Introduction
A mesenchymal-epithelial transition (MET) gene
encodes a receptor tyrosine kinase and its activation promotes the
tumor cell proliferation. MET exon 14 (METex14) is crucial for the
ubiquitination and degradation of MET proteins, and its skipping
produces incomplete MET proteins, which grow abnormally and become
cancerous (1).
In non-small cell lung cancer (NSCLC), METex14
skipping mutation is observed in 3-4% of cases and typically occurs
in the absence of other driver mutations (2). The incidence of METex14 skipping is
2% in adenocarcinoma, 6% in adenosquamous cell carcinoma and 13% in
pulmonary sarcomatoid carcinoma (2,3).
METex14 skipping is more closely associated with old age, the
female gender and never-smoking histories compared with patients
without METex14 skipping (3). Some
oral MET kinase inhibitors, such as crizotinib (4), tepotinib (1), capmatinib (5) and savolitinib (ClinicalTrials.gov Identifier: NCT02897479) have been
found to be effective based on clinical trials. Of these oral MET
kinase inhibitors, tepotinib and capmatinib have been approved for
use in Japan (3). The VISION
trial, which investigated the effectiveness of tepotinib in
patients with MET ex14 skipping, demonstrated an overall response
rate of 46% and a median duration of response of 11.1 months
(1).
ArcherMET, AmoyDx test and Oncomine Dx target test
(DxTT, Thermo Fisher Scientific, Inc.) are next-generation
sequencing platforms used for the detection of oncogenic driver
mutations and can detect METex14 skipping. However, for the use of
MET kinase inhibitors in Japan, positive results are required on
ArcherMET or AmoyDx test, but not on Oncomine DxTT, as companion
diagnostics (CDx) (6). The present
study reports the case of a patient with NSCLC with METex14
skipping who was successfully treated with tepotinib, although
METex14 skipping was positive on Oncomine DxTT, but not on
ArcherMET.
Case report
A 60-year-old male with a 38-pack-year history of
smoking was referred to the authors' hospital, Kanagawa
Cardiovascular and Respiratory Center (Yokohama, Japan) due to
cough and abnormal chest shadows. He underwent left upper lobectomy
for the resection of lung adenocarcinoma stage IIB (pT1bN1M0). The
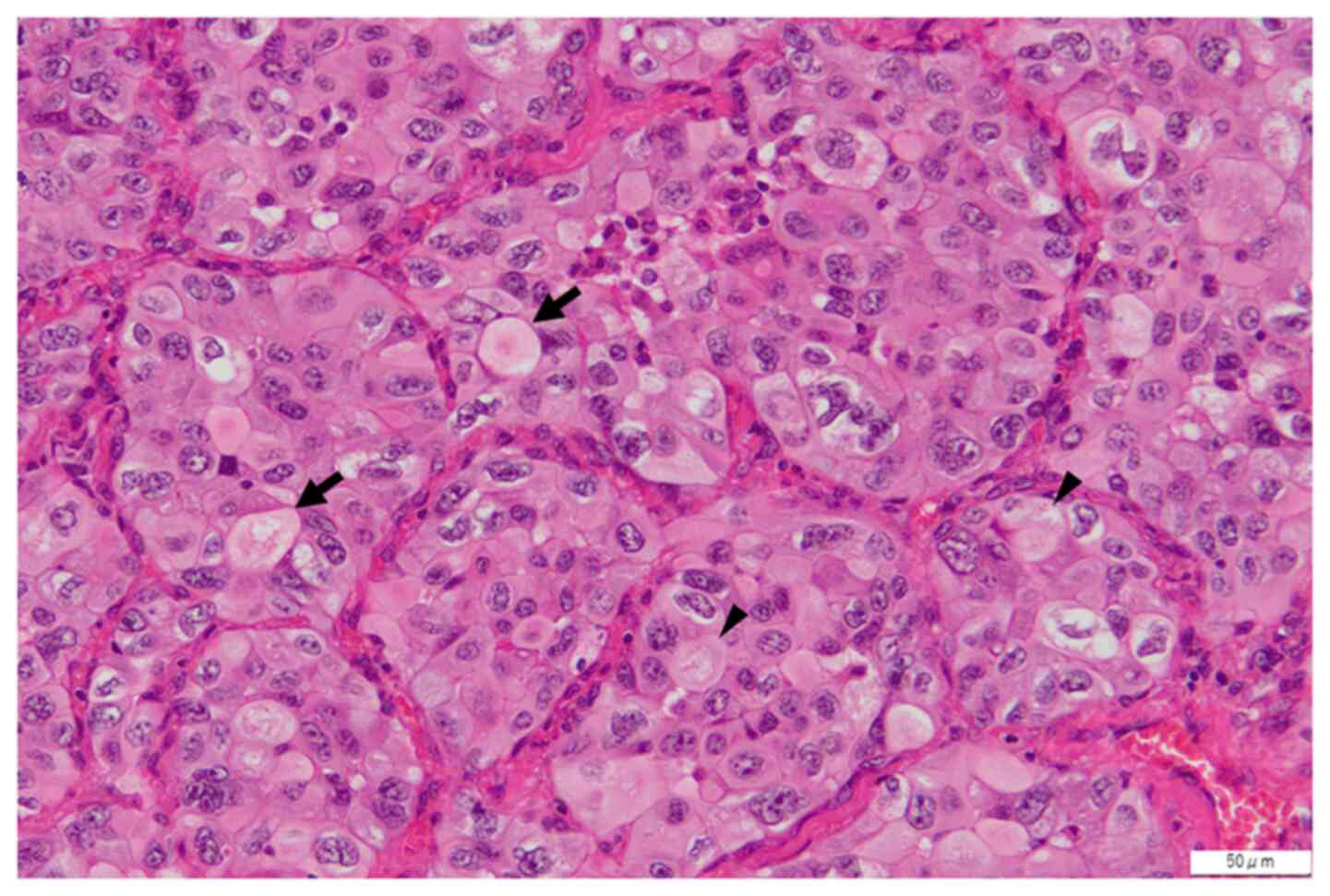
largest diameter of the tumor was 15 mm, and its histological
subtype was solid, as illustrated in Fig. 1. The left lobar lymph node (#12)
was pathologically positive, whereas the other lymph nodes were
free of cancer cells. He received two cycles of adjuvant
chemotherapy consisting of cisplatin and vinorelbine. At 6 months
after surgery, the mediastinal lymph node (#4) was enlarged.
Post-operative recurrence was confirmed using endobronchial
ultrasound-guided transbronchial needle aspiration. Whereas other
oncogenic driver mutations such as epidermal growth factor receptor
gene (EGFR) mutation was negative, METex14 skipping was positive on
Oncomine DxTT. However, it was negative on ArcherMET, although the
same residual RNA sample was used. In this sample used for Oncomine
DxTT, the read count of MET(13)-MET(15) products were only 46. The
patient then received four lines of anticancer drug therapy
serially for 2 years: first, cisplatin pemetrexed and
pembrolizumab; second, TS-1; third, pembrolizumab; fourth,
pemetrexed. However, the tumor grew along with pericardial
effusion, causing cardiac tamponade, which required immediate
drainage. Under the circumstances of limited treatment options, he
was admitted to the authors' hospital in June 2022 and tepotinib
was administered at a dose of 500 mg once daily under careful
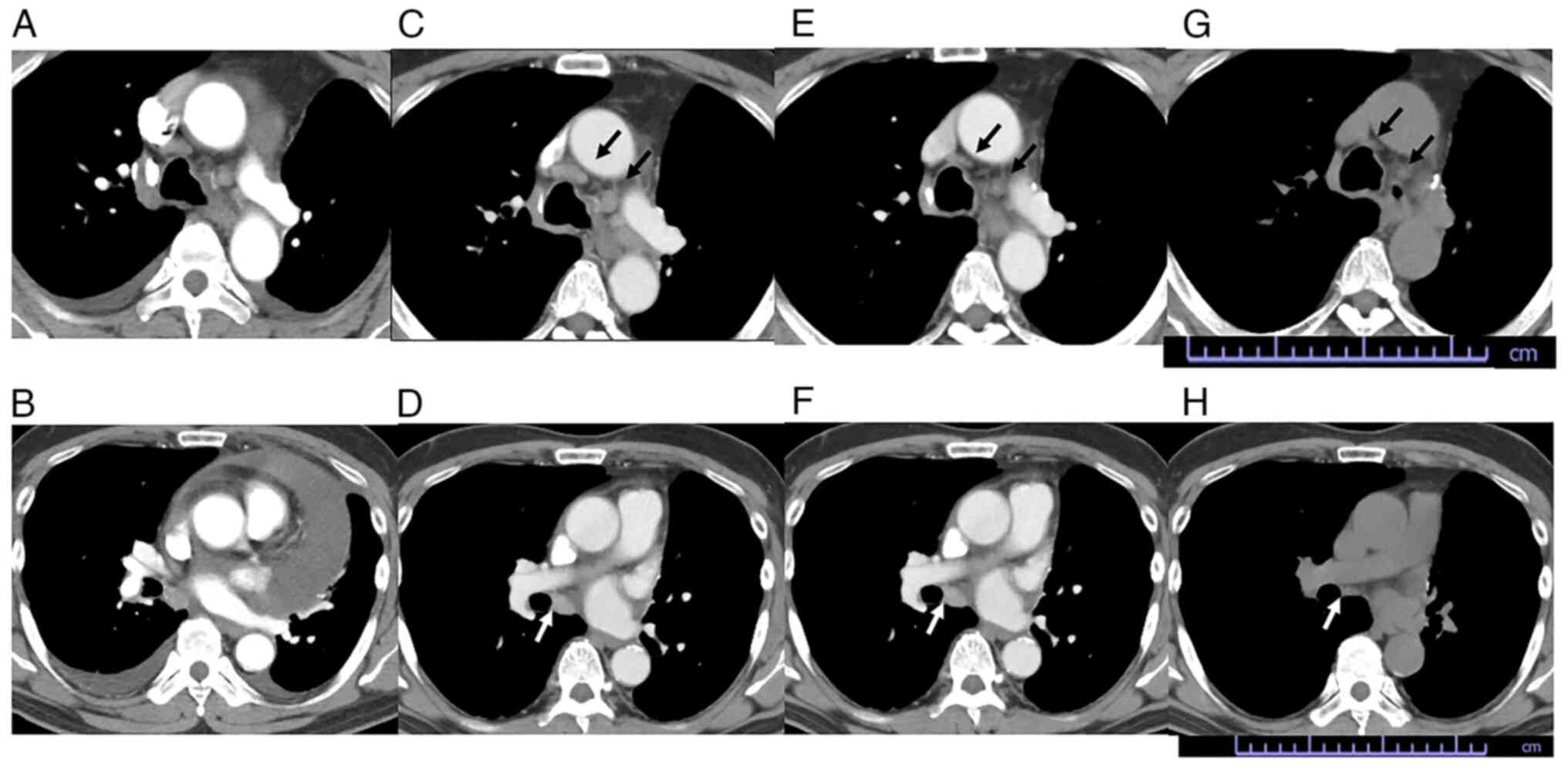
observation. After 4 weeks, a chest computed tomography scan
confirmed a partial response by shrinking of the enlarged lymph
nodes (Fig. 2) according to RECIST
(7). After 2 months, the patient
developed grade 2 peripheral edema as a treatment-related adverse
effect, and the dose was reduced to 250 mg and administration was
continued. The response still continued 7 months following the
initiation of tepotinib treatment.
Discussion
In the present case MET kinase inhibitor was not
used initially, as METex14 skipping was positive with Oncomine
DxTT, but negative with ArcherMET. However, cancer progression
caused cardiac tamponade after several rounds of chemotherapy, and
therefore the treatment options are limited. Tepotinib was
administered with careful observation and all lesions were
successfully treated. This clinical course strongly indicates a
false negative result with ArcherMET.
To date, Oncomine DxTT is used worldwide for lung
cancer diagnosis to detect several oncogenic drivers as CDx. This
panel test can also detect METex14 skipping through RNA-based
amplicon sequencing using Ion PGM Dx. However, in Japan, CDx for
MET kinase inhibitors are limited to ArcherMET, FoundationOne or
AmoyDx test, but not Oncomine DxTT (6), although no specific molecular assays
have been assigned for MET kinase inhibitors in the United States
and Europe. Among several studies reporting high rates of false
negatives for METex14 skipping (8-12),
there were two studies referring to the discordance between
Oncomine DxTT and ArcherMET. A previous study demonstrated a case
of successful treatment with the MET kinase inhibitor although
METex14 skipping was positive with ArcherMET and negative with
Oncomine DxTT (13). In another
study, 26 samples were positive in Oncomine DxTT results, but eight
samples (30.8%) did not reveal METex14 skipping with ArcherMET
(12). The majority of the
discordant samples had read counts <800 of MET(13)-MET(15)
products. This discordance was concluded to be a false positive for
Oncomine DxTT in samples with relatively low read counts, although
the effectiveness of MET inhibitor was not documented (12). In the present case, the sample
obtained had only 46 read counts, which supports this study.
However, all tumor lesions exhibited rapid shrinkage after
initiating tepotinib treatment, and the clinical course in the case
described herein strongly indicates a false negative for ArcherMET
due to relatively low read counts.
In conclusion, the present study reports a case of
successful treatment with tepotinib, although METex14 skipping was
only positive with Oncomine DxTT and not with ArcherMET. It may
thus be necessary to consider the use of MET kinase inhibitors in
patients with METex14 skipping proven only with Oncomine DxTT,
particularly when the sample has relatively low read counts.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YO and AS analyzed and interpreted the data and
wrote the manuscript. YO, AS, EH, SY, SI, ET, HK, TB, SK and TO
evaluated the patient and participated in the therapy. KO was
involved in the pathological diagnosis. EH and SY confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The patient provided his written informed consent
for participation in the present study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and any associated
images.
Competing interests
TB has received consultation fees from Merck Pharma
Japan (the supplier of tepotinib distributed in Japan). All
remaining authors declare that they have no competing
interests.
References
|
1
|
Paik PK, Felip E, Veillon R, Sakai H,
Cortot AB, Garassino MC, Mazieres J, Viteri S, Senellart H, Van
Meerbeeck J, et al: Tepotinib in non-small-cell lung cancer with
MET Exon 14 skipping mutations. N Engl J Med. 383:931–943.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Heist RS, Shim HS, Gingipally S,
Mino-Kenudson M, Le L, Gainor JF, Zheng Z, Aryee M, Xia J, Jia P,
et al: MET Exon 14 skipping in non-small cell lung cancer.
Oncologist. 21:481–486. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Socinski MA, Pennell NA and Davies KD: MET
Exon 14 skipping mutations in non-small-cell lung cancer: An
overview of biology, clinical outcomes, and testing considerations.
JCO Precis Oncol. 5(PO.20.00516)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Drilon A, Clark JW, Weiss J, Ou SI,
Camidge DR, Solomon BJ, Otterson GA, Villaruz LC, Riely GJ, Heist
RS, et al: Antitumor activity of crizotinib in lung cancers
harboring a MET exon 14 alteration. Nat Med. 26:47–51.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wolf J, Seto T, Han JY, Reguart N, Garon
EB, Groen HJM, Tan DSW, Hida T, de Jonge M, Orlov SV, et al:
Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell
lung cancer. N Engl J Med. 383:944–957. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pharmaceuticals and Medical Devices
Agency: Information on in vitro diagnostic products or medical
devices approved for the purpose of determining the indication of a
drug. Accesed Dec 23, 2022. https://www.pmda.go.jp/files/000239775.pdf.
|
|
7
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schrock AB, Frampton GM, Suh J, Chalmers
ZR, Rosenzweig M, Erlich RL, Halmos B, Goldman J, Forde P,
Leuenberger K, et al: Characterization of 298 Patients with Lung
Cancer Harboring MET Exon 14 Skipping Alterations. J Thorac Oncol.
11:1493–1502. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Poirot B, Doucet L, Benhenda S, Champ J,
Meignin V and Lehmann-Che J: MET Exon 14 Alterations and new
resistance mutations to tyrosine kinase inhibitors: Risk of
inadequate detection with current amplicon-based NGS panels. J
Thorac Oncol. 12:1582–1587. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Davies KD, Lomboy A, Lawrence CA, Yourshaw
M, Bocsi GT, Camidge DR and Aisner DL: DNA-Based versus RNA-Based
Detection of MET Exon 14 skipping events in lung cancer. J Thorac
Oncol. 14:737–741. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Champagnac A, Bringuier PP, Barritault M,
Isaac S, Watkin E, Forest F, Maury JM, Girard N and Brevet M:
Frequency of MET exon 14 skipping mutations in non-small cell lung
cancer according to technical approach in routine diagnosis:
Results from a real-life cohort of 2,369 patients. J Thorac Dis.
12:2172–2178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Teishikata T, Shiraishi K, Shinno Y,
Kobayashi Y, Kashima J, Ishiyama T, Yoshida T, Mori T and Yatabe Y:
An alert to possible false positives with a commercial assay for
MET Exon 14 skipping. J Thorac Oncol. 16:2133–2138. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Takamori S, Seto T, Yamaguchi M, Kinoshita
F, Fujishita T, Ito K, Toyozawa R, Shoji F and Okamoto T: Case
report: Success of tepotinib therapy in overcoming resistance to
osimertinib in a patient with EGFR-mutant lung adenocarcinoma with
a potential acquired MET exon 14 skipping mutation. Front Oncol.
12(965741)2022.PubMed/NCBI View Article : Google Scholar
|