarin brings only a limited benefit.
Furthermore, based on the data obtained, it could not be determined
whether a lower initial dose of silymarin would result in
comparable effectivity. In addition, the further escalation of the
silymarin dose at first assessment (after 1 month of treatment)
cannot be recommended. To the best of our knowledge, this is the
first study aiming to determine the optimal dose of silymarin in
CILI using the association between the IDoS and the levels of
parameters from LFTs. Considering the aforementioned limitations of
the present study, further investigations using randomized
controlled trials are warranted.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to legal restrictions applied in Slovakia
but may be requested from the corresponding author.
Authors' contributions
FK and BB contributed to the retrospective study by
performing data collection and evaluation. Statistical analysis and
the final preparation of the manuscript were performed by FK. FK
and BB confirm the authenticity of all the raw data. Both authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Faculty Hospital Trencin (Trencin, Slovakia). Since
the present study was retrospective and non-interventional in
nature, the requirement to obtain patient informed consent for
participation was waived by the Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ramadori G and Cameron S: Effects of
systemic chemotherapy on the liver. Ann Hepatol. 9:133–143.
2010.PubMed/NCBI
|
|
2
|
Grigorian A and O'Brien CB: Hepatotoxicity
secondary to chemotherapy. J Clin Transl Hepatol. 2:95–102.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Veitch Z, Khan OF, Tilley D, Tang PA,
Ribnikar D, Stewart DA, Kostaras X, King K and Lupichuk S: Impact
of cumulative chemotherapy dose on survival with adjuvant FEC-D
chemotherapy for breast cancer. J Natl Compr Canc Netw. 17:957–967.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nielson CM, Bylsma LC, Fryzek JP, Saad HA
and Crawford J: Relative dose intensity of chemotherapy and
survival in patients with advanced stage solid tumor cancer: A
systematic review and meta-analysis. Oncologist. 26:e1609–e1618.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gillessen A and Schmidt HHJ: Silymarin as
supportive treatment in liver diseases: A narrative review. Adv
Ther. 37:1279–1301. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Federico A, Dallio M and Loguercio C:
Silymarin/silybin and chronic liver disease: A marriage of many
years. Molecules. 22(191)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Luangchosiri C, Thakkinstian A, Chitphuk
S, Stitchantrakul W, Petraksa S and Sobhonslidsuk A: A
double-blinded randomized controlled trial of silymarin for the
prevention of antituberculosis drug-induced liver injury. BMC
Complement Altern Med. 15(334)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Marjani M, Fahim F, Sadr M, Kazempour
Dizaji M, Moniri A, Khabiri S, Tabarsi P and Velayati AA:
Evaluation of Silymarin for management of anti-tuberculosis drug
induced liver injury: A randomized clinical trial. Gastroenterol
Hepatol Bed Bench. 12:138–142. 2019.PubMed/NCBI
|
|
9
|
Moezian GSA, Javadinia SA, Sales SS,
Fanipakdel A, Elyasi S and Karimi G: Oral silymarin formulation
efficacy in management of AC-T protocol induced hepatotoxicity in
breast cancer patients: A randomized, triple blind,
placebo-controlled clinical trial. J Oncol Pharm Pract. 28:827–835.
2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mohaghegh F, Solhi H and Kazemifar AM:
Silymarin (Milk Thistle) can revoke liver enzyme changes during
chemotherapy of breast cancer with Taxanes. Eur J Integr Med.
7:650–652. 2015.
|
|
11
|
Fathalah WF, Abdel Aziz MA, Abou El Soud
NH and El Raziky MES: High dose of silymarin in patients with
decompensated liver disease: A randomized controlled trial. J
Interferon Cytokine Res. 37:480–487. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sohail I, Malkani N, Tahir N, Khalil A,
Attar R and Mumtaz S: Silymarin protects the liver from
α-naphthylisothiocyanate-induced cholestasis by modulating the
expression of genes involved in bile acid homeostasis. Cell Mol
Biol (Noisy-le-grand). 68:208–212. 2022.PubMed/NCBI
|