Introduction
Gastric cancer (GC) remains one of the most common
and deadly cancers worldwide, with over 50% of GC cases prevalent
in Eastern Asia. Based on GLOBOCAN 2020 data, stomach cancer is the
fifth most common neoplasm and the fourth most deadly cancer, with
an estimated 769,000 deaths in 2020(1).
The invention of immune checkpoint inhibitors (ICIs)
has paved the way for a new era in cancer immunotherapy. Notably,
inhibition of the programmed death-1 (PD-1) and programmed death
ligand-1 (PD-L1) axis with ICIs, such as nivolumab and
pembrolizumab, in combination with cytotoxic drugs, is emerging as
a new treatment strategy for advanced GC. PD-L1 plays a vital role
in tumor cells evading antitumor immunity in various types of
cancer. PD-L1 is widely expressed in various cells and tissues,
including cancer and immune cells. It is upregulated by multiple
inflammatory cytokines such as IL-6, TNF-α, and interferon-γ, and
likely functions as a negative feedback loop during inflammation.
These inflammatory cytokines are thought to play a major role in
cancer cachexia (2).
The 2011 consensus definition described cachexia as
a ‘multifactorial syndrome characterized by an ongoing loss of
skeletal muscle mass (with or without loss of fat mass) that cannot
be fully reversed by conventional nutritional support and leads to
progressive functional impairment’ (3). Cachexia is observed in advanced
malignancy as well as in the terminal course of many chronic
diseases, such as cardiac or renal failure and chronic obstructive
pulmonary disease. Common clinical symptoms include muscle wasting,
anemia, decreased caloric intake, and altered immune function,
contributing to disability, fatigue, decreased quality of life, and
decreased survival in patients with advanced GC (4-6).
Soluble PD-L1 (sPD-L1) is reportedly generated
mainly by proteolysis of membrane-bound PD-L1(7) and has recently been identified in
blood samples of patients and is reportedly useful as a factor of
poor prognosis in various cancers, such as hepatocellular carcinoma
(8,9), lung cancer (10), and GC (11-14).
sPD-L1 may impair host immunity and contribute to systemic
immunosuppression, leading to cancer progression and poor clinical
outcomes (15). However, there are
only a few reports of sPD-L1 expression profiles in cancer and host
in clinical practice for GC, especially in correlation to cancer
cachexia.
Therefore, we explored the utility of sPD-L1 as a
biomarker in patients with GC and its integrated analysis with
clinicopathological factors, including cancer cachexia.
Materials and methods
Clinical samples
This study was approved by the Ethics Committee of
the Graduate School of Medicine, Chiba University (assignment
number 1103), and informed consent was obtained from all patients.
Blood samples were collected from 173 patients with histologically
proven gastric adenocarcinoma during their first visit to
Department of Esophageal-Gastro-Intestinal Surgery, Chiba
University Hospital, Chiba, Japan between January 2012 and December
2017. No inclusion criteria such as age or performance status were
used. Exclusion criteria were defined as the coexistence of active
other cancers.
sPD-L1 levels were measured using enzyme-linked
immunosorbent assay (ELISA), as described under ‘Measurement of
sPD-L1 levels,’ and integrated with clinicopathological factors
such as age, sex, body mass index (BMI), stage, pathological
findings, and blood test results. Clinical data were collected from
the clinical database.
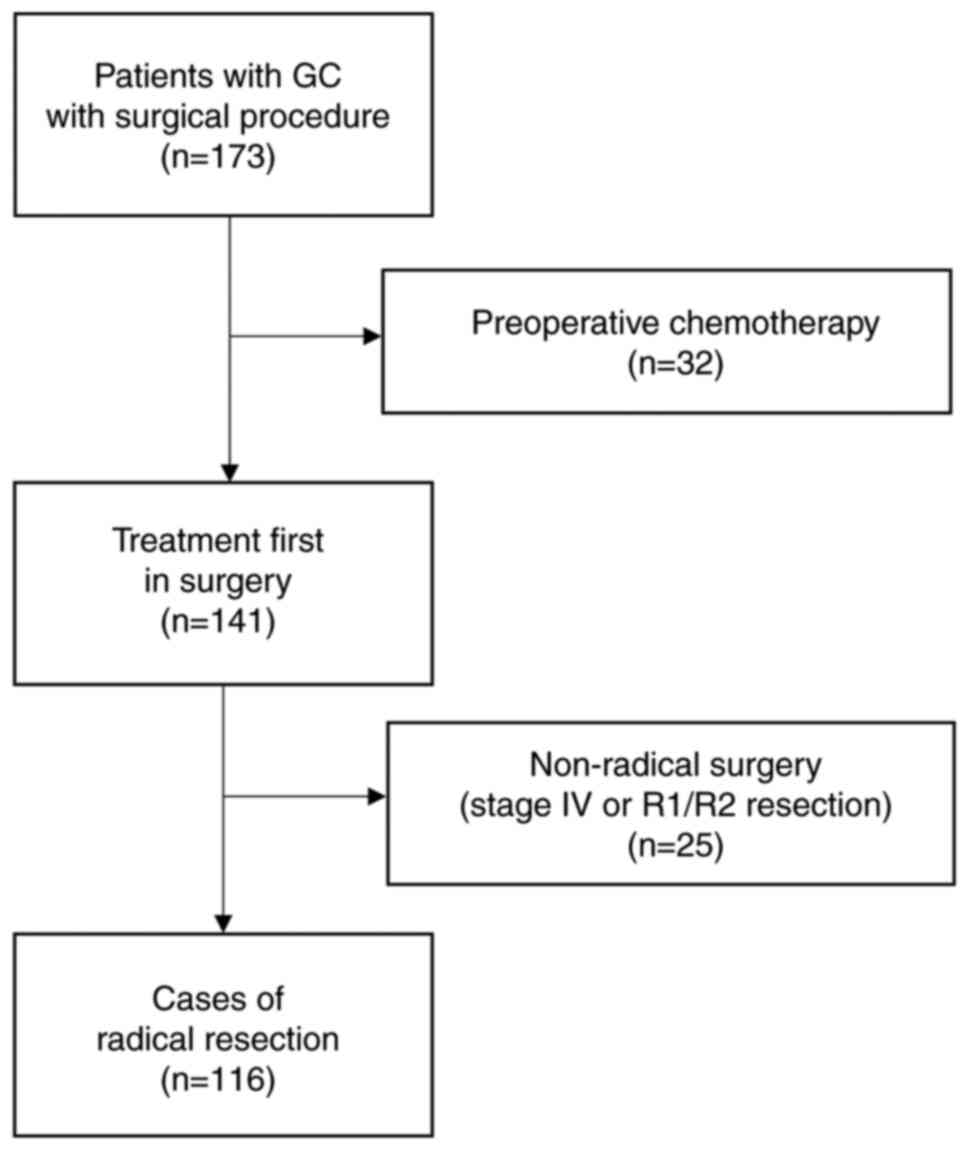
Among the 173 cases, 32 were pretreated, and 141
were preceded by surgical treatment. Twenty-five of the 141
patients underwent non-radical surgery, and 116 underwent radical
surgery with no preoperative treatment. Patients undergoing radical
GC surgery (n=116) were divided into two groups according to the
high and low sPD-L1 levels, and patient background, laboratory
values, and prognosis (overall and relapse-free survival) were
compared in each group. The pathological factors were tested in the
non-preoperative treatment group (n=141).
Disease classification
The staging classification was confirmed by the
International Union Against Cancer TNM staging system, 8th edition.
Histological classification was performed according to the Japanese
gastric cancer classification. As previously reported (16), the histological types were divided
into intestinal predominant, diffuse predominant, and diffuse mixed
intestinal types. The latter two were designated diffuse type.
Measurement of sPD-L1 levels
The serum concentrations of sPD-L1 were measured as
previously reported (17), using a
commercially available ELISA kit for human PD-L1 (R&D Systems,
Inc., Minneapolis, MN, USA), according to the manufacturer's
protocol. Briefly, 100 µl of serum samples and standards were added
to each well and incubated for 2 h at room temperature on a
horizontal orbital microplate shaker. After the liquid was removed
and washed four times, 200 µl of Human/Cynomolgus Monkey B7-H1
conjugate was added and incubated for 2 h at room temperature on a
shaker. After the liquid was removed and washed four times, 200 µl
of the substrate solution was added to each well and incubated for
30 min at room temperature on the benchtop and protected from
light. The stop solution was added to each well, and the optical
density was measured using a microplate reader. All experiments
were performed with technical duplicates for each sample, and the
average values were calculated. The median (57 pg/ml) was used as
the cut-off value, and the two groups were compared.
Factors governing cancer cachexia
To evaluate the association with host cachexia
status, we analyzed (1) low body
weight (BMI <18.5), (2) anemia
(Hb <12 g/dl), (3) malnutrition
(Alb <3.2 g/dl), and (4)
chronic inflammation (CRP >0.5 mg/dl) as factors of cancer
cachexia, and the relationship between the number of items
satisfying these criteria and sPD-L1 was evaluated. Data from 148
cases with no missing items in the initial blood draw or clinical
information were used for the analysis. The association between
pathological Stage (pStage) and the number of cachexia items,
excluding the preoperative treatment group, was examined.
Statistical analysis
Continuous variables were denoted by the median
(min-max). The range of sPD-L1 levels in each item was indicated by
the median and interquartile range. The Wilcoxon rank-sum tests and
chi-square tests were used to compare the two groups, and the
Kruskal-Wallis and Steel-Dwass tests were used to compare three or
more groups. Univariate and multivariate Cox regression analyses
were performed. Survival rates were evaluated using the
Kaplan-Meier curve and log-rank test. The neutrophil-to-lymphocyte
ratio (NLR) cut-off values were set using receiver operating
characteristic (ROC) curves with five-year recurrence-free survival
as the outcome. Statistical analysis was performed using JMP ver.
15(SAS Institute Inc., Cary, NC, USA), and statistical significance
was set at P<0.05.
Results
Characteristics of included cases
The flowchart of the patients included in this
analysis is displayed in Fig. 1.
The clinicopathological features of all the patients (n=173) are
listed in Table I, and values are
expressed as medians (min-max). The success or failure of 14
patients with a history of Helicobacter pylori (H. pylori)
eradication was unclear, and for those without a history of
eradication, the results were determined by IgG antibody in blood:
61 patients were negative and 96 patients were positive. Surgical
procedures included distal, total, and proximal gastrectomy in 88,
55, and 4 cases, respectively. Bypass and trial laparotomy were
performed in 18 cases, and endoscopic treatment in eight cases.
 | Table IClinicopathological features of all
patients (n=173). |
Table I
Clinicopathological features of all
patients (n=173).
| Variables | Value |
|---|
| Median age, years
(min-max) | 70 (28-93) |
| Sex, n | |
|
Male | 120 |
|
Female | 53 |
| Median BMI,
kg/m2 (min-max) | 22.5 (15.5-33.1) |
| Median WBC, /µl
(min-max) | 6,400
(3,300-15,700) |
| Median NLR
(min-max) | 2.3 (0.2-18.6) |
| Median Hb, g/dl
(min-max) | 13.2 (6.6-17.4) |
| Median Plt,
x103/µl (min-max) | 233 (13-544) |
| Median T-Cho, mg/dl
(min-max) | 199 (106-325) |
| Median Alb, g/dl
(min-max) | 4.2 (2.0-5.2) |
| Median CRP, mg/dl
(min-max) | 0.1 (0.0-13.4) |
| Median CEA, ng/ml
(min-max) | 2.5 (0.3-776.0) |
| Median CA19-9, U/ml
(min-max) | 14.2
(0.1-2860.0) |
| Median sPD-L1,
pg/ml (min-max) | 57.1
(3.2-400.5) |
| H. pylori
infection, n | |
|
Negative | 61 |
|
Positive | 96 |
|
Eradication
history | 14 |
| Clinical staging,
n | |
|
Tumor
depth | |
|
T1 | 41 |
|
T2 | 29 |
|
T3 | 42 |
|
T4 | 58 |
|
LN
metastasis | |
|
N0 | 97 |
|
N1-3 | 73 |
|
Distant
metastasis | |
|
M0 | 149 |
|
M1 | 21 |
| Procedure, n | |
|
DG | 88 |
|
TG | 55 |
|
PG | 4 |
|
ESD | 8 |
|
Bypass,
other | 18 |
The median value of sPD-L1 in GC patients was 57
pg/ml and was used as the cut-off value to compare the two
groups.
sPD-L1 and pathological factors
The pathological features and sPD-L1 levels were
compared between the 141 patients who did not receive preoperative
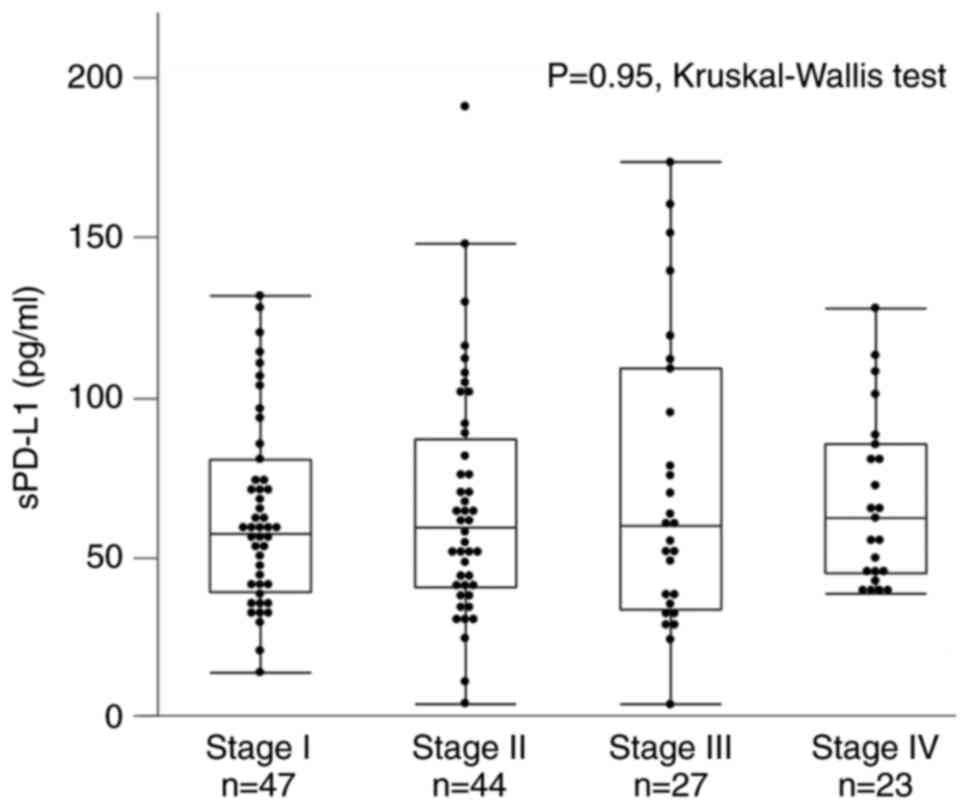
treatment. The number of cases in the pStage I, II, III, and IV was
47, 44, 27, and 23, with sPD-L1 values of 57.1 [39.0-80.6] pg/ml,
59.2 [40.4-87.0] pg/ml, 59.6 [33.4-109.2] pg/ml, and 62.1
[44.7-85.3] pg/ml, respectively. There were no differences in
sPD-L1 levels at each stage (P=0.95, Kruskal-Wallis test) (Fig. 2).
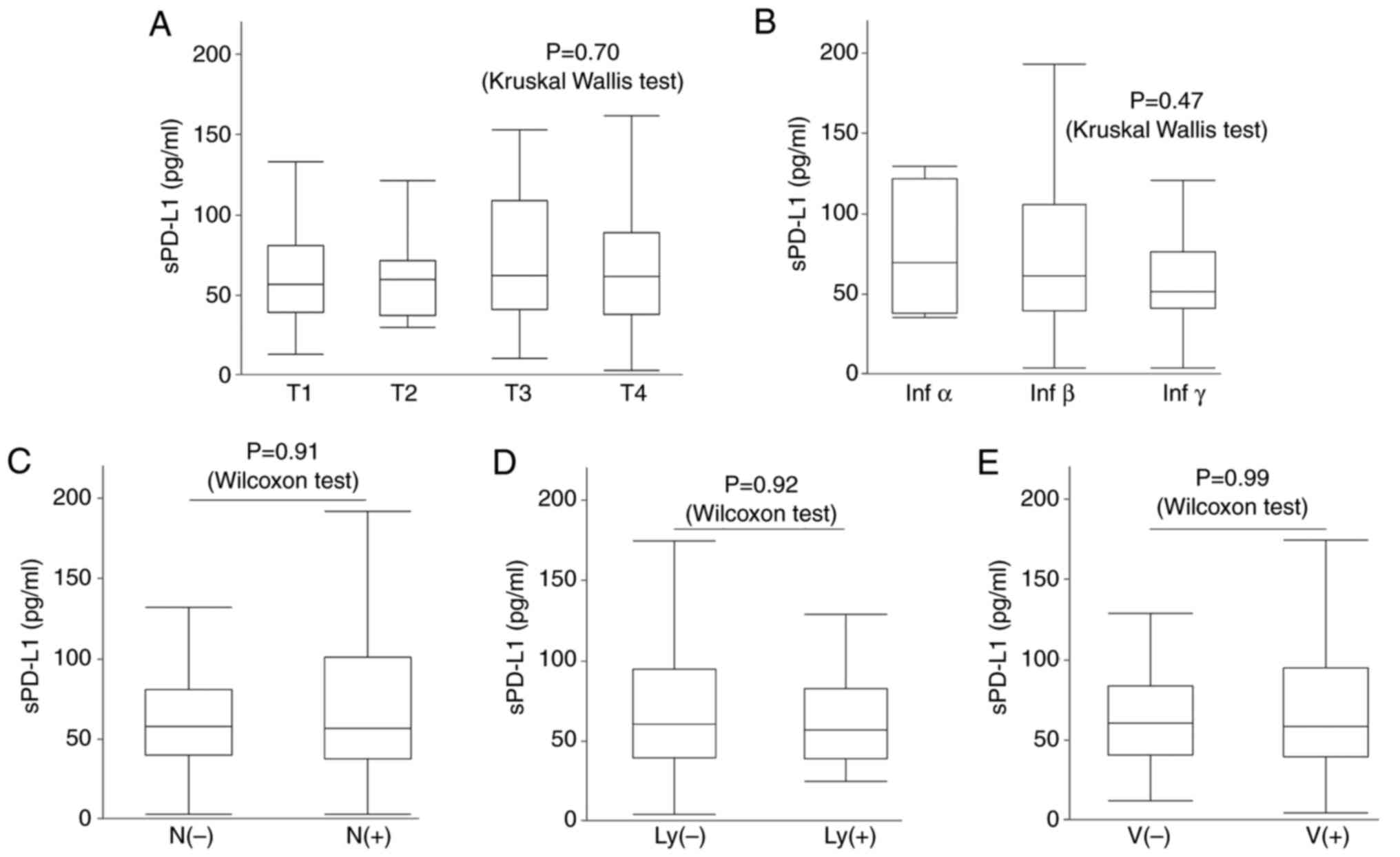
Histological features, such as tumor depth (T1: 56.7
[38.9-80.6] pg/ml, T2: 59.2 [37.2-71.6] pg/ml, T3: 61.7
[41.1-108.5] pg/ml, T4: 61.1 [38.0-88.8] pg/ml, P=0.70,
Kruskal-Wallis test), mode of invasion (infα: 68.6 [37.5-120.6]
pg/ml, infβ: 60.7 [38.6-104.7] pg/ml, infγ: 50.5 [40.4-75.6] pg/ml,
P=0.47, Kruskal-Wallis test), lymph node metastasis (N(-): 58.0
[40.4-81.4] pg/ml, N(+): 57.1 [38.0-101.1] pg/ml, P=0.91, Wilcoxon
test), lymph vessel invasion (ly(-): 59.6 [38.9-94.0] pg/ml, ly(+):
56.3 [38.4-82.0] pg/ml, P=0.92, Wilcoxon test), and vessel invasion
(v(-): 59.6 [39.4-82.6] pg/ml, v(+): 57.4 [38.0-94.0] pg/ml,
P=0.99, Wilcoxon test) did not significantly differ (Fig. 3).
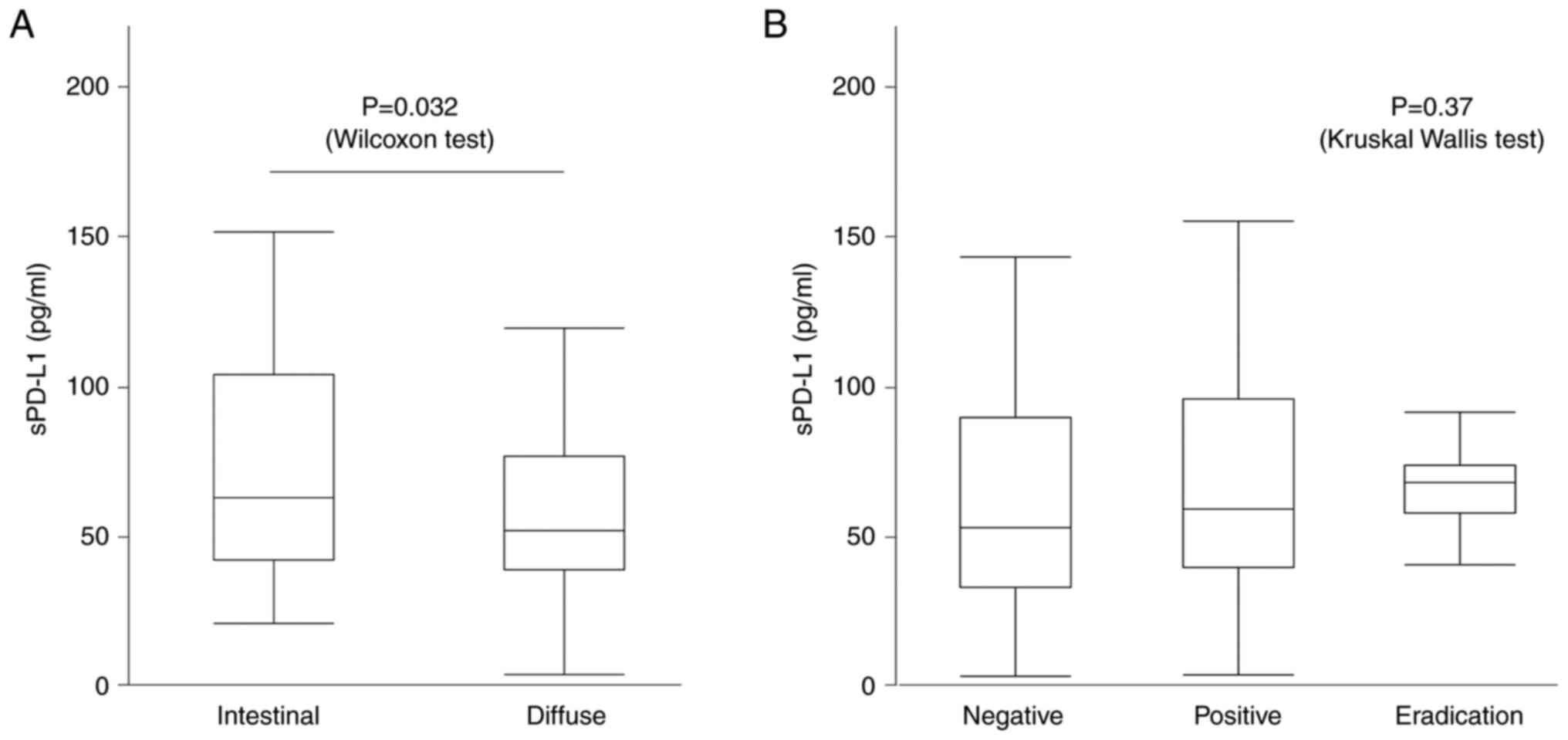
The intestinal type had high sPD-L1 levels compared
with that of the diffuse type (68.3 [41.5-104.1] pg/ml vs. 56.4
[38.2-76.6] pg/ml, P=0.032, Wilcoxon test) (Fig. 4A). However, for H. pylori
infection, the median sPD-L1 was 51.8 [37.4-95.3] pg/ml for H.
pylori negative, 57.7 [38.9-80.7] pg/ml for positive, and 66.4
[59.3-75.0] pg/ml for those with history of eradication, which were
not significant (P=0.37, Kruskal-Wallis test) (Fig. 4B).
sPD-L1 and patient
characteristics
Patient background, laboratory values, and survival
for groups with high and low median sPD-L1 values are listed in
Table II.
 | Table IIPatient background, laboratory values
and survival in the high and low sPD-L1 groups. |
Table II
Patient background, laboratory values
and survival in the high and low sPD-L1 groups.
| Variables | sPD-L1 high
(n=60) | sPD-L1 low
(n=56) | P-value |
|---|
| Median age, years
(min-max) | 72.5
(47.4-93.0) | 68.0
(46.0-83.7) | 0.0289a |
| Sex, n | | | 0.0678b |
|
Male | 43 | 31 | |
|
Female | 17 | 25 | |
| Median BMI
(min-max) | 22.6
(15.5-33.1) | 22.8
(18.7-30.2) | 0.4370a |
| Median WBC, /µl
(min-max) | 6,200
(3,900-13,000) | 6,050
(3,300-15,700) | 0.8160a |
| Median NLR
(min-max) | 2.7 (0.8-8.1) | 1.8 (0.2-18.6) | 0.0018a |
| Median Hb, g/dl
(min-max) | 12.5
(7.0-16.6) | 13.6
(6.9-16.7) | 0.0080a |
| Median Plt,
x103/µl (min-max) | 228 (45-419) | 226 (13-515) | 0.8186a |
| Median T-Cho, mg/dl
(min-max) | 187 (107-325) | 210 (140-308) | 0.0016a |
| Median Alb, g/dl
(min-max) | 4.1 (2.0-4.9) | 4.3 (3.5-5.2) | 0.0027a |
| Median CRP, mg/dl
(min-max) | 0.1 (0.0-13.4) | 0.1 (0.0-5.0) | 0.0307a |
| Median CEA, ng/ml
(min-max) | 2.7 (0.5-14.2) | 2.4 (0.6-8.5) | 0.3340a |
| Median CA19-9, U/ml
(min-max) | 12.2
(0.1-232.9) | 12.2
(0.1-228.9) | 0.6495a |
| H. pylori,
n | | | 0.3924b |
|
Negative | 19/ | 25 | |
|
Positive | 30 | 28 | |
| Procedure, n | | | 0.2131b |
|
TG | 19 | 12 | |
|
No TG | 41 | 44 | |
| Histology, n | | | 0.3359b |
|
Intestinal | 26 | 19 | |
|
Diffuse | 32 | 34 | |
| pStage, n | | | 0.9929b |
|
I | 24 | 23 | |
|
II | 23 | 21 | |
|
III | 13 | 12 | |
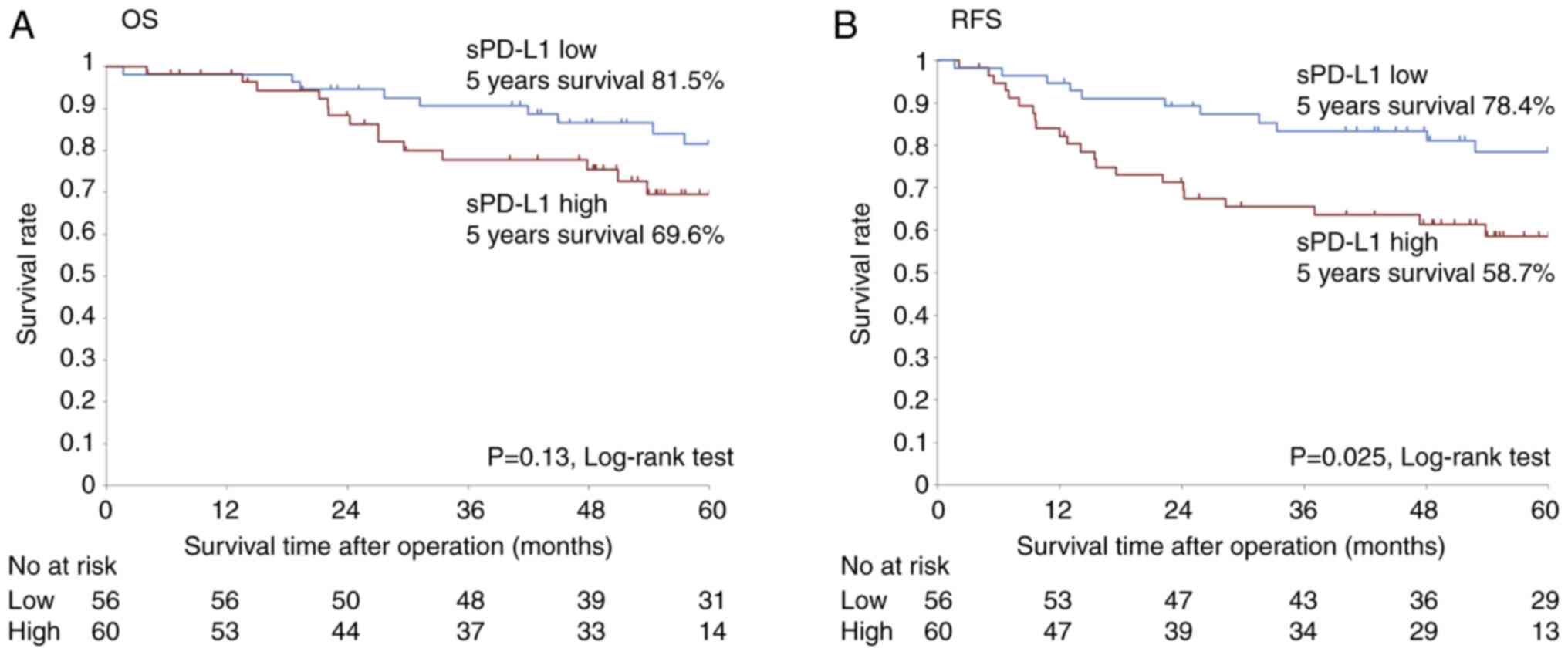
| 5-year OS, % | 69.6 | 81.5 | |
| 5-year RFS, % | 58.7 | 78.4 | |
No consistent trends in sex, BMI, H. pylori
infection, procedure, or pStage were observed (P=0.0678, 0.4370,
0.3924, 0.2131, and 0.9929, respectively). However, the group with
higher sPD-L1 included patients with advanced age (72.5 vs. 68,
P=0.0289), higher NLR (2.74 vs. 1.95, P=0.0015), lower Hb (12.5
g/dl vs. 13.6 g/dl, P=0.012), lower Alb (4.0 g/dl vs. 4.3 g/dl,
P=0.0006), lower total cholesterol (T-Cho) (186 mg/dl vs. 206
mg/dl, P=0.0005), and higher CRP (0.2 mg/dl vs. 0.1 mg/dl,
P=0.008). Among the tumor markers, carcinoembryonic antigen levels
tended to be higher (2.85 ng/ml vs. 2.25 ng/ml, P=0.037), whereas
that of cancer antigen CA19-9 did not differ.
sPD-L1 can predict the survival of GC
patients
The Kaplan-Meier curve and log-rank test were used
to evaluate the overall and recurrence-free survival in high and
low sPD-L1 groups (Fig. 5). The
five-year overall survival rate was lower in the high sPD-L1 group
(69.6%) than that in the low sPD-L1 group (81.5%); however, the
difference was not statistically significant (P=0.13, log-rank
test). The five-year relapse-free survival rate in the high sPD-L1
group was 58.7%, which was significantly lower than the 78.4% in
the low sPD-L1 group (P=0.025, log-rank test).
In univariate analysis, older age (≥70), high CA19-9
(>35.4 U/ml), high sPD-L1 (≥57 pg/ml), diffuse type, pStage
II/III were poor prognostic factors for poor relapse-free survival
(P=0.0311, 0.0095, 0.0211, 0.0496, 0.0010, respectively), and
multivariate Cox regression analyses revealed that high sPD-L1 was
an independent prognostic factor for poor relapse-free survival
after radical surgery (HR, 3.54; 95% CI 1.54-8.15, P=0.0029),
independent of pStage II/III (HR 44.31, 95% CI, 5.60-350.50,
P=0.0003), and high CA19-9 (HR 7.13, 95% CI 2.72-18.65,
P<0.0001). Older age was associated with high sPD-L1 and was a
significant poor prognostic factor in univariate analysis for
relapse free survival, but not in multivariate analysis. (Table III).
 | Table IIIUnivariate and multivariate Cox
regression analyses of relapse-free survival after radical
surgery. |
Table III
Univariate and multivariate Cox
regression analyses of relapse-free survival after radical
surgery.
| | Univariate | Multivariate |
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | | | | | | |
|
≥70 | 2.22 | 1.08-4.58 | 0.0311 | 1.72 | 0.79-3.75 | 0.1751 |
|
<70 | Ref. | | | | | |
| Sex | | | | | | |
|
Female | 1.21 | 0.61-2.41 | 0.5916 | 2.21 | 0.97-5.03 | 0.0582 |
|
Male | Ref. | | | | | |
| BMI | | | | | | |
|
<22 | 1.98 | 0.95-4.10 | 0.0673 | - | - | - |
|
≥22 | Ref. | | | | | |
| CEA, ng/ml | | | | | | |
|
>4.8 | 0.79 | 0.60-1.92 | 0.6002 | - | - | - |
|
≤4.8 | Ref. | | | | | |
| CA19-9, U/ml | | | | | | |
|
>35.4 | 2.63 | 1.27-5.47 | 0.0095 | 7.13 | 2.72-18.65 | <0.0001 |
|
≤35.4 | Ref. | | | | | |
| NLR | | | | | | |
|
>1.9 | 2.06 | 0.92-4.60 | 0.0795 | - | - | - |
|
≤1.9 | Ref. | | | | | |
| sPD-L1, pg/ml | | | | | | |
|
≥57 | 2.35 | 1.14-4.84 | 0.0211 | 3.54 | 1.54-8.15 | 0.0029 |
|
<57 | Ref. | | | | | |
| Procedure | | | | | | |
|
TG | 1.30 | 0.63-2.68 | 0.4777 | - | - | - |
|
DG, PG,
other | Ref. | | | | | |
| Histology | | | | | | |
|
Diffuse
type | 2.22 | 1.00-4.92 | 0.0496 | 1.97 | 0.83-4.69 | 0.1261 |
|
Intestinal
type | Ref. | | | | | |
| pStage | | | | | | |
|
II/III | 28.57 | 3.90-209.28 | 0.0010 | 44.31 | 5.60-350.50 | 0.0003 |
|
I | Ref. | | | | | |
sPD-L1 reflects cachexia status in GC
patients
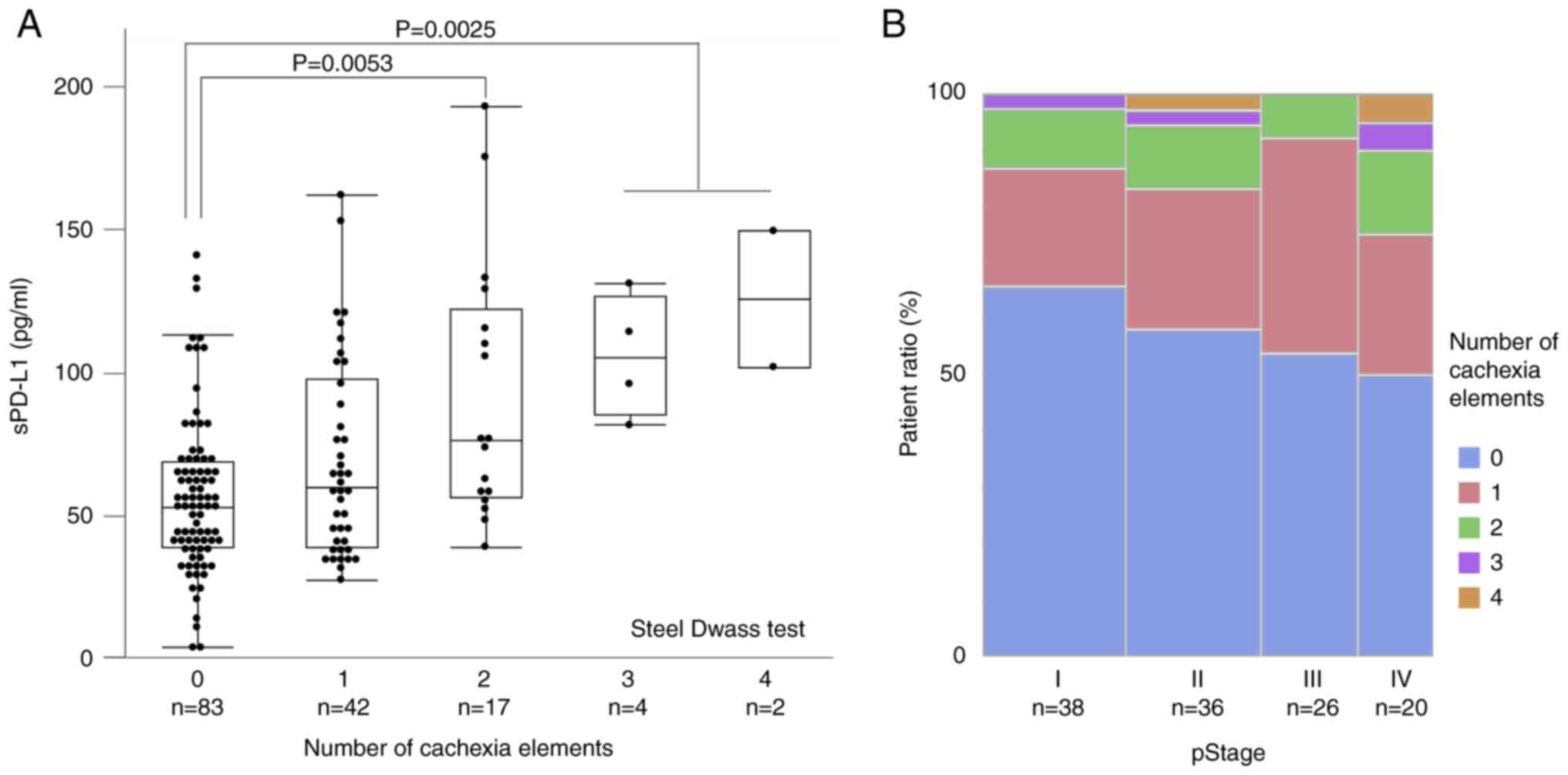
Our findings suggested that the sPD-L1 levels were
52.0 [38.0-68.3] pg/ml for zero items (n=83), 59.1 [38.5-97.0]
pg/ml for one item (n=42), 75.7 [55.6-121.4] pg/ml for two items
(n=17), 104.3 [84.4-126.0] pg/ml for three items (n=4), and 124.8
[101.1-148.6] pg/ml for four items (n=2). sPD-L1 values also tended
to increase with the increasing number of matched items, with
significantly higher results for two, three, and four items
compared with that of zero items (two items vs. zero item,
P=0.0025; three and four items vs. zero item, P=0.0053; Steel-Dwass
test) (Fig. 6A). Thus, sPD-L1
likely reflects elements of cachexia in GC patients.
On the other hand, with respect to the association
between the number of cachexia items and tumor progression, the
percentage of patients with 0 items tends to decrease in pStage I,
II, III, and IV to 65.8, 58.3, 53.9, and 50.0%, respectively, but
there is no statistically significant difference in the
distribution (P=0.793, Chi-square test) (Fig. 6B).
Discussion
In this study, we observed that sPD-L1 was higher in
patients with a higher number of factors, such as inflammation,
anemia, malnutrition, and cachexia-related weight loss, and the
effect on relapse-free survival in radical resection cases of GC
was clarified. Additionally, sPD-L1 was an independent prognostic
factor for postoperative recurrence-free survival in patients with
GC, independent of tumor markers CA19-9 and pStage. Although the
possibility that age, which is considered a prognostic factor for
gastric cancer in multiple literatures (18-20),
may be a confounding factor for sPD-L1 being a prognostic factor
could be considered, older age was not significant in the
multivariate analysis of this study.
PD-L1 is permanently expressed in normal peripheral
tissues, antigen-presenting cells, and vascular endothelial cells
in various organs, and its expression is upregulated by
inflammation. PD-L1 is upregulated by multiple inflammatory
cytokines (IL-6, TNF-α, interferon-γ, and others) (2). In GC, IFN-γ treatment reportedly
increases the expression of intracellular and membrane-bound PD-L1
in vitro (21). During an
immune response, most immunocompetent cells, including activated
lymphocytes, express PD-L1, which binds to PD-1 on T cells and
inhibits T cell function, induces immune tolerance, suppresses
excessive immune responses, and serves as a negative feedback
mechanism to protect the body from tissue injury. This may lead to
immune escape in tumor immunity. Therefore, sPD-L1 is thought to be
a marker of immune exhaustion (22-24).
We previously reported that sPD-L1 concentration is
proportional to the expression of PD-L1 in tissues (17) and described the efficacy of sPD-L1
in peripheral blood as a valuable biomarker in esophageal squamous
cell carcinoma (25). However,
another report on GC found no significant correlation (11). Although there are reports of PD-L1
being highly expressed in H. pylori-infected gastric cancer tissues
(26), there are no reports
showing an association with sPD-L1. sPD-L1 is more highly expressed
in the intestinal type than in the diffuse type, and its
involvement in chronic inflammation caused by H. pylori infection
was suspected, but no significant difference was found between
sPD-L1 and H. pylori infection.
Various factors associated with sPD-L1 have been
reported (27,28); however, discussion on host factors
is limited. Our analysis revealed that sPD-L1 was not significantly
different in stage progression but was lower in the diffuse type,
which is consistent with previous reports (28). Wei et al (29) reported that the value of sPD-L1 did
not change before and after surgery, suggesting that host-derived
sPD-L1 accounts for some of the value. In our study, high sPD-L1
levels were associated with older age, high NLR, low Hb, low T-Cho,
low Alb, and high CRP levels, which may be related to nutritional
and inflammatory indicators and is consistent with a PD-L1
activation mechanism. Cachexia is often associated with chronic
inflammatory diseases, various cancers such as gastrointestinal and
lung cancer, and chronic infections, and inflammatory cytokines
have been shown to play a major role in these diseases (2). Therefore, it is logical that sPD-L1
is associated with cachexia.
However, this study had the following limitations:
We focused mainly on relapse-free survival and did not examine the
regimen and duration of postoperative adjuvant therapy and the
effect of post-relapse therapy, especially ICI. Many patients
treated before ICI were covered by public insurance; we would like
to analyze the relationship between sPD-L1 and ICI efficacy in the
future. Various researchers have reported the usefulness of sPD-L1
or circulating exosomal PD-L1 biomarkers. The correlation between
PD-L1 expression in tissues and sPD-L1 expression in the blood is
controversial. Additionally, we did not compare PD-L1 expression
between tissues and blood. Tumor proportion and combined positive
scores are related to some extent according to previous
immunostaining studies; however, such analysis is complicated and
requires a pathologist. Therefore, such associations were not
performed in this study. The analysis of cachexia was based
primarily on changes (loss) in body weight and skeletal muscle
mass, whereas low body weight (BMI <18.5) was a surrogate factor
in this study.
Recently, ghrelin-like drugs have been launched as
therapeutic interventions for cancer cachexia (30); however, multiple laboratory tests
are required to confirm their indications. Our findings suggest
that sPD-L1 may be a driving force and contribute to the early
detection of pre-cachexia. sPD-L1 is associated with indices
related to cachexia in patients with GC and may be a predictive
marker for recurrence-free survival rate after radical surgery.
Acknowledgements
The authors would like to thank Ms. Keiko Iida
(Department of Frontier Surgery, Chiba University Graduate School
of Medicine, Chiba, Japan) for her assistance with the
experiments.
Funding
Funding: The present study was supported by JSPS KAKENHI (grant
nos. 21K16414 and 22K08747).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM, MK, HS, KM, TT, RO, KH and YK were involved in
the study design and surgical treatment. HH and HMa checked and
revised the study design. YM, TSa, TSh, KM, KK, SI and HMo
performed the clinical data extraction and analysis. TSa and TSh
performed sPD-L1 measurements. Statistical analyses were performed
by YM and YN, with validation by HH and HMa. YM and TSa confirm the
authenticity of all the raw data. The manuscript was written by YM
under the supervision of HH and HMa. All authors revised the
manuscript critically for important intellectual content. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Graduate School of Medicine, Chiba University
(Chiba, Japan), and written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Baazim H, Antonio-Herrera L and Bergthaler
A: The interplay of immunology and cachexia in infection and
cancer. Nat Rev Immunol. 22:309–321. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fukahori M, Shibata M, Hamauchi S,
Kasamatsu E and Machii K: A retrospective cohort study to
investigate the incidence of cancer-related weight loss during
chemotherapy in gastric cancer patients. Support Care Cancer.
29:341–348. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Namikawa T, Marui A, Yokota K, Fujieda Y,
Munekage M, Uemura S, Maeda H, Kitagawa H, Kobayashi M and Hanazaki
K: Frequency and prognostic impact of cachexia during drug
treatment for unresectable advanced gastric cancer patients. Surg
Today. 52:1560–1567. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Morimoto K, Uchino J, Yokoi T, Kijima T,
Goto Y, Nakao A, Hibino M, Takeda T, Yamaguchi H, Takumi C, et al:
Impact of cancer cachexia on the therapeutic outcome of combined
chemoimmunotherapy in patients with non-small cell lung cancer: A
retrospective study. OncoImmunology. 10(1950411)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nielsen C, Ohm-Laursen L, Barington T,
Husby S and Lillevang ST: Alternative splice variants of the human
PD-1 gene. Cell Immunol. 235:109–116. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Finkelmeier F, Canli Ö, Tal A, Pleli T,
Trojan J, Schmidt M, Kronenberger B, Zeuzem S, Piiper A, Greten FR
and Waidmann O: High levels of the soluble programmed death-ligand
(sPD-L1) identify hepatocellular carcinoma patients with a poor
prognosis. Eur J Cancer. 59:152–159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chang B, Huang T, Wei H, Shen L, Zhu D, He
W, Chen Q, Zhang H, Li Y, Huang R, et al: The correlation and
prognostic value of serum levels of soluble programmed death
protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in
patients with hepatocellular carcinoma. Cancer Immunol Immunother.
68:353–363. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Okuma Y, Hosomi Y, Nakahara Y, Watanabe K,
Sagawa Y and Homma S: High plasma levels of soluble programmed cell
death ligand 1 are prognostic for reduced survival in advanced lung
cancer. Lung Cancer. 104:1–6. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shigemori T, Toiyama Y, Okugawa Y,
Yamamoto A, Yin C, Narumi A, Ichikawa T, Ide S, Shimura T, Fujikawa
H, et al: Soluble PD-L1 expression in circulation as a predictive
marker for recurrence and prognosis in gastric cancer: Direct
comparison of the clinical burden between tissue and serum PD-L1
expression. Ann Surg Oncol. 26:876–883. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ito M, Oshima Y, Yajima S, Suzuki T,
Nanami T, Shiratori F, Funahashi K, Nemoto T and Shimada H: Is high
serum programmed death ligand 1 level a risk factor for poor
survival in patients with gastric cancer? Ann Gastroenterol Surg.
2:313–318. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Takahashi N, Iwasa S, Sasaki Y, Shoji H,
Honma Y, Takashima A, Okita NT, Kato K, Hamaguchi T and Yamada Y:
Serum levels of soluble programmed cell death ligand 1 as a
prognostic factor on the first-line treatment of metastatic or
recurrent gastric cancer. J Cancer Res Clin Oncol. 142:1727–1738.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Park W, Bang JH, Nam AR, Jin MH, Seo H,
Kim JM, Oh KS, Kim TY and Oh DY: Prognostic value of serum soluble
programmed death-ligand 1 and dynamics during chemotherapy in
advanced gastric cancer patients. Cancer Res Treat. 53:199–206.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Frigola X, Inman BA, Lohse CM, Krco CJ,
Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H and Kwon
ED: Identification of a soluble form of B7-H1 that retains
immunosuppressive activity and is associated with aggressive renal
cell carcinoma. Clin Cancer Res. 17:1915–1923. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tanaka H, Yoshii M, Imai T, Tamura T,
Toyokawa T, Muguruma K, Hirakawa K and Ohira M: Clinical
significance of coexisting histological diffuse type in stage
II/III gastric cancer. Mol Clin Oncol. 15(234)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shiraishi T, Toyozumi T, Sakata H,
Murakami K, Kano M, Matsumoto Y, Yokoyama M, Okada K, Kamata T,
Ryuzaki T, et al: Soluble PD-L1 concentration is proportional to
the expression of PD-L1 in tissue and is associated with a poor
prognosis in esophageal squamous cell carcinoma. Oncology.
100:39–47. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park JC, Lee YC, Kim JH, Kim YJ, Lee SK,
Hyung WJ, Noh SH and Kim CB: Clinicopathological aspects and
prognostic value with respect to age: An analysis of 3,362
consecutive gastric cancer patients. J Surg Oncol. 99:395–401.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Song P, Wu L, Jiang B, Liu Z, Cao K and
Guan W: Age-specific effects on the prognosis after surgery for
gastric cancer: A SEER population-based analysis. Oncotarget.
7:48614–48624. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alshehri A, Alanezi H and Kim BS:
Prognosis factors of advanced gastric cancer according to sex and
age. World J Clin Cases. 8:1608–1619. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Imai Y, Chiba T, Kondo T, Kanzaki H,
Kanayama K, Ao J, Kojima R, Kusakabe Y, Nakamura M, Saito T, et al:
Interferon-γ induced PD-L1 expression and soluble PD-L1 production
in gastric cancer. Oncol Lett. 20:2161–2168. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Iwai Y, Terawaki S and Honjo T: PD-1
blockade inhibits hematogenous spread of poorly immunogenic tumor
cells by enhanced recruitment of effector T cells. Int Immunol.
17:133–144. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Akutsu Y, Murakami K, Kano M, Toyozumi T,
Matsumoto Y, Takahashi M, Otsuka R, Sekino N, Yokoyama M, Shiraishi
T and Matsubara H: The concentration of programmed cell
death-ligand 1 in the peripheral blood is a useful biomarker for
esophageal squamous cell carcinoma. Esophagus. 15:103–108.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu Y, Zhu F, Ba H, Chen J and Bian X:
Helicobacter pylori infection and PD-L1 expression in gastric
cancer: A meta-analysis. Eur J Clin Invest.
53(e13880)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Oh SY, Kim S, Keam B, Kim TM, Kim DW and
Heo DS: Soluble PD-L1 is a predictive and prognostic biomarker in
advanced cancer patients who receive immune checkpoint blockade
treatment. Sci Rep. 11(19712)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wei H, Wu F, Mao Y, Zhang Y, Leng G, Wang
J, Zhang W and Wang T: Measurement of soluble PD-1 and soluble
PD-L1 as well as PD-L1 and PD-1 from perioperative patients with
gastric carcinoma. Jpn J Clin Oncol. 52:331–345. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Naito T, Uchino J, Kojima T, Matano Y,
Minato K, Tanaka K, Mizukami T, Atagi S, Higashiguchi T, Muro K, et
al: A multicenter, open-label, single-arm study of anamorelin
(ONO-7643) in patients with cancer cachexia and low body mass
index. Cancer. 128:2025–2035. 2022.PubMed/NCBI View Article : Google Scholar
|