Introduction
Being the most prevalent type of malignant tumor,
lung cancer has the highest cancer-related mortality worldwide with
2093876 new cases and 1761007 cases died of lung cancer in
2018(1). Non-small cell lung
cancer (NSCLC) is the most common pathological type of lung cancer,
accounting for ~85% of lung cancer cases (2). Widespread application of chest CT in
physical examination facilitates diagnosis of early-stage NSCLC.
Currently, surgery is the first-line treatment option for
early-stage NSCLC (3). Lobectomy
with systematic lymph node dissection (SLND) has been the standard
surgical treatment for lung cancer for several years (4,5).
However, there are new options for LND in early-stage NSCLC, among
which lobe-specific selective LND (LSLND) is one of the most
discussed surgical methods (6-9).
Studies have confirmed that LSLND could shorten the operation time
and decrease perioperative complications without affecting overall
survival (OS) and disease-free survival rate of patients after
surgery (8-10).
In addition, some studies suggested that for early-stage NSCLC,
lymph node sampling (LNS) achieves a prognosis similar to SLND
(11,12). The subcarinal lymph node, also
known as the #7 lymph node, has been regarded as an essential N2
lymph node in LND of lung cancer in the past and it was required to
be removed in SLND. The extent of SLND involves the inclusion of
subcarinal lymph nodes, while LSLND and LNS do not require
mandatory subcarinal LND (8-10,12).
There is still controversy about whether the subcarinal lymph nodes
must be dissected in early-stage NSCLC. The present study focused
on stage IB NSCLC and investigated if the subcarinal LND is
necessary for stage IB NSCLC.
Materials and methods
Patient selection
Data were collected from patients with lung cancer
who had surgery at Sun Yat-Sen University Cancer Center, Guangzhou,
China between January 1999 and December 2009. The medical records
and the follow-up system were evaluated for patient information,
postoperative pathological information, surgical conditions and
follow-up outcomes. The inclusion criteria were as follows: i) No
neoadjuvant therapy; ii) patients treated with lobectomy and iii)
postoperative pathological diagnosis was stage IB NSCLC, which was
validated independently by two pathologists. The exclusion criteria
were as follows: i) Patients who had preoperative chemotherapy or
radiation; ii) patients with other types of tumor and iii) no
detailed follow-up information. Finally, 597 patients (age range
19-85; male/female 425/172) were enrolled.
Follow-up
Follow-up began when patients underwent surgery and
was performed every 3 months for the first 2 years, every 6 months
between 3-5 years and annually after 5 years. The evaluation of the
patient condition included routine blood, blood biochemistry, tumor
marker, abdominal ultrasound and chest CT. Brain MRI was conducted
to determine brain metastasis once per year . The patient survival
and recurrence were recorded at each visit. All cases were followed
up until January 2013.
Statistical analysis
The variables of the two groups were compared using
χ2 or Fisher's exact (>20% of cells with expected
frequency <5) test as appropriate. OS and recurrence-free
survival (RFS) were calculated using the Kaplan-Meier method. For
univariate and multivariate analysis, the Cox proportional hazard
regression model was utilized. Variables with P<0.1 in
univariate analysis were used in the multivariate analysis.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed with the R software
(version 4.2.0; R-project.org/). The ‘matchit’
(version 4.4.0; cran.r-project.org/web/packages/MatchIt/vignettes/MatchIt.html)
package was used for propensity score matching (PSM). The
‘survival’ (version 3.3-1 cran.r-project.org/package=survival) and ‘survminer’
(version 0.4.9 cran.r-project.org/web/packages/survminer/readme/README.html)
packages were used to generate survival curves and forest plots.
Due to crossover in the survival curves, the two-stage test was
used to calculate the P-value using the R package TSHRC (cran.r-project.org/web/packages/TSHRC/index.html)
(13). X-tile (version 3.6.1; Yale
University, USA; medicine.yale.edu/lab/rimm/research/software/)
was used to determine the optimal cutoff values for the number of
resected lymph nodes.
Results
Baseline characteristics
The current study included a total of 597 patients
with stage IB NSCLC. The baseline characteristics of the study
cohort are shown in Table I. The
majority of patients were male (71.2%) and 32.2% of the patients
were >65 years of age. Among all the included cases, 58.3% had
smoking history and 16.1% had family history of malignancy. In
terms of postoperative pathology, patients with grades I + II
accounted for 60.5%. The most common pathological types were
adenocarcinoma (64.2%), followed by squamous cell carcinoma
(33.5%), while the remaining 2.3% included large cell carcinoma,
sarcomatoid carcinoma and mucoepidermoid carcinoma. The proportion
of cases with visceral pleural invasion was 60%, while that of
bronchial invasion was 25%. In addition, 54% of the patients had
>12 lymph nodes resected, while 28.3% of the patients had ≥6
lymph node stations resected. Following surgery, ~16.1% of patients
received adjuvant chemotherapy.
 | Table IClinicopathological characteristics of
original and matched data. |
Table I
Clinicopathological characteristics of
original and matched data.
| | Original data
set | Matched data set |
|---|
| | | Resection of
subcarinal lymph nodes | | | Resection of
subcarinal lymph nodes | |
|---|
| Characteristic | Total | No (n=185) | Yes (n=412) | P-value | Total | No (n=146) | Yes (n=146) | P-value |
|---|
| Sex (%) | | | | 0.348 | | | | 0.524 |
|
Male | 425 (71.2) | 137 (74.1) | 288 (69.9) | | 204 (69.9) | 105 (71.9) | 99 (67.8) | |
|
Female | 172 (28.8) | 48 (25.9) | 124 (30.1) | | 88 (30.1) | 41 (28.1) | 47 (32.2) | |
| Age, years (%) | | | | 0.088 | | | | 1.000 |
|
≤65 | 405 (67.8) | 116 (62.7) | 289 (70.1) | | 203 (69.5) | 102 (69.9) | 101 (69.2) | |
|
>65 | 192 (32.2) | 69 (37.3) | 123 (29.9) | | 89 (30.5) | 44 (30.1) | 45 (30.8) | |
| Smoking status
(%) | | | | 0.906 | | | | 0.475 |
|
No | 249 (41.7) | 76 (41.1) | 173 (42.0) | | 119 (40.8) | 56 (38.4) | 63 (43.2) | |
|
Yes | 348 (58.3) | 109 (58.9) | 239 (58.0) | | 173 (59.2) | 90 (61.6) | 83 (56.8) | |
| Family history of
cancer (%) | | | | 0.856 | | | | 1.000 |
|
No | 501 (83.9) | 154 (83.2) | 347 (84.2) | | 250 (85.6) | 125 (85.6) | 125 (85.6) | |
|
Yes | 96 (16.1) | 31 (16.8) | 65 (15.8) | | 42 (14.4) | 21 (14.4) | 21 (14.4) | |
| Grade (%) | | | | 0.402 | | | | 0.100 |
|
I + II | 361 (60.5) | 117 (63.2) | 244 (59.2) | | 159 (54.5) | 87 (59.6) | 72 (49.3) | |
|
III +
IV | 236 (39.5) | 68 (36.8) | 168 (40.8) | | 133 (45.5) | 59 (40.4) | 74 (50.7) | |
| Histology (%) | | | | 0.378 | | | | 0.658 |
|
Adenocarcinoma | 383 (64.2) | 126 (68.1) | 257 (62.4) | | 188 (64.4) | 97 (66.4) | 91 (62.3) | |
|
Squamous
cell carcinoma | 200 (33.5) | 56 (30.3) | 144 (35.0) | | 99 (33.9) | 46 (31.5) | 53 (36.3) | |
|
Other | 14 (2.3) | 3 (1.6) | 11 (2.6) | | 5 (1.7) | 3 (2.1) | 2 (1.4) | |
| Visceral pleura
invasion (%) | | | | 0.919 | | | | 0.406 |
|
No | 239 (40.0) | 73 (39.5) | 166 (40.3) | | 122 (41.8) | 57 (39.0) | 65 (44.5) | |
|
Yes | 358 (60.0) | 112 (60.5) | 246 (59.7) | | 170 (58.2) | 89 (61.0) | 81 (55.5) | |
| Bronchial invasion
(%) | | | | 0.031 | | | | 0.668 |
|
No | 445 (74.5) | 149 (80.5) | 296 (71.8) | | 230 (78.8) | 117 (80.1) | 113 (77.4) | |
|
Yes | 152 (25.5) | 36 (19.5) | 116 (28.2) | | 62 (21.2) | 29 (19.9) | 33 (22.6) | |
| Resected lymph node
stations (%) | | | | <0.001 | | | | 1.000 |
|
<6 | 428 (71.7) | 171 (92.4) | 257 (62.4) | | 264 (90.4) | 132 (90.4) | 132 (90.4) | |
|
≥6 | 169 (28.3) | 14 (7.57) | 155 (37.6) | | 28 (9.59) | 14 (9.59) | 14 (9.59) | |
| Resected lymph node
number (%) | | | | <0.001 | | | | 1.000 |
|
<12 | 269 (45.1) | 141 (76.2) | 128 (31.1) | | 204 (69.9) | 102 (69.9) | 102 (69.9) | |
|
≥12 | 328 (54.9) | 44 (23.8) | 284 (68.9) | | 88 (30.1) | 44 (30.1) | 44 (30.1) | |
| Chemotherapy
(%) | | | | 1.000 | | | | 0.610 |
|
No | 501 (83.9) | 155 (83.8) | 346 (84.0) | | 252 (86.3) | 124 (84.9) | 128 (87.7) | |
|
Yes | 96 (16.1) | 30 (16.2) | 66 (16.0) | | 40 (13.7) | 22 (15.1) | 18 (12.3) | |
Among all the cases included in the present study,
185 patients did not undergo subcarinal lymph node resection,
whereas 412 did. Table I shows
statistically significant differences in bronchial invasion,
resected lymph node stations and resected lymph node numbers
between the two groups.
Prognostic factors for OS and RFS
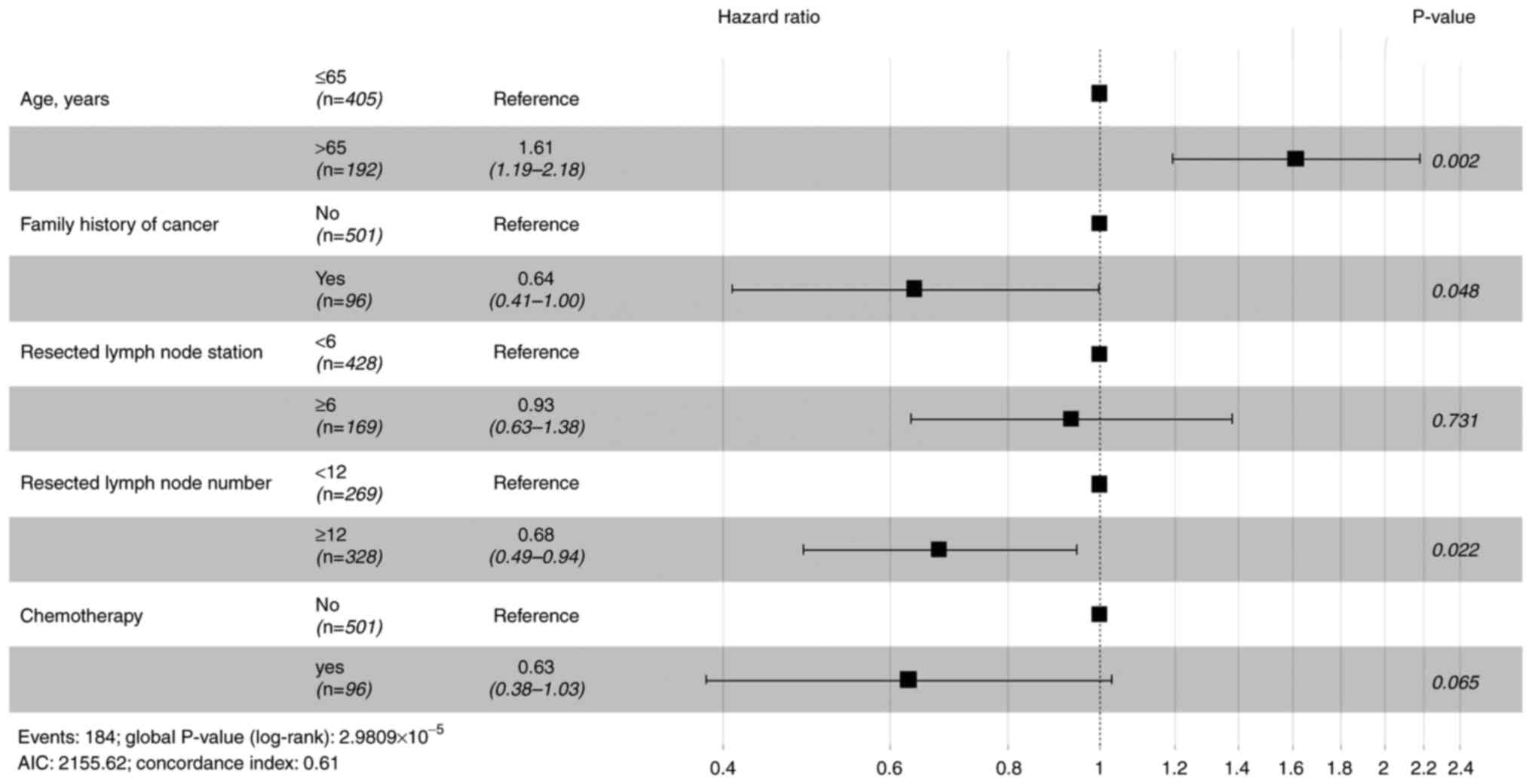
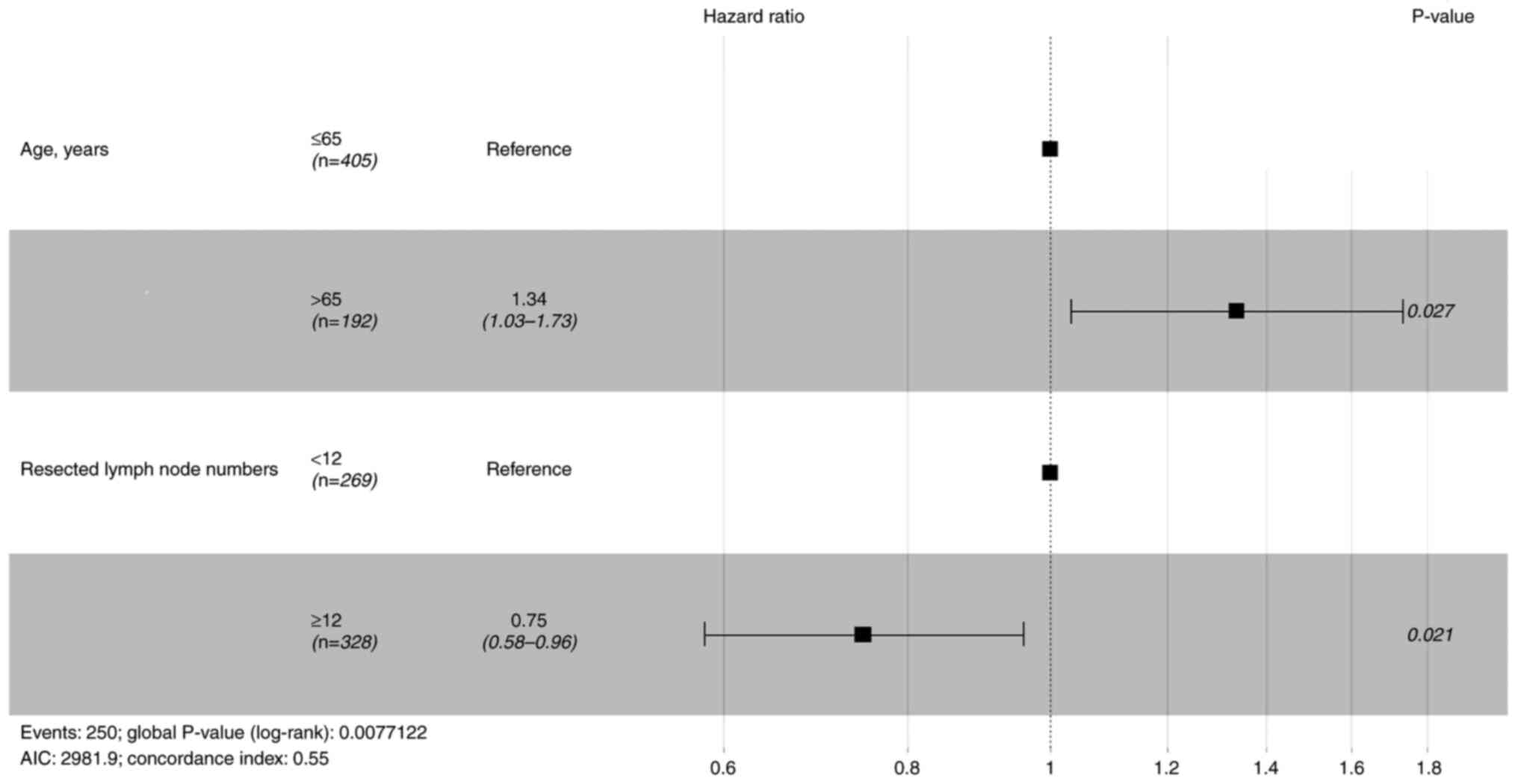
Before PSM, prognostic factors were evaluated with
Cox proportional hazards regression models. Table II shows univariate analysis for OS
and RFS. Variables with P<0.1 were included in the multivariate
analysis. Age, family history of cancer and the resected lymph
nodes number were significant prognostic factors for OS (Fig. 1), whereas only age and resected
lymph node number were significant prognostic factors for RFS
(Fig. 2). Cox regression analysis
of all included cases suggested that resection of subcarinal lymph
nodes was not a prognostic factor for OS and RFS.
 | Table IIUnivariate analysis of overall
survival and recurrence-free survival before propensity score
matching. |
Table II
Univariate analysis of overall
survival and recurrence-free survival before propensity score
matching.
| | Overall
survival | Recurrence-free
survival |
|---|
| Characteristic | Total | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (%) | | | 0.419 | | 0.587 |
|
Male | 425 (71.2) | Reference | | Reference | |
|
Female | 172 (28.8) | 0.87
(0.63-1.22) | | 0.93
(0.70-1.22) | |
| Age, years (%) | | | 0.001 | | 0.032 |
|
≤65 | 405 (67.8) | Reference | | Reference | |
|
>65 | 192 (32.2) | 1.68
(1.25-2.26) | | 1.33
(1.02-1.72) | |
| Smoking status
(%) | | | 0.101 | | 0.594 |
|
No | 249 (41.7) | Reference | | Reference | |
|
Yes | 348 (58.3) | 1.28
(0.95-1.73) | | 1.07
(0.83-1.38) | |
| Family history of
cancer (%) | | | 0.035 | | 0.118 |
|
No | 501 (83.9) | Reference | | Reference | |
|
Yes | 96 (16.1) | 0.62
(0.40-0.97) | | 0.75
(0.52-1.08) | |
| Grade (%) | | | 0.324 | | 0.378 |
|
I + II | 361 (60.5) | Reference | | Reference | |
|
III +
IV | 236 (39.5) | 1.16
(0.86-1.55) | | 1.12
(0.87-1.44) | |
| Histology (%) | | | 0.514 | | 0.142 |
|
Adenocarcinoma | 383 (64.2) | Reference | | Reference | |
|
Squamous
cell carcinoma | 200 (33.5) | 0.84
(0.61-1.15) | | 0.77
(0.58-1.01) | |
|
Other | 14 (2.3) | 0.76
(0.24-2.40) | | 0.68
(0.25-1.84) | |
| Visceral pleura
invasion (%) | | | 0.882 | | 0.344 |
|
No | 239 (40.0) | Reference | | Reference | |
|
Yes | 358 (60.0) | 0.98
(0.73-1.31) | | 1.13
(0.88-1.46) | |
| Bronchial invasion
(%) | | | 0.131 | | 0.169 |
|
No | 445 (74.5) | Reference | | Reference | |
|
Yes | 152 (25.5) | 0.75
(0.52-1.09) | | 0.81
(0.60-1.10) | |
| Resection of
subcarinal lymph nodes (%) | | | 0.255 | | 0.237 |
|
No | 185 (31.0) | Reference | | Reference | |
|
Yes | 412 (69.0) | 0.84
(0.62-1.13) | | 0.86
(0.66-1.11) | |
| Resected lymph node
station (%) | | | 0.061 | | 0.286 |
|
<6 | 428 (71.7) | Reference | | Reference | |
|
≥6 | 169 (28.3) | 0.72
(0.51-1.02) | | 0.86
(0.65-1.14) | |
| Resected lymph node
number (%) | | | 0.004 | | 0.025 |
|
<12 | 269 (45.1) | Reference | | Reference | |
|
≥12 | 328 (54.9) | 0.65
(0.49-0.87) | | 0.75
(0.59-0.97) | |
| Chemotherapy
(%) | | | 0.014 | | 0.177 |
|
No | 501 (83.9) | Reference | | Reference | |
|
Yes | 96 (16.1) | 0.55
(0.34-0.89) | | 0.78
(0.54-1.12) | |
Survival analysis
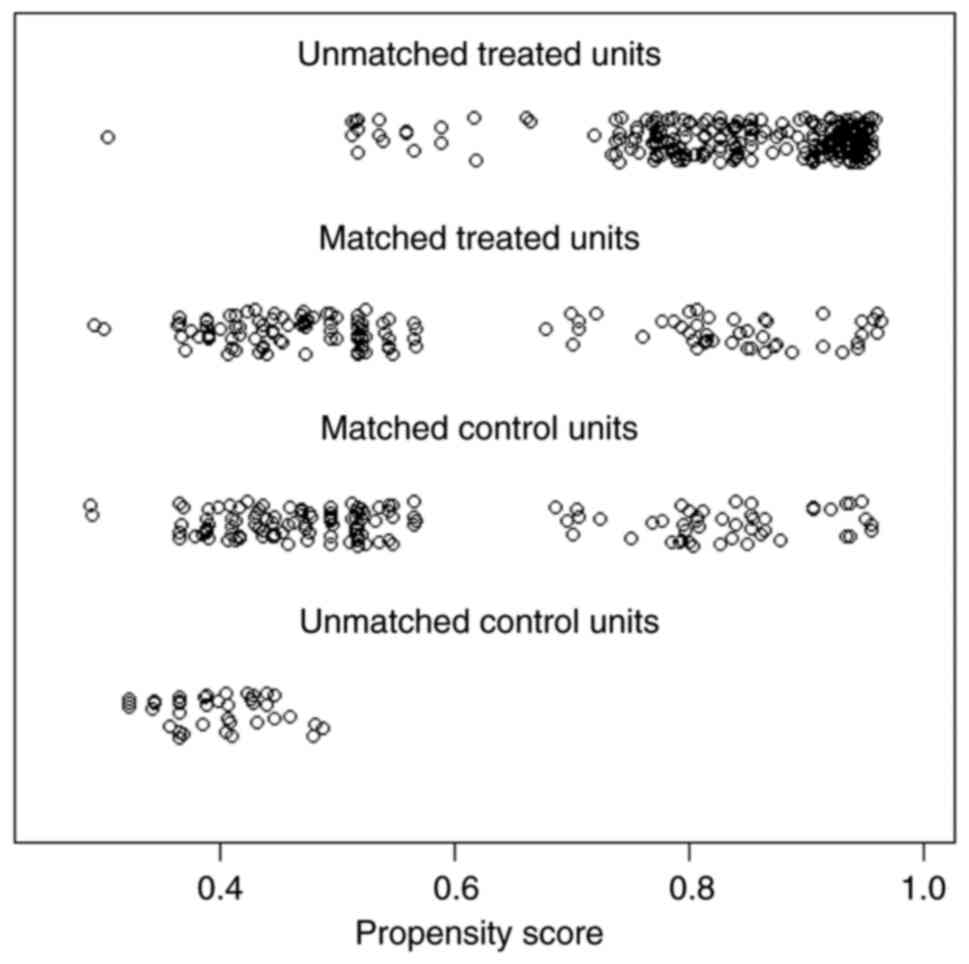
To compare the effect of subcarinal lymph node
resection on OS and RFS, 1:1 PSM was performed for all variables. A
total of 252 cases, 146 for each group, were selected after PSM
(Table I). χ2 indicated
that all variables were not statistically different between the two
groups. Fig. 3 shows the
distribution of propensity scores before and after matching. A good
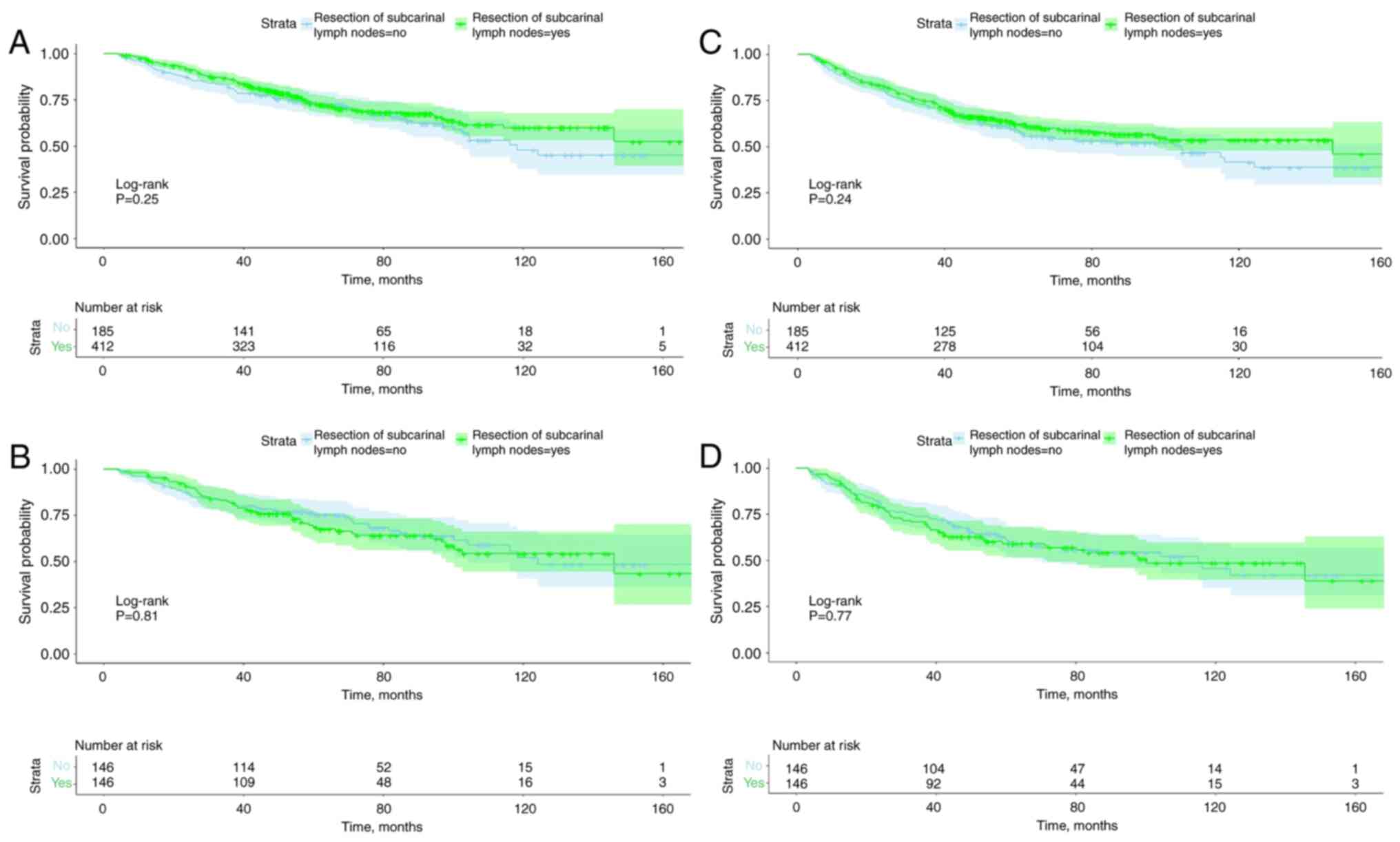
match was obtained between the two groups. Fig. 4 shows OS and RFS curves stratified
with or without subcarinal lymph node resection before and after
PSM. All four survival curves were not statistically significant,
indicating that resection of subcarinal lymph nodes was not
associated with OS and RFS.
Discussion
For LND in NSCLC, SLND (including subcarinal LND)
has been the gold standard surgical treatment (14-16).
However, with the increase in number of patients undergoing surgery
for early-stage NSCLC (10),
research has focused on new surgical approaches to LND in
early-stage lung cancer. Among them, LSLND and LNS have been
proposed by studies for the treatment of early-stage NSCLC
(9,10,17,18).
However, these LND methods do not emphasize the need to remove
subcarinal lymph nodes. To explore the effect of subcarinal lymph
node resection on prognosis in early-stage NSCLC, a total of 597
patients with stage IB NSCLC were included in the present study.
Cox regression was used to investigate the prognostic factors and
resection of the subcarinal lymph node did not affect OS and RFS.
To decrease the influence of bias and confounding variables,
survival analysis was performed after PSM, which suggested that the
resection of subcarinal lymph nodes was not associated with OS and
RFS.
International Association for the Study of Lung
Cancer proposed the concept of SLND (19,20).
In 2006, the European Society of Thoracic Surgeons guidelines
defined the extent of resection in SLND as removal of ≥6 stations
of lymph nodes, including >3 stations of mediastinal lymph nodes
on the same side and the subcarinal lymph nodes (#7 lymph nodes)
(14). Studies have found that
SLND could improve the survival of patients with advanced NSCLC
(12,21-23).
SLND may increase surgical complications such as lymphatic fistula,
recurrent laryngeal nerve injury, surgical blood loss and prolonged
hospitalization (19); however,
necessity of SLND remains controversial for early-stage NSCLC
(24-26).
For early NSCLC, several studies have suggested that LSLND and LNS
achieve the same therapeutic effect as SLND (6,8,10-12,27).
LSLND is defined as the removal of specific mediastinal and hilar
lymph nodes according to the lobe of the tumor (9). LNS is defined as the resection of
abnormal lymph nodes found preoperatively and intraoperatively
(28). Neither of these two
methods of LND for early-stage NSCLC require dissection of
subcarinal lymph nodes and, according to the present findings, LNS
and LSLND achieve the same therapeutic effect as SLND. The present
study found that for stage IB NSCLC, there was no association
between subcarinal lymph node dissection and OS or RFS. These
results were consistent with the aforementioned previous findings
on early-stage NSCLC.
Since the subcarinal lymph nodes are adjacent to the
carina, esophagus and vagus nerve, these structures are prone to
damage during dissection. Therefore, for early-stage NSCLC,
surgeons may choose not to remove subcarinal lymph nodes. According
to previous studies and the present study, the absence of
subcarinal LND for early-stage lung cancer has no significant
effect on OS and RFS. In summary, for early-stage NSCLC, not
dissecting the subcarinal lymph nodes can decrease potential
surgical damage, speed up surgery time and enable patients to
recover faster (18,24,28).
However, SLND could allow more accurate staging and provide an
opportunity for postoperative adjuvant therapy (11,29).
Therefore, SLND (including resection of subcarinal lymph nodes) may
be recommended in patients who are considered at risk of lymph node
metastasis in preoperative examination.
The present analysis suggested that subcarinal lymph
node resection was not associated with OS or RFS. It could also be
concluded that number of resected lymph node stations was not
associated with OS or RFS. However, the number of resected lymph
nodes in the multivariate analysis was a statistically significant
prognostic factor for OS and RFS., while the resection of
subcarinal lymph nodes and the resected lymph node stations were
not statistically significant. These results indicated that for
stage IB NSCLC, the number of dissected lymph nodes may be more
meaningful in terms of improving prognosis than resection of the
subcarinal lymph nodes and number of lymph node stations. Previous
studies on NSCLC have suggested that this view remains
controversial and needs to be confirmed by more studies in the
future (30-32).
If this conclusion is established, it will challenge the current
principle of LND in lung cancer.
The current study had limitations. Firstly, this was
a single-center retrospective study and multi-center prospective
studies are needed to confirm the present conclusions. Secondly,
this study enrolled 597 patients; this relatively small sample size
could lead to bias. Due to the small sample size, no further
subgroup analysis was performed for different pathological subtypes
of NSCLC. Finally, the enrolled population of this study included
only patients with postoperative pathologically confirmed stage IB;
it is difficult to obtain the accurate pathological staging of
patients before surgery. Therefore, the conclusions of this study
have limitations in their future clinical application.
For stage IB NSCLC, there was no statistically
significant association between resection of the subcarinal lymph
node and patient survival. Subcarinal lymph node resection in
surgery of stage IB NSCLC may be considered optional.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Scientific Research
improvement program of Beijing Chest Hospital (grant no.
Kj2021cx009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW, YH and XY wrote the manuscript. YH, SL, LZ and
FW participated in design of the study. FW, XY and LZ were involved
in acquisition of data. FW, SL and XY participated in analysis and
interpretation of data. FW and SL confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The studies involving human participants were
reviewed and approved (approval no B2018-011) by Ethics Committee
of Sun Yat-Sen University Cancer Center (Guangzhou, P.R. China and
Beijing Chest Hospital Institutional Review Board (Beijing, P.R.
China). The participants provided written informed consent to
participate in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Masuda M, Kuwano H, Okumura M, Arai H,
Endo S, Doki Y, Kobayashi J, Motomura N, Nishida H, Saiki Y, et al:
Thoracic and cardiovascular surgery in Japan during 2013: Annual
report by The Japanese association for thoracic surgery. Gen Thorac
Cardiovasc Surg. 63:670–701. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
De Leyn P, Lardinois D, Van Schil P,
Rami-Porta R, Passlick B, Zielinski M, Waller D, Lerut T and Weder
W: European trends in preoperative and intraoperative nodal
staging: ESTS guidelines. J Thorac Oncol. 2:357–361.
2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Howington JA, Blum MG, Chang AC, Balekian
AA and Murthy SC: Treatment of stage I and II non-small cell lung
cancer: Diagnosis and management of lung cancer, 3rd ed: American
College of Chest Physicians evidence-based clinical practice
guidelines. Chest. 143:e278S–e313S. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ishiguro F, Matsuo K, Fukui T, Mori S,
Hatooka S and Mitsudomi T: Effect of selective lymph node
dissection based on patterns of lobe-specific lymph node metastases
on patient outcome in patients with resectable non-small cell lung
cancer: A large-scale retrospective cohort study applying a
propensity score. J Thorac Cardiovasc Surg. 139:1001–1006.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Maniwa T, Okumura T, Isaka M, Nakagawa K,
Ohde Y and Kondo H: Recurrence of mediastinal node cancer after
lobe-specific systematic nodal dissection for non-small-cell lung
cancer. Eur J Cardiothorac Surg. 44:e59–e64. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hishida T, Miyaoka E, Yokoi K, Tsuboi M,
Asamura H, Kiura K, Takahashi K, Dosaka-Akita H, Kobayashi H, Date
H, et al: Lobe-specific nodal dissection for clinical stage I and
II NSCLC: Japanese multi-institutional retrospective study using a
propensity score analysis. J Thorac Oncol. 11:1529–1537.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Adachi H, Sakamaki K, Nishii T, Yamamoto
T, Nagashima T, Ishikawa Y, Ando K, Yamanaka K, Watanabe K,
Kumakiri Y, et al: Lobe-specific lymph node dissection as a
standard procedure in surgery for non-small cell lung cancer: A
propensity score matching study. J Thorac Oncol. 12:85–93.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Okada M, Sakamoto T, Yuki T, Mimura T,
Miyoshi K and Tsubota N: Selective mediastinal lymphadenectomy for
clinico-surgical stage I non-small cell lung cancer. Ann Thorac
Surg. 81:1028–1032. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Darling GE, Allen MS, Decker PA, Ballman
K, Malthaner RA, Inculet RI, Jones DR, McKenna RJ, Landreneau RJ,
Rusch VW and Putnam JB Jr: Randomized trial of mediastinal lymph
node sampling versus complete lymphadenectomy during pulmonary
resection in the patient with N0 or N1 (less than hilar) non-small
cell carcinoma: Results of the American college of surgery oncology
group z0030 trial. J Thorac Cardiovasc Surg. 141:662–670.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hughes MJ, Chowdhry MF, Woolley SM and
Walker WS: In patients undergoing lung resection for non-small cell
lung cancer, is lymph node dissection or sampling superior?
Interact Cardiovasc Thorac Surg. 13:311–315. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li H, Han D, Hou Y, Chen H and Chen Z:
Statistical inference methods for two crossing survival curves: A
comparison of methods. PLoS One. 10(e0116774)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lardinois D, De Leyn P, Van Schil P, Porta
RR, Waller D, Passlick B, Zielinski M, Lerut T and Weder W: ESTS
guidelines for intraoperative lymph node staging in non-small cell
lung cancer. Eur J Cardiothorac Surg. 30:787–792. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rice D, Chansky K, Nowak A, Pass H,
Kindler H, Shemanski L, Opitz I, Call S, Hasegawa S, Kernstine K,
et al: The IASLC mesothelioma staging project: Proposals for
revisions of the N descriptors in the forthcoming eighth edition of
the TNM classification for pleural mesothelioma. J Thorac Oncol.
11:2100–2111. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chansky K, Detterbeck FC, Nicholson AG,
Rusch VW, Vallières E, Groome P, Kennedy C, Krasnik M, Peake M,
Shemanski L, et al: The IASLC lung cancer staging project: External
validation of the revision of the TNM stage groupings in the eighth
edition of the TNM classification of lung cancer. J Thorac Oncol.
12:1109–1121. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jett JR, Schild SE, Kesler KA and
Kalemkerian GP: Treatment of small cell lung cancer: Diagnosis and
management of lung cancer, 3rd ed: American College of Chest
Physicians evidence-based clinical practice guidelines. Chest.
143:e400S–e419S. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Adachi H, Maehara T, Nakayama H and Masuda
M: Mediastinal lymph node dissection in surgical treatment for
early stage non-small-cell lung cancer: Lobe-specific or
systematic? J Thorac Dis. 9:2728–2731. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ma K, Chang D, He B, Gong M, Tian F, Hu X,
Ji Z and Wang T: Radical systematic mediastinal lymphadenectomy
versus mediastinal lymph node sampling in patients with clinical
stage IA and pathological stage T1 non-small cell lung cancer. J
Cancer Res Clin Oncol. 134:1289–1295. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Goldstraw P: Report on the international
workshop on intrathoracic staging. London, October 1996. Lung
Cancer. 18:107–111. 1997.
|
|
21
|
Wu Y, Huang ZF, Wang SY, Yang XN and Ou W:
A randomized trial of systematic nodal dissection in resectable
non-small cell lung cancer. Lung Cancer. 36:1–6. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Keller SM, Adak S, Wagner H and Johnson
DH: Mediastinal lymph node dissection improves survival in patients
with stages II and IIIa non-small cell lung cancer. Eastern
cooperative oncology group. Ann Thorac Surg. 70:358–365; discussion
365-356. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Misthos P, Sepsas E, Kokotsakis J, Skottis
I and Lioulias A: Prognosis of stage pIIIA non small cell lung
cancer after mediastinal lymph node dissection or sampling. J BUON.
14:45–49. 2009.PubMed/NCBI
|
|
24
|
Doddoli C, Aragon A, Barlesi F, Chetaille
B, Robitail S, Giudicelli R, Fuentes P and Thomas P: Does the
extent of lymph node dissection influence outcome in patients with
stage I non-small-cell lung cancer? Eur J Cardiothorac Surg.
27:680–685. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Allen MS, Darling GE, Pechet TT, Mitchell
JD, Herndon JE II, Landreneau RJ, Inculet RI, Jones DR, Meyers BF,
Harpole DH, et al: Morbidity and mortality of major pulmonary
resections in patients with early-stage lung cancer: Initial
results of the randomized, prospective ACOSOG Z0030 trial. Ann
Thorac Surg. 81:1013–1019; discussion 1019-1020. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bille A, Woo KM, Ahmad U, Rizk NP and
Jones DR: Incidence of occult pN2 disease following resection and
mediastinal lymph node dissection in clinical stage I lung cancer
patients. Eur J Cardiothorac Surg. 51:674–679. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang X, Wang J, Chen Q and Jiang J:
Mediastinal lymph node dissection versus mediastinal lymph node
sampling for early stage non-small cell lung cancer: A systematic
review and meta-analysis. PLoS ONE. 9(e109979)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Watanabe S: Lymph node dissection for lung
cancer: Past, present, and future. Gen Thorac Cardiovasc Surg.
62:407–414. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Meng D, Zhou Z, Wang Y, Wang L, Lv W and
Hu J: Lymphadenectomy for clinical early-stage non-small-cell lung
cancer: A systematic review and meta-analysis. Eur J Cardiothorac
Surg. 50:597–604. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Samayoa AX, Pezzi TA, Pezzi CM, Gay EG,
Asai M, Kulkarni N, Carp N, Chun SG and Putnam JB Jr: Rationale for
a minimum number of lymph nodes removed with non-small cell lung
cancer resection: Correlating the number of nodes removed with
survival in 98,970 patients. Ann Surg Oncol. 23:1005–1011.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liang W, He J, Shen Y, Shen J, He Q, Zhang
J, Jiang G, Wang Q, Liu L, Gao S, et al: Impact of examined lymph
node count on precise staging and long-term survival of resected
non-small-cell lung cancer: A population study of the US SEER
database and a Chinese multi-institutional registry. J Clin Oncol.
35:1162–1170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Riquet M, Arame A and Pricopi C:
Subcarinal lymph node importance revisited. Ann Thorac Surg.
105:666–667. 2018.PubMed/NCBI View Article : Google Scholar
|