Introduction
Breast cancer is a prevalent health concern
affecting a significant number of women worldwide (1). Targeted therapies, such as
trastuzumab, have been widely studied and shown to be effective in
the treatment of HER2-positive breast cancer, leading to improved
survival outcomes for patients (2). Despite advances in treatment,
recurrence remains a significant challenge for patients with
metastatic breast cancer (3). The
human epidermal growth factor receptor 2 (HER2)
proto-oncogene regulates cell growth, survival, and
differentiation. HER2 overexpression, commonly referred to
as HER2 positivity, is a poor prognostic sign in patients
with breast cancer (2,4), and HER2-positive breast cancer
accounts for 15-20% of annual breast cancer-associated deaths. The
recurrence rate in HER2-positive metastatic breast cancer varies,
but it is a significant factor affecting patient outcomes and
prognosis. Studies have shown that the recurrence rate in
HER2-positive metastatic breast cancer ranges from 20-30% (3). However, the introduction of
anti-HER2 agents has revolutionized the standard of care for
patients with HER2-positive breast cancer.
Pertuzumab is a recombinant human immunoglobulin G
(IgG) monoclonal antibody that targets HER2 and acts by
blocking HER2 dimerization with other HER family
members including HER1, HER3, and HER4 (5). Trastuzumab is another targeted
therapy that binds similarly to HER2. However, while
pertuzumab binds to subdomain II of the HER2 extracellular
domain epitope, trastuzumab binds to subdomain IV (6). Although these agents bind to
different HER2 epitopes, they have complementary mechanisms
of action. The combined administration of trastuzumab and
pertuzumab offers a more comprehensive blockade of the HER2
signaling pathway and results in more antitumor activity (7).
The CLEOPATRA phase III trial demonstrated the
efficacy of adding pertuzumab to trastuzumab-docetaxel combination
therapy as a first-line agent in patients with HER2-positive
metastatic breast cancer (mBC). The trial revealed that adding
pertuzumab resulted in a significant improvement in overall
survival (OS) compared with trastuzumab and docetaxel combined with
a placebo (8,9). In addition, the toxicity profiles of
patients receiving pertuzumab and those receiving placebo were
similar and manageable. Based on the significantly improved OS in
the CLEOPATRA trial, the combination of pertuzumab, trastuzumab,
and docetaxel has become the standard of care for first-line
therapy for patients with mBC. However, docetaxel is associated
with significant toxicity. Therefore, several studies have reported
that the combination of pertuzumab-trastuzumab with other
chemotherapy agents, including weekly paclitaxel or nanoparticle
albumin-bound (nab)-paclitaxel, is effective and more tolerable
than regimens that include docetaxel (10-12).
Therefore, the combination of pertuzumab-trastuzumab with either
taxane- or vinorelbine-based chemotherapy agents is the new
standard first-line therapy for patients with HER2-positive
mBC.
In patients with HER2-positive mBC who have
recurrence or disease progression following first-line therapy with
pertuzumab-trastuzumab, physicians may choose to re-target the
HER2 receptor, though data regarding the safety and efficacy
of this practice are relatively limited (9,13-15).
Therefore, this retrospective, observational study involving
physicians from major Swiss oncology centers aimed to assess the
therapeutic regimens, toxicities, and clinical outcomes following
second- or later-line pertuzumab therapy in patients with mBC who
did not receive pertuzumab as a first-line chemotherapy agent.
Materials and methods
Data source
Patients with HER2-positive mBC who received
second- or later-line pertuzumab therapy without having received
pertuzumab as a first-line therapy at nine major Swiss oncology
centers (Canton Hospital Winterthur, Med. Oncology, Winterthur,
Switzerland; Department of Gynecology, Canton Hospital Baden,
Baden; Switzerland; Canton Hospital Olten, Division of Internal
Medicine, Olten, Switzerland; Tumor Center ZeTuP, Rapperswil;
Canton Hospital Aarau, Aarau, Switzerland; Canton Hospital
Muensterlingen, Münsterlingen; Switzerland; Oncology Private
Practice Basel, Affiliate of the Department of Medical Oncology,
University Hospital Basel, Basel; Switzerland; Medical University
Clinic, Canton Hospital Baselland, Liestal; University of Basel,
Basel, Switzerland) were retrospectively identified. The patients'
demographic, clinical, and therapeutic data were extracted from the
medical records. Physicians of these patients were asked to
complete a questionnaire regarding treatment regimens, safety, and
survival for each included patient. The questionnaire was
previously developed by one of the authors with the support of a
statistical team.
Patient selection criteria
Pertuzumab-naive patients with HER2-positive
mBC who had a relapse of mBC following first-line therapy between
2001 and 2016 and were subsequently treated with at least one dose
of second- or later-line pertuzumab therapy were included in this
study. Only female patients aged ≥18 years who were treated at one
of the included Swiss oncology centers were included. All eligible
patients had mBC with known HER2-positive status. The study
end-date was September 12, 2017. Patients who were male, diagnosed
or treated for another primary cancer during the study period, or
enrolled in other clinical trials and those who had previously been
administered pertuzumab as a first-line treatment were
excluded.
Clinical study measures
The initial date of pertuzumab administration was
defined as the index date. The primary endpoint was OS, calculated
from the index date to death or the date of the last follow-up.
Data regarding disease progression, adverse events (AEs), and
co-administered treatments were retrieved from the medical records.
AEs were assessed according to the National Cancer Institute Common
Terminology Criteria for Adverse Events (NCI CTCAE, version 4.0).
The duration of pertuzumab therapy was defined as the time from the
index date to the date of the last administration of pertuzumab,
death, or the end of the study. Data of patients who were alive or
lost to follow-up at the end of the study were censored.
Statistical analysis
Descriptive statistics were used to summarize
patient demographics, clinical characteristics, treatment patterns,
and AEs. Categorical variables are presented as frequency and
percentage, and continuous variables are presented as mean and
standard deviation or median and range.
A survival analysis was performed using the
Kaplan-Meier methodology, using the LIFETEST procedure. All
statistical analyses were performed using SPSS 17.0 statistical
software.
Results
Patient characteristics
Overall, 35 female patients (median age: 49 years;
range: 35-87 years) with HER2-positive mBC were included in
the study. The clinicopathological characteristics of the patients
are summarized in Table I.
Overall, 33 patients (94%) had invasive ductal carcinoma and two
(6%) had invasive lobular carcinoma. Twenty-four patients (69%) had
ER-positive or PR-positive tumors, 17 (49%) had stage IV cancer,
and five (14%) had stage III cancer at diagnosis. Sixteen patients
(46%) received trastuzumab therapy prior to pertuzumab therapy. The
most common metastatic sites were the bone (n=22; 63%), liver
(n=15; 43%), and lymph nodes (n=14; 43%).
 | Table IPatient characteristics (n=35). |
Table I
Patient characteristics (n=35).
| Variables | No. (%) |
|---|
| Histological
subtype | |
|
Invasive
ductal carcinoma | 33(94) |
|
Invasive
lobular carcinoma | 2(6) |
| Estrogen- and/or
progesterone receptor-positive | 24(69) |
| Stage IV cancer at
first diagnosis | 17(49) |
| Prior (neo)adjuvant
chemotherapy | 15(43) |
| Prior
trastuzumab | 16(46) |
| Metastatic sites | |
|
Bone | 22(63) |
|
Liver | 15(43) |
|
Lymph
nodes | 15(43) |
Ten patients (29%) underwent primary
breast-conserving procedures and 16 (46%) underwent ablative
(mastectomy) procedures. Thirteen patients (37%) underwent
radiotherapy and 15 (43%) underwent chemotherapy.
Treatments
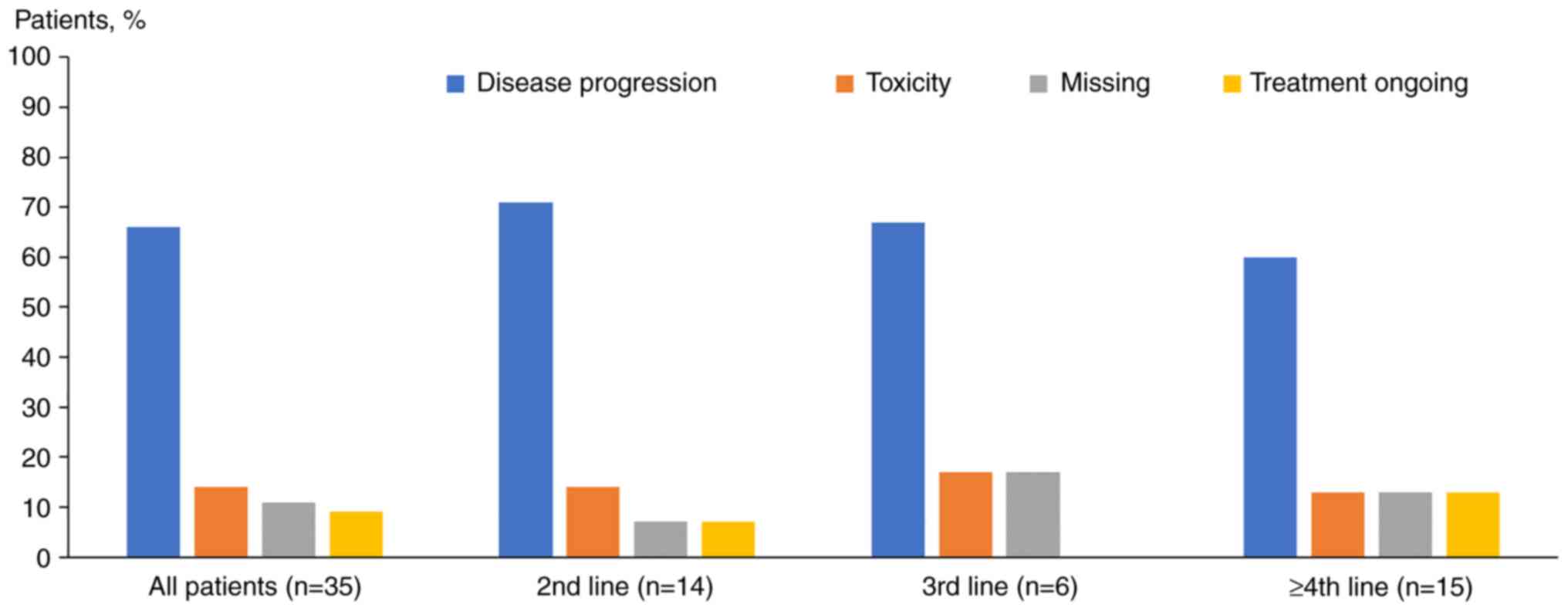
A total of 14 patients (40%) received pertuzumab as
a second-line agent, six (17%) as a third-line agent, and 15 (43%)
as a fourth- or later-line agent (Table II).
 | Table IIChemotherapy regimens. |
Table II
Chemotherapy regimens.
| Regimen | All patients, n (%)
(n=35) | Second-line, n (%)
(n=14) | Third-line, n (%)
(n=6) | Fourth- or
later-line, n (%) (n=15) |
|---|
| Pertuzumab +
trastuzumab + chemotherapy | 29(83) | 12(86) | 5(83) | 12(80) |
| Taxane | 19(54) | 8(57) | 4(67) | 7(47) |
| Vinorelbine | 6(17) | 3(21) | 1(17) | 2(13) |
| Gemcitabine | 1(3) | 0 (0) | 0 (0) | 1(7) |
| Carboplatin | 1(3) | 0 (0) | 0 (0) | 1(7) |
| Anthracycline | 2(6) | 1(7) | 0 (0) | 1(7) |
| Pertuzumab +
trastuzumab + endocrine therapy | 1(3) | 1(7) | 0 (0) | 0 (0) |
| Pertuzumab +
trastuzumab alone | 5(14) | 1(7) | 1(17) | 3(20) |
The median duration of pertuzumab administration was
6 months (range: 2-60 months). The median duration of pertuzumab
therapy was 5.5 months (range: 2-30 months) for patients who
received pertuzumab as a second-line agent (n=24), 6 months (range:
2-60 months) for patients who received pertuzumab as a third-line
agent (n=6), and 11 months (range: 2-40 months) for patients who
received pertuzumab as a fourth- or later-line agent (n=15).
Most patients (n=29; 83%) received a combination of
pertuzumab-trastuzumab with another chemotherapeutic agent,
including taxane (n=19; 54%), vinorelbine (n=6; 17%),
anthracyclines (n=2; 6%), and gemcitabine and carboplatin (n=1;
3%). Among the remaining six patients, one (3%) was administered
pertuzumab-trastuzumab with endocrine therapy and five (14%) were
administered pertuzumab-trastuzumab alone.
Pertuzumab was discontinued mainly due to disease
progression (n=23; 66%) and toxicity (n=5; 14%) (Fig. 1). However, pertuzumab-associated
toxicities were noted in one patient (3%).
Safety outcomes
G3 toxicities were reported in five patients (14%)
(Table III). No patients had G4
toxicities. The most commonly-recorded AE was fatigue (overall:
n=16, 46%; G3: n=4, 11%), followed by congestive heart failure
(overall: n=5, 14%; G3: n=2, 6%), nausea (overall: n=5, 14%; G3:
n=0), and myelosuppression (overall: n=4, 11%; G3: n=2, 6%).
 | Table IIIAdverse events. |
Table III
Adverse events.
| Adverse events | All grades, n
(%) | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|
| Fatigue | 16(46) | 7(20) | 5(14) | 4(11) | 0 (0) |
| Congestive heart
disease | 5(14) | 1(3) | 2(6) | 2(6) | 0 (0) |
| Nausea | 5(14) | 5(14) | 0 (0) | 0 (0) | 0 (0) |
|
Myelosuppression | 4(11) | 1(3) | 1(3) | 2(6) | 0 (0) |
| Diarrhea | 3(9) | 3(9) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 1(3) | 0 (0) | 1(3) | 0 (0) | 0 (0) |
| Mucositis | 1(3) | 0 (0) | 1(3) | 0 (0) | 0 (0) |
Overall survival
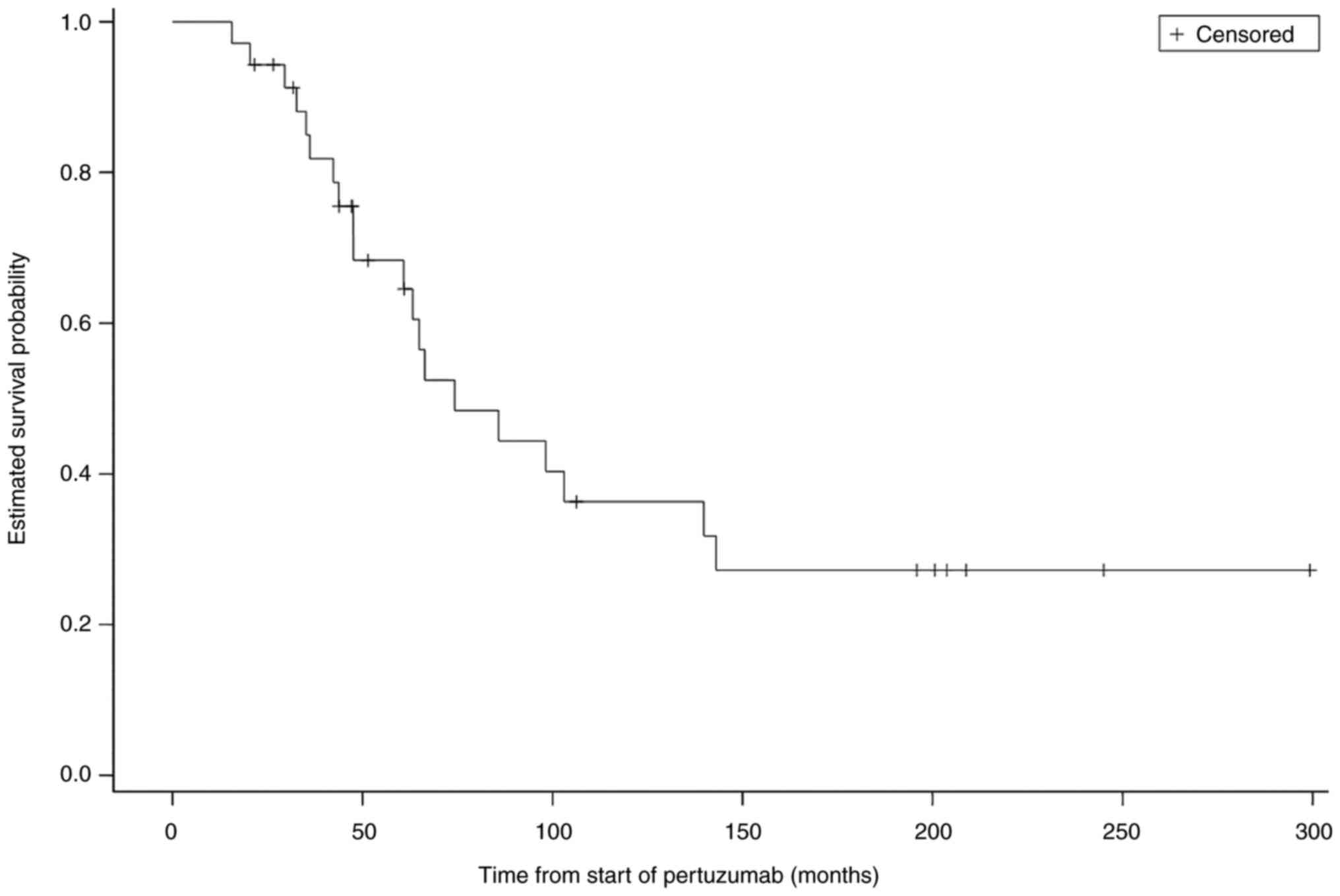
At the final follow-up, 20 patients (57%) had died.
The median OS was 74.2 months (95% confidence interval: 47.6-139.8
months) (Fig. 2).
Discussion
HER2-positive breast cancer has been linked
to more aggressive tumor behavior and poorer outcomes than
HER2-negative breast cancer (16). However, the use of trastuzumab
significantly improves the OS in patients with advanced
HER2-positive breast cancer. Several new chemotherapeutic
agents are currently being developed or are undergoing clinical
investigation, including trastuzumab-emtansine,
trastuzumab-deruxtecan, neratinib, and tucatinib. Most patients
with mBC experience disease progression following first-line
treatment (17-21).
The continuation of trastuzumab administration in patients who
experience disease progression has been associated with improvement
in the time to progression without an increased risk of
treatment-related toxicity (22).
In 2010, a phase II trial conducted by Baselga et al
(23) revealed that patients with
mBC who experienced disease progression during prior trastuzumab
therapy tolerated and responded well to the addition of pertuzumab
to their therapeutic regimen. Combining pertuzumab-trastuzumab with
taxane-based chemotherapy has become the new standard first-line
therapy for patients with HER2-positive mBC based on the
findings of the CLEOPATRA trial (9). The combination of
pertuzumab-trastuzumab with vinorelbine-based chemotherapy is as
effective as and less toxic than the combination with taxane-based
therapy (10). However, as a
second-line treatment, the administration of pertuzumab-trastuzumab
combined with capecitabine was not superior to the combination of
soletrastuzumab and capecitabine (24).
In the CLEOPATRA trial, the median OS was
significantly higher in patients who received pertuzumab compared
to patients who received placebo (9). These previous results are consistent
with the results of this study. In this study, the first dose of
later-line pertuzumab was administered on the OS index date, rather
than on the first treatment date for mBC. Therefore, these results
may reflect a patient selection bias.
As in the CLEOPATRA trial, chemotherapy accounted
for most of the AEs reported in this study. Symptoms that developed
during pertuzumab therapy in this study did not differ from those
reported in previous studies. The addition of pertuzumab to the
patients' therapeutic regimens did not increase cardiac toxicity in
this study.
The pertuzumab-trastuzumab combination has a higher
anti-cancer activity than either drug alone (7,25).
The combination therapy has been reported as effective against
advanced breast cancer following disease progression (8) and for patients receiving neoadjuvant
therapy. Pertuzumab, trastuzumab, and docetaxel have been
associated with higher complete response rates in pathological
samples than trastuzumab-docetaxel, pertuzumab-docetaxel, or
pertuzumab-trastuzumab combinations (25). The combination of
pertuzumab-trastuzumab and chemotherapy has been approved for the
neoadjuvant treatment of HER2-positive early breast cancer
with a high risk of recurrence by the Food and Drug Administration
in the United States and the Swiss Medic in Switzerland (26). Therefore, an increasing number of
patients with metastatic HER2-positive breast cancer will be
pretreated with pertuzumab in the future.
More clinical uses of combination therapy are
currently being investigated. A randomized phase III trial (Detect
V/CHEVENDO; NCT02344472) comparing the safety and efficacy of the
pertuzumab-trastuzumab combination with either endocrine therapy or
chemotherapy is being conducted in patients with hormone
receptor-positive and HER2-positive mBC.
HER2-/neu-targeted combinations may help patients avoid
potential chemotherapy-related toxicities while achieving high
efficacy, which will improve the patients' quality of life
(9,27-29).
This study has several limitations, including its
retrospective nature and small sample size. In addition, the OS
outcomes may have been overestimated as the index date for the OS
calculation was much later in the disease course in this study
compared to that in previous studies. This difference may have
resulted in a patient selection bias toward favorable prognosis
factors (long-term response in first-line therapy), similar to the
bias in a previous study regarding trastuzumab-deruxtecan (19). However, the remarkably long OS
observed in this study warrants further prospective investigations
of the use of pertuzumab in later-line regimens.
In conclusion, the use of trastuzumab has been shown
to significantly improve overall survival in patients with advanced
HER2-positive breast cancer. The pertuzumab-trastuzumab combination
has been approved for the neoadjuvant treatment of high-risk
HER2-positive early breast cancer and has been shown to have a
higher anti-cancer activity than either drug alone. This study
investigates the use of pertuzumab in later-line treatment regimens
for metastatic HER2-positive breast cancer. The median OS and
safety profile of second- or later-line pertuzumab therapy are
consistent with those reported for the first-line use of
pertuzumab. These results indicate that the combination of
pertuzumab and trastuzumab may have a positive impact on overall
survival in later-line regimens for patients with metastatic
HER2-positive breast cancer. However, due to its retrospective
nature and small sample size, further prospective investigations
are warranted to confirm these findings.
Acknowledgements
The authors would like to thank Professor Liwei Wang
(State Key Laboratory of Oncogenes and Related Genes, Shanghai
Cancer Institute, Department of Oncology, Renji Hospital, School of
Medicine, Shanghai Jiao Tong University, Shanghai, China) for his
support in editing the manuscript. Roche Pharma (Switzerland)
provided administrative support for the analysis.
Funding
Funding: The study was funded by F. Hoffmann-La Roche Ltd.,
Basel, Switzerland.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EB, CMS, AM, CL, CUN, DK, AS, CT, DT and MV
conducted the research. EB, ELGM and MV wrote and revised the
manuscript. EB and ELGM interpreted the data, and prepared the
figures and tables. CMS, AM, CL, CUN, DK, AS, CT and DT analyzed
the data. MV supervised and managed the research activity. EB and
MV confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
This retrospective, observational questionnaire was
conducted according to the Declaration of Helsinki and local
legislation. Ethics approval was not required for the present study
as retrospective questionnaire-based studies on records do not
require formal approval.
Patient consent for publication
Not applicable.
Competing interests
AM: Honoraria: Roche Switzerland, Novartis, Pfizer,
Amgen, and Tesaro; Consulting/advisory role: Roche Switzerland,
Novartis, AstraZeneca, Pfizer, and Amgen; Expert testimony: Roche
Switzerland; CL: Honoraria: Pfizer and AstraZeneca;
Consulting/advisory role: Pfizer and AstraZeneca; AS: Honoraria:
Amgen, Roche, Pfizer, MSD, BMS, Lilly, Celgene, and Merck; CT:
Consulting/advisory role: Celgene, Amgen, and Janssen; Research
funding: Celgene. DT: Honoraria: Roche; Consulting/advisory role:
Roche; MV: Honoraria: Roche, Novartis, and Pfizer;
Consulting/advisory role: Roche, Novartis, and Pfizer; Research
funding: Roche. EB was supported by Krebsliga Schweiz, BIL KFS
4261-08-2017. All other authors declare that they have no competing
interests.
References
|
1
|
World Health Organization, Breast cancer,
2020. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/.
|
|
2
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel members.
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Andrulis IL, Bull SB, Blackstein ME,
Sutherland D, Mak C, Sidlofsky S, Pritzker KP, Hartwick RW, Hanna
W, Lickley L, et al: neu/erbB-2 amplification identifies a
poor-prognosis group of women with node-negative breast cancer.
Toronto breast cancer study group. J Clin Oncol. 16:1340–1349.
1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Adams CW, Allison DE, Flagella K, Presta
L, Clarke J, Dybdal N, McKeever K and Sliwkowski MX: Humanization
of a recombinant monoclonal antibody to produce a therapeutic HER
dimerization inhibitor, pertuzumab. Cancer Immunol Immunother.
55:717–727. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hudis CA: Trastuzumab-mechanism of action
and use in clinical practice. N Engl J Med. 357:39–51.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Scheuer W, Friess T, Burtscher H,
Bossenmaier B, Endl J and Hasmann M: Strongly enhanced antitumor
activity of trastuzumab and pertuzumab combination treatment on
HER2-positive human xenograft tumor models. Cancer Res.
69:9330–9336. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Baselga J, Cortés J, Kim SB, Im SA, Hegg
R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al:
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast
cancer. N Engl J Med. 366:109–119. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Swain SM, Baselga J, Kim SB, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Heeson S, et al: Pertuzumab, trastuzumab, and docetaxel in
HER2-positive metastatic breast cancer. N Engl J Med. 372:724–734.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bachelot T, Ciruelos E, Schneeweiss A,
Puglisi F, Peretz-Yablonski T, Bondarenko I, Paluch-Shimon S,
Wardley A, Merot JL, du Toit Y, et al: Preliminary safety and
efficacy of first-line pertuzumab combined with trastuzumab and
taxane therapy for HER2-positive locally recurrent or metastatic
breast cancer (PERUSE). Ann Oncol. 30:766–773. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Perez EA, López-Vega JM, Petit T, Zamagni
C, Easton V, Kamber J, Restuccia E and Andersson M: Safety and
efficacy of vinorelbine in combination with pertuzumab and
trastuzumab for first-line treatment of patients with HER2-positive
locally advanced or metastatic breast cancer: VELVET Cohort 1 final
results. Breast Cancer Res. 18(126)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Andersson M, López-Vega JM, Petit T,
Zamagni C, Easton V, Kamber J, Restuccia E and Perez EA: Efficacy
and safety of pertuzumab and trastuzumab administered in a single
infusion bag, followed by vinorelbine: VELVET Cohort 2 final
results. Oncologist. 22:1160–1168. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A and
Kaufman B: Lapatinib plus capecitabine for HER2-positive advanced
breast cancer. N Engl J Med. 355:2733–2743. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
National Comprehensive Cancer Network,
NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, 2021.
https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
|
|
15
|
Rimawi MF and Osborne CK: Revisiting the
use of HER2-targeted therapies in the adjuvant treatment of early
breast cancer. J Clin Oncol. 33:1236–1238. 2015.
|
|
16
|
Biserni GB, Engstrøm MJ and Bofin AM: HER2
gene copy number and breast cancer-specific survival.
Histopathology. 69:871–879. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gradishar WJ, Anderson BO, Abraham J, Aft
R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD,
et al: Breast cancer, version 3.2020, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 18:452–478.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sledge GW Jr, Toi M, Neven P, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
MONARCH 2: Abemaciclib in combination with fulvestrant in women
with HR+/HER2-advanced breast cancer who had progressed while
receiving endocrine therapy. J Clin Oncol. 35:2875–2884.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Modi S, Saura C, Yamashita T, Park YH, Kim
SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive breast
cancer. N Engl J Med. 382:610–621. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Barok M, Tanner M, Köninki K and Isola J:
Trastuzumab-DM1 causes tumour growth inhibition by mitotic
catastrophe in trastuzumab-resistant breast cancer cells in vivo.
Breast Cancer Res. 13(R46)2011.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Murthy RK, Loi S, Okines A, Paplomata E,
Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, et
al: Tucatinib, trastuzumab, and capecitabine for HER2-positive
metastatic breast cancer. N Engl J Med. 382:597–609.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Waddell T, Kotsori A, Constantinidou A,
Yousaf N, Ashley S, Parton M, Allen M, Starling N, Papadopoulos P,
O'Brien M, et al: Trastuzumab beyond progression in HER2-positive
advanced breast cancer: The Royal Marsden experience. Br J Cancer.
104:1675–1679. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Baselga J, Gelmon KA, Verma S, Wardley A,
Conte P, Miles D, Bianchi G, Cortes J, McNally VA, Ross GA, et al:
Phase II trial of pertuzumab and trastuzumab in patients with human
epidermal growth factor receptor 2-positive metastatic breast
cancer that progressed during prior trastuzumab therapy. J Clin
Oncol. 28:1138–1144. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Urruticoechea A, Rizwanullah M, Im SA,
Ruiz ACS, Láng I, Tomasello G, Douthwaite H, Badovinac Crnjevic T,
Heeson S, Eng-Wong J and Muñoz M: Randomized phase III trial of
trastuzumab plus capecitabine with or without pertuzumab in
patients with human epidermal growth factor receptor 2-positive
metastatic breast cancer who experienced disease progression during
or after trastuzumab-based therapy. J Clin Oncol. 35:3030–3038.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gianni L, Pienkowski T, Im YH, Roman L,
Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J,
Im SA, et al: Efficacy and safety of neoadjuvant pertuzumab and
trastuzumab in women with locally advanced, inflammatory, or early
HER2-positive breast cancer (NeoSphere): A randomised multicentre,
open-label, phase 2 trial. Lancet Oncol. 13:25–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Amiri-Kordestani L, Wedam S, Zhang L, Tang
S, Tilley A, Ibrahim A, Justice R, Pazdur R and Cortazar P: First
FDA approval of neoadjuvant therapy for breast cancer: Pertuzumab
for the treatment of patients with HER2-positive breast cancer.
Clin Cancer Res. 20:5359–5364. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
ClinicalTrials.gov: Phase III trial of pertuzumab and
trastuzumab plus endocrine therapy versus chemotherapy plus
trastuzumab and pertuzumab in patients with hormone
receptor-positive, HER2-positive primary breast cancer (Detect
V/CHEVENDO), 2015. https://clinicaltrials.gov/ct2/show/NCT02344472.
|
|
28
|
Kute T, Lackey KA and Sutton LM: Targeted
therapy in HER2-positive metastatic breast cancer: A review of the
literature. J Adv Pract Oncol. 9:401–414. 2018.
|
|
29
|
Huober J, Fasching PA, Barsoum M,
Petruzelka L, Wallwiener M, Thomssen C and Untch M: Higher efficacy
of the combination of trastuzumab and pertuzumab in the neoadjuvant
setting compared with the adjuvant setting in patients with
HER2-positive breast cancer. J Clin Oncol. 33:983–990. 2015.
|